properties of water essay and scoring
advertisement
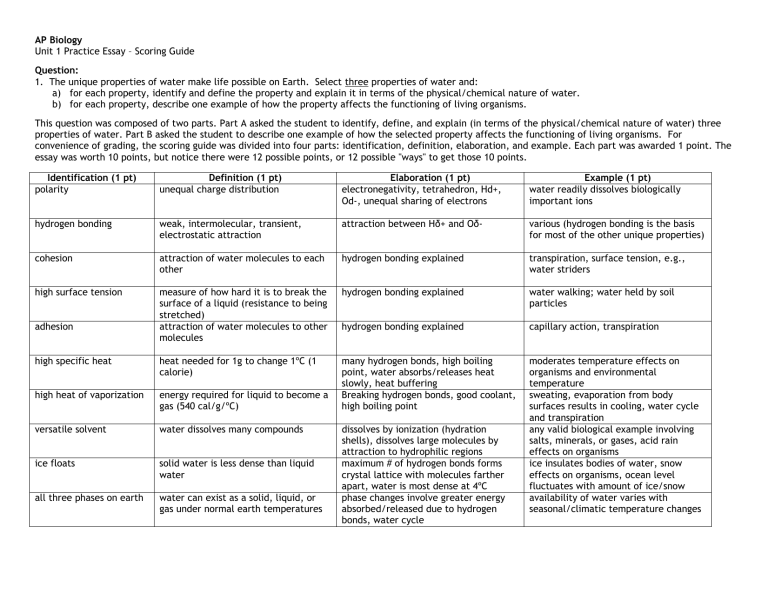
AP Biology Unit 1 Practice Essay – Scoring Guide Question: 1. The unique properties of water make life possible on Earth. Select three properties of water and: a) for each property, identify and define the property and explain it in terms of the physical/chemical nature of water. b) for each property, describe one example of how the property affects the functioning of living organisms. This question was composed of two parts. Part A asked the student to identify, define, and explain (in terms of the physical/chemical nature of water) three properties of water. Part B asked the student to describe one example of how the selected property affects the functioning of living organisms. For convenience of grading, the scoring guide was divided into four parts: identification, definition, elaboration, and example. Each part was awarded 1 point. The essay was worth 10 points, but notice there were 12 possible points, or 12 possible "ways" to get those 10 points. Identification (1 pt) polarity Definition (1 pt) unequal charge distribution Elaboration (1 pt) electronegativity, tetrahedron, Hd+, Od-, unequal sharing of electrons Example (1 pt) water readily dissolves biologically important ions hydrogen bonding weak, intermolecular, transient, electrostatic attraction attraction between Hδ+ and Oδ- various (hydrogen bonding is the basis for most of the other unique properties) cohesion attraction of water molecules to each other hydrogen bonding explained transpiration, surface tension, e.g., water striders high surface tension measure of how hard it is to break the surface of a liquid (resistance to being stretched) attraction of water molecules to other molecules hydrogen bonding explained water walking; water held by soil particles hydrogen bonding explained capillary action, transpiration many hydrogen bonds, high boiling point, water absorbs/releases heat slowly, heat buffering Breaking hydrogen bonds, good coolant, high boiling point moderates temperature effects on organisms and environmental temperature sweating, evaporation from body surfaces results in cooling, water cycle and transpiration any valid biological example involving salts, minerals, or gases, acid rain effects on organisms ice insulates bodies of water, snow effects on organisms, ocean level fluctuates with amount of ice/snow availability of water varies with seasonal/climatic temperature changes adhesion high specific heat heat needed for 1g to change 1ºC (1 calorie) high heat of vaporization energy required for liquid to become a gas (540 cal/g/ºC) versatile solvent water dissolves many compounds ice floats solid water is less dense than liquid water all three phases on earth water can exist as a solid, liquid, or gas under normal earth temperatures dissolves by ionization (hydration shells), dissolves large molecules by attraction to hydrophilic regions maximum # of hydrogen bonds forms crystal lattice with molecules farther apart, water is most dense at 4ºC phase changes involve greater energy absorbed/released due to hydrogen bonds, water cycle





