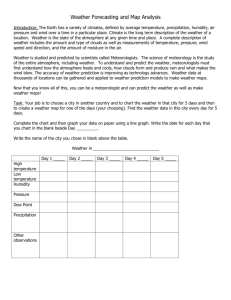
Dew Point Scan 7.4
advertisement

r.esson 7-4: Atmospheric Moisture
Water enters the atmosphere by evaporation. To understand what is
• voived with evaporation we need to revisit what temperature means.
in
. energy of a material. In other words,
Temperature
is the average k'1netic
some of the molecules of water are hotter and some are cooler. In a cup
of water, there will be a few molecules of water that are moving so fast
that they have enough energy to turn into the gas phase. If these molecules are at the surface, they will break free and escape into the atmosphere, taking its extra heat
with it. Evaporation has just
There are two ways that water can
taken place. This cools the
enter the atmosphere: evaporation and
water that is left behind,
sublimation (boiling is "fast
which is why we feel cool
evaporation"). Sublimation happens
when water evaporates off
when molecules of a solid go directly
of our skin. The warmer the
into the gas phase without first
water is to begin with, the
becoming a liquid. Once again, this has
more molecules there wilJ
to do with the "average kinetic energy."
be that have enough energy
In an ice cube, most of the molecules of
to evaporate.
water will have very little movement.
If you look at the molHowever, there will be a few that are
very energetic-energetic enough to
ecules of water vapor in the
become a gas. These molecules will
air with the same perspecescape the ice and become atmospheric
tive, there will be some molmoisture. Ice cubes in your freezer will
ecules that no longer have
sublime. This is why old ice cubes often
enough energy to stay in the
look as if someone only filled the tray
gaseous phase. These n1olhalf-way. The 1noisture escaped into
ecules will condense on the
the atmosphere in your freezer and you
nearest surface and prosee a cloud of moisture spill out when
duce a tiny droplet of liqyou open the door.
uid water (condensation).
1
)~-----H_o_m_ew_o_r_k_H_e_lp_e_rs_:_E_art_h_Sc_ie_n_ce_ _ _ _ ____
212
Hun1idity in the air is a constant balance between evaporation and
condensation. The primary factors a re how much ene rgy and water are
available. In a warm environment, there will be more evaporation. Conde nsation will still happen, but at a lesser rate.
There are two types of humidity that are used to describe the moi~ture content of the atmosphere: absolute humidity and relative hum idity.
Absolute humidity is a measure of the mass of water in a volume of ai r.
Absolute humidity is not used very much in meteorology, so relative humidity is the one we will concentrate on here.
The amount of water that the air can hold is called its capacity. Capacity depends on the temperature. Because the highest-energy molecules
are the ones that evaporate and get into the air, warmer air can hold more
moisture than cooler air. In other words, warm air has a higher capacity of
moisture than cold air.
Relative Humidity
Relative humidity tells us how close the air is to being " full " of moisture and has more practical applications than absolute humidity. Relative
humidity is a percentage of how much moisture is currently in the air as
compared to the capacity of the air at that temperature. It is actually inaccurate to say that the air is " full" of moisture-air that is full of moisture is
called a lake. The air reaches its ca pacity when the rate of evaporation
and condensation equal each other. At this point the air is saturated.
To measure relative humidity, you need an instrument called a sling
psychrometer. The psychrometer is simply two thermometers: a regular
one ( called the "dry bulb" thermometer) and one with a moiste ned cloth
over the bulb at the bottom (called the "wet bulb" thermometer). In prii~ciple, here's how it works: When water evaporates off of the wl't l'"•' th 11
also takes away some of the heat in the thermometer. This makl'S thl' thermometer colder tha n the air and " depresses" the te mperature. Thl' amount
of the wet bulb de pressio n directly de pe nds on how much 11H,iscu rL' r a~i
evaporate into the air. If the tempe rature dropped by a littll'. thl' t"l' \\ ;t~ n~
. n ancI t I1c air
. .1s c Iose to saturat1nn
. . 11· t Iw 1·LI 11p·l r-t(lll
l
muc h e va poratio
•
dropped by several degrees, the air is dry.
Dew Point
.
.
. .
1·1 '\ 1,l,th
Dew po111t an d rc la t1 vc liun11d1t y an.· very dn"-IL' I\' 1L·bt L·d. H. .
.
1
.
.
1·
.
.
·1
.
.
I>
,i
nt
1..,
l
ll
give an Ill <. 1ca t1 0 11 o l 10w muc h wa ll·r is in thl· alml1, plll' rt..'. ~,, P4
·
,
.
.
l1lllll 1' ·
tc rnpl'. r:1t u r e t ha t Y" ll will 11 L'.l' d l o d 11II t lw a ir, u11lkr L' ll t n : 111 Ll llll
Meteorology and Energy in the Atmosphere
213
.
der for dew (condensation) to form. If you drink a glass of soda, the
of the glass will "sweat" once the temperature of the o utside of the
outs1 reaches
e
.
If it
. ts
. current Iy d ry at ground level o n a 700F
the dew pomt.
glass nd the dew point is S0°F, clouds will be forming at an altitude where
day a
.
o
1
und-level
air
has
coo
ed
to
50
F.
the gro
10 0 ~d
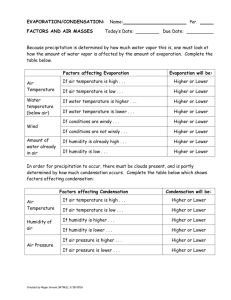
To Use a Relative Humidity Chart
The important thing to do when using this chart is to read every
word on the chart before you start work. The big mistake that
people make is to read the numbers at the top of the chart and
think that they are the wet-bulb or the dew point values. At the top
of the chart, it says "difference between wet-bulb and dry-bulb."
You must read and understand that this means that you have to
subtract. Once you get past that trap, the rest is easy.
Relative Humidity (%)
Dry-Bulb
Dtlference Between Wet-Bulb and Dry-Bulb Tempenlwes (~)
Tempera-
tur• re>
-20
0
1
2
~
...
s
6
7
8
100
100
100
100
28
55
11
1nn
61
7-1
66
71
33
_,
100
100
100
100
48
54
13
20
32
11
-2
1nn
73
77
79
AA
37
20
1
0
81
63
45
28
11
83
67
70
51
36
20
56
6
100
100
100
100
59
27
35
22
8
100
87
72
74
42
46
51
~Q
?R
17
6
10
12
100
100
100
100
88
88
89
76
78
79
54
-43
33
24
38
28
,nn
90
91
100
100
100
100
91
92
92
13
19
25
29
33
36
40
-42
45
-18
-16
-14
-12
-10
-8
-6
2
4
14
16
18
20
22
24
26
28
1nn
100
30
9
10
11
12
13
15
14
40
48
85
86
92
93
93
41
"~'
65
6
14
10
57
.a
60
62
50
54
41
33
80
67
69
71
45
37
81
72
R4
40
82
74
75
76
58
60
62
51
44
53
64
57
46
49
51
AR
77
78
66
68
69
70
71
""
.ii.A
53
86
79
72
""
66
'-,Q
61
55
83
84
8S
55
47
49
-4
10
16
21
?Ei
30
33
36
39
42
«
2
8
14
19
23
27
30
1
7
12
1
6
17
21
25
11
15
34
28
20
23
'lR
31
76
39
34
29
5
10
14
-4
g
4
?1
13
17
'\2
25
20
16
18
9
Figure 7. 7
Example:
Air Temperature
=
l8°C
Wet-bulb
=
14°C
t> Take the current air temperature, that's the dry-bulb,
and slide down the side of the chart until you see that
temperature. If it is an odd number, just go down to
the next printed value for now.
)~----~H~o~m~e_w_or_k_H_e_lp~e_r_s:_E_a_rt_h_S_c_ie_nce
_ _ _ _ _ _ __
214
Slide across to the difference betwee n the two
thermometers: 4°C.
I> The box you are in is the relative humidity: 64%
t>
I> If you had an odd number for the tempe rature, just
average this number and the one in the box directly
above.
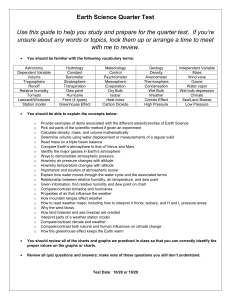
To Use a Dew Point Temperature Chart
The dew point temperature chart works exactly the same, so there's
no new reading here. Very often, problems will ask you to solve for
the relative humidity and dew point temperature for a set of temperatures. The beauty of this is that you only have to work the charts
once, and both answers will be in the same square on each chart.
Dewpoint Temperatures (°C)
Ory-Bulb
Temperatwe fC)
0
1
- 20
-20 -33
-18
-1 8 -28
-16
-14
-12
10
8
-6
- 14 - 21
-12 -18
10 14
8 -1 2
-6 -1 0
4
2
4
6
2
4
6
8
8
10
20
22
24
26
28
JO
2
3
'
5
11
12
13 21
-9 -14
-5 -9 -14 -28
2
5
9 16
1 -2 -5 -10 -17
4
1 -1 -6 -10 -17
7
4
2
2
5 -10
10
7
4
2 ~
5
12 10
8
5
3
-1
14 12 10
8
6
2
17 15
13 11
9
6
19
17
16 14
11
9
21
19
18 16 14 12
19
6
7
8
9
10
cc•>
13
1,
15
-16 - 24
-4
2
0
12
14
16
18
Dlffere.nce Between Wet-Bulb and Dry-Bulb Temperaturn
2
0
10
12
14
16
18
20
22
24
26
28
30
-36
-28
22
-18 -29
-14 -22
-7 -12 17 29
5
8 13 20
-3 -6 - 9 -15 -24
- 1 -3 - 6 -11 -17
1
1 -4
7 -11
4
1 -1
4 -7
1 -2
6
3
5
8
6
4
1
2
10
8
6
4
1
12 11
9
6
4
14 13 11
9
7
16 15 13 11
9
19 17 15 14 12
21 19 17 16 14
23
21 20 18 16
25 23 22 20 18
27 25 24 22 21
29 27 26 24
23
19
10 19
-5 - 10 -1 9
_,
3
7
10
s
0
4
8
_..
- 10 -1 8
1
9
-3
5
1
Figure 7.8
Example ( same conditions as the . 1 .
. .
now we 're finding the d
.
i e at1ve hum1d1ty e:xampk. hul
.
ew point temperature):
Air Temperature = t 8oC
W et-bulb = 1➔ c
0
!> T ake the current air tcm , .. .
.
·ind sl1'de J
I ·
pctctlute, thats thl.' drv-bulb
..
·
( own t 1c side of t I
·
·
temperature If ·t ,: .
1c cha rt unt il yo u Sl.'l.' thar
•
I ts ctn ndd numb , . . .
the next prinl ed v· I , 1· .
l: t , .rust !,!.l) dl)\\ll lll
c1 llL
01
now.
--<C 215
__ _ _ _ _ _ _M_e_t_eo_r_ol_o_igy_an_d_E_ne_r~
gy_in_t_h_
e _A_tm_os~p_h_
er_e_ _ _
r>
Slide across to the diffe rence between the two
thennome te rs: 4°C.
r>
The box you are in is the dew point tempe rature :
11 °C.
r>
If you had an odd number for the temperature, just
average this nun1ber and the one in the box directly
above.
In order for condensation to occur, water needs a surface on wh ich to
condense. At ground level, you can see this as dew on blades of grass or on
the hoods of cars. However, in the air, you rarely find blades of grass or
car hoods, yet condensation still happens in large amounts. The surfaces
that water condenses onto are usually small particles of dust, smoke, or
salt (from sea spray). When acting as a surface for condensation, these
particles are called condensation nuclei. Normally, the atmosphere has
enough condensation nuclei to allow cloud formation. However, artificial
nuclei can be added by "seeding" the clouds with smoke, which may stimulate more condensation. After a rain, the atmosphere is usuaIJy cleaner
and clearer because dust and other pollutants have been stripped from
the air.
Homework Helpers: Earth Science
~16 ) } -- - - - - - . : , _ _ - -- - - - ~ - - -
---- -
CLesson 7-4 Review :>----------- - -----E,C'rcisc- .-'\
C(,mpktc th"-' fl,l l\)\\ in g. c hart u~inµ th e l"l' l.tt ivc humiuity and d ew
.
P<11nt
tcmpcratun.' charts.
/
D~· Bulb
\\·l't
Bulb
\.~oc
7cc
-·
..,
22''('
2n°c
-"·
..
l (''° C
12°c
4.
6°C
s c
5.
22~c
6.
20::,C
-
I •
19cc
19°c
8.
17°c
13°c
9.
26~c
24°c
10.
l6°C
12°c
\.
Rdati, c Humidity
1
Dew Point Tem p.
-
-
0
21°c
66%
Exercise B
For each of the following, write either evaporation or condensation.
11 . The process by which a substance changes from a liquid to a gas is
called - - - - - - - - 12. During _ _ _ _ _ _ _ _ more molecules break free from a
liqu id than join it.
13. When more molecules join a liquid than leave, _ _ _ _ _ _ __
ta ke~ place.
14 . When molecull:s of water vapor collide and stick togethe r in the air.
- -- - - - - -- occurs.
Meteorology and Energy in the Atmosphere
217
From the following list, choose the term that best completes each
sentence.
evaporation
sublimation
melting
freezing
kinetic energy
condensation
15. The higher the temperature of something, the greater its _ _ __
16. The change in state from gas to liquid is called _ _ _ _ _ _ __
17. Liquid changing to gas only at the surface is called
18. The change in state from solid to liquid is called _ _ _ _ _ __
19. The change in state from liquid to solid is called _ _ _ _ _ __
20. In
--------
particles pass directly from solid to gas.