16005030
advertisement

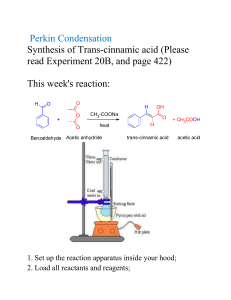
N Acetylation: The Acetylation of Aniline Introduction In an earlier experiment, we saw that the oxygen atom in an alcohol or phenol could be acetylated with acetic anhydride or acetyl chloride. These are examples of acylation reactions that produce acetate esters. If we substitute nitrogen plus an H (to account for the trivalent N), for oxygen in an alcohol we get an amine. For example, ethanol would become ethylamine. Likewise, the substitution of NH for O in phenol makes aniline, a primary aromatic amine. Figure 1 shows the structural relationships among these compounds. In these structures, nitrogen requires two hydrogen atoms to satisfy its trivalence. CH3CH2OH CH3CH2NH2 ethanol ethyl alcohol OH phenol ethanamine ethylamine NH2 aniline Figure 1. Alcohols and amines. Though the compounds in Figure 1 differ in some properties (e.g., ethanol is a neutral compound, phenol is a weak acid, and ethylamine and aniline are weak bases), they can all serve as nucleophiles because of the pair of non-bonding electrons on the heteroatom. Thus, all of these compounds react similarly with acetic anhydride or acetyl chloride. As we have seen before, acetic anhydride is formed by heating (i.e., pyrolysis) of acetic acid. Two molecules of acetic acid lose one molecule of water to form one molecule of the anhydride. When two molecules join by the loss of a small molecule such as water, the reaction is called a condensation reaction. Acetic anhydride is formed by a condensation reaction between two acetic acid molecules as shown in Figure 2. Lab 10 1 O 2 CH3COH O O CH3COCCH3 + H2O acetic anhydride acetic acid Figure 2. Preparation of acetic anhydride. Mechanism of Acetylation of Aniline Figure 3 shows the mechanism of N acylation, using acetic anhydride as the acylating agent and aniline as the substrate. O O CH3COCCH3 NH2 + O- O CH3C O CCH3 NH2 acetic anhydride aniline transfer of H+ OH O CH3C O CCH3 NH di-ion O H CH3C NH O -O CCH3 + O N CCH3 H acetanilide acetate + O HOCCH3 acetic acid Figure 3. Mechanism of N acylation. The product of an acetylation of an amine is an acetamide, which is an analog of an acetate ester. When the amine is aniline, the acetamide is called Lab 10 2 acetanilide. Thus, amides contain a nitrogen atom next to a carbonyl group. Compounds that contain a heteroatom next to a carbonyl group are acids or acid derivatives. Amides are acid derivatives. Acid derivatives are composed of an acid part and a second part, which depends on the heteroatom and overall structure. For example, esters are a combination of an acid and an alcohol, and amides are a combination of an acid and an amine. Both esters and amides are acid derivatives because they both contain an acid part. The acidic hydrolysis of acid derivatives produce the acid and the other part. Basic hydrolysis produces the acid salt and the other part. The salt must be acidified to produce the acid. Thus, amides, esters, acyl halides, and anhydrides are all acid derivatives because hydrolysis of them yields an acid. Figure 4 shows the acid hydrolysis of four derivatives of acetic acid, acetamide, ethyl acetate, acetyl chloride and acetic anhydride. Acetic acid is a product in each case, because acetic acid is the acid part of each starting compound. The second part depends on the starting material. For example, acetamide yields ammonia, ethyl acetate yields ethanol, acetyl chloride yields hydrogen chloride, and acetic anhydride yields acetic acid. Lab 10 3 O CH3CNH2 O HOH H+ acetamide an amide O CH3COCH2CH3 O HOH ethyl acetate an ester CH3CCl acetic anhydride CH3COH + HOCH2CH3 ethanol an alcohol acetic acid O HOH H+ acetyl chloride an acyl chloride or acid chloride O O CH3COCCH3 ammonia acetic acid H+ O CH3COH + NH3 CH3COH acetic acid O HOH H+ CH3COH acetic acid + HCl hydrochloric acid O + CH COH 3 acetic acid Figure 4. Hydrolysis of acid derivatives. Lab 10 4 Procedure 1. Tare a 25-mL Erlenmeyer flask on a microanalytic balance. 2. Carefully transfer 0.46-g aniline to the Erlenmeyer flask. 3. Take the Erlenmeyer flask to your bench; add 10 drops of concentrated hydrochloric acid, HCl, to the flask. 4. Add 10-mL distilled water to the Erlenmeyer flask and mix the contents. 5. Prepare a solution of sodium acetate (NaOAc) by dissolving 0.5-g anhydrous sodium acetate in 3-mL distilled water in a small beaker. 6. Add, in quick succession, 15 drops of acetic anhydride and the solution from Step 5 to the 25-mL Erlenmeyer flask. 7. Swirl the Erlenmeyer flask to mix the contents and place it in an ice-water bath. 8. Acetanilide will crystallize from the solution. When the crystallization is complete, filter the crystals on a Büchner funnel. 9. Weigh the dry crystals and then show them to the instructor. 10. Clean and return all glassware to the storages areas. Check the balance area. Return any chemicals to their original locations and turn off all balances. Lab 10 5 Questions N Acetylation Stu No___ Sec___ Last name____________________________, First _____________________ 1. Draw structures of isopropylamine, acetamide, sec-butyl alcohol, and methyl acetate. ________________ _________________ ______________ _____________ isopropylamine acetamide sec-butyl alcohol methyl acetate Write complete chemical equations for the reactions indicated below. 2. The pyrolysis (strong heating) of acetic acid. 3. The preparation of propanoyl chloride, starting with propanoic acid. 4. 2-Nitrophenol plus acetic anhydride at room temperature. 5. Phenol plus acetyl chloride, heated in the presence of aluminum chloride. 6. Aniline plus acetic anhydride at room temperature. 7. Ethanol plus propanoyl chloride at room temperature. Lab 10 6 8. Acetic anhydride plus water in an acidic medium. 9. Aniline plus acetyl chloride, when heated in the presence of aluminum chloride. 10. What family is produced by the reaction indicated in problem 6? __A. amine __B. amide __C. lactam __D. imide Lab 10 7