worksheet 1
advertisement

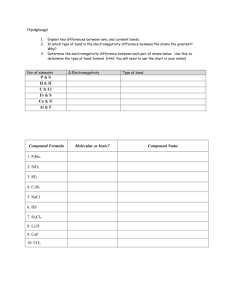
BIO 201: Work sheet 1- Lesson 1 (atoms, bonds, pH, isomers) Answer the following questions by referring to your text book, lesson presentation and other resources Set 1 Fill in the blanks with following wordsPositive element(s) compound(s) energy level(s) number(s) force(s) atom(s) ion(s) charged nucleus electron(s) negative 1. An atom is chemically stable when its outer ---------------is completely filled with------------2. A chemical bond is a --------------that holds together --------------in a compound. 3. An --------------that has lost or gained ---------is called an ion. 4. An ionic bond is the ---------------of attraction between the opposite charges of the --------in an ionic compound. 5. The attraction that forms between ---------------when they share------------is known as a covalent bond. 6. A polar molecule has a slightly -----------------end and a slightly----------------end. 7. A nonpolar molecule does not have oppositely --------------ends. 8. Protons have a _________ charge and are found here in atoms: Set 2 1. Which element in water has a slightly negative charge? 2. A----------------- bond is the force of attraction between the opposite charges of the ions in an ionic compound. 3. ---------------sharing of electrons in covalent bonds results in polar molecules. 4. An ion is a -- --------particle that has either more or fewer electrons than protons. 5. ____ ___________ -an ion with a positive charge 6. ---------------the force that holds cations (metals) and anions (nonmetals) together. Answer the following1. 2. 3. 4. 5. CH3OH has ___ _____ elements in it. How many carbon atoms does the compound CO2 have? How many oxygen atoms does the compound H2O have? How many hydrogen atoms does the compound H2O have? How many carbon atoms are in: C2H4? Set 3 1. Which of the following statements about chemical bonds is true? A. B. C. D. Covalent bonds are stronger than ionic bonds. Hydrogen bonds occur between two atoms of hydrogen. Bonding readily occurs between nonpolar and polar molecules. A molecule of water is unlikely to bond with an ion. 2. A. B. C. What kind of isomers are these? Cis/Trans Optical Structural 3. A. B. C. What kind of isomers are these? Cis/Trans Optical Structural 4. In condensation /dehydration reactionsA. Water is lost from both reactants B. Water is added to both reactants C. Water is lost from products D. Water is added to products 5. Which statement about Hydrolysis reactions is NOT true A. In hydrolysis large molecules are broken into smaller ones B. Polymers are broken into monomers C. Water is added to a large molecule D. Water is added to two small molecules to make a large molecule 6. Carboxyl groups make the compound moreA. Acidic B. Basic C. Soluble D. Insoluble 7. Hydroxyl groups make the compound moreA. Acidic B. Basic C. Soluble D. Insoluble Set 4- Acids and bases 1. Give an example of an acid found in something you eat 2. A. B. C. What is the environment like inside your stomach? Acidic Basic Neutral 3. The pH scale is from ---------------- to ---------------4. Acids have numbers ----------than 7 A. Greater B. Lesser 5. Bases have numbers ----------than 7 A. Greater B. Lesser 6. A substance with pH 7 like distilled water is called ---------7. Acidic solutions have lot of --------- ions present 8. Basic solutions have lot of--- ------ ions present Set 5- Identify functional groups- H C H H 3 CO H O C H 3 C H O C H 3 C O H