electron config
advertisement

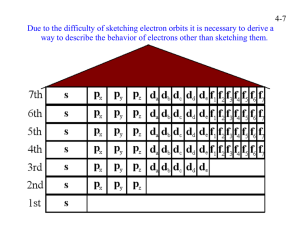
Periodic Table Puzzle 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 G H 18 I F B C E J D Place the letter of each of the above elements next to its description below. 1. An alkali metal _____ 2. An alkaline earth metal _____ 3. An inert gas _____ 4. An active nonmetal _____ 5. A semi-metal or metalloid _____ 6. A lanthanide series element _____ 7. Its most common oxidation state (charge) is –2 _____ 8. A metal with more than one oxidation state _____ 9. Metal with an oxidation number of +3 _____ 10. Has oxidation numbers of +1 and –1 _____ A Electron Configuration (Level 1, Level 2, Level 3) Electrons are distributed in the electron cloud into principle energy levels (1, 2, 3…), sublevels (s, p, d, f), orbitals (s has 1, p has 3, d has 5, f as 7) and spin (two electrons allowed per orbital; spin up, spin down). These constitute the 4 quantum numbers of an electron. The electron configurations level 1, 2 and 3 are was to simplify writing all the quantum numbers for each electron. Level 1: This is also known as the box and arrow diagram. Here, boxes or lines are drawn to represent each orbital and the corresponding level and sublevel is written below it. Level 2: This is the condensed for of level one. Here just the level and sublevel symbols are written and the total number of electrons in that sublevel is written as an exponent. Level 3: This is also known as the noble gas configuration. Here you backtrack numerically to find the closest noble gas. Write the symbol for it in braces [ x ] and write out the level 2 information after where the noble gas is. Example: Sodium (11 electrons) 1. 1s 2. 1s22s22p63s1 3. [Ne]3s1 2s 2p 3s Draw the electron configurations (Level 1, 2 and 3) for the following atoms. Level 1 Level 2 Level 3 Br P Ge Ba Cu* (borrows) Orbital Filling Rules The rules that you have been applying in order to determine the electronic configuration of an atom are summarized below. A. Lowest energy orbitals are filled first. THE AUFBAU PRINCIPLE. B. Orbitals can only contain a maximum of two electrons and when two electrons enter the same orbital they must have opposite spins (+ ½ or – ½). (In the electrons in boxes diagram they must be drawn NOT OR ). THE PAULI EXCLUSION PRINCIPLE. C. When orbitals of identical energy (degenerate) are available electrons enter these orbitals singly before any spin pairing takes place. HUNDS RULE. D. There are some notable exceptions. For example Cr and Cu achieve extra stability by forming a half-filled and completely filled d sub-shell respectively by using one of their 4s electrons. Consider each of the elements listed and the INCORRECT electronic configuration associated with each one. In each case identify which of the above rules or principles (A, B, C or D) is violated and insert the correct electronic configuration. Then add a possible set of quantum numbers for the outer most electron. An example is completed for you. POSSIBLE SET OF QUANTUM INCORRECT CORRECT NUMBERS FOR ELEMENT CONFIGURATION VIOLATION CONFIGURATION OUTERMOST ELECTRON N 1s2 2s2 2px2 2py1 Al B P Cu Mg 1s2 2s2 2p6 3p3 1s2 2s3 2 1s 2s2 2p6 3p5 [Ar] 4s2 3d9 [Ne] 1s2 2s1 2px1 2py1 2pz1 1s2 2s2 2px2 [Kr] 5s2 4d9 [Ar] 4s1 3d6 [Ar] 4s2 3dxy2 3dxz2 3dyz2 3dz22 3dx2-y20 [Ne] [Ar] 3d3 2 1s 2s1 2px1 2py1 1s1 2s2 2p6 3s2 [Ne] 3s2 3px2 3py2 [Ar] 3d5 [Ne] 3s2 3px2 3py1 [Ar] 4s2 3d16 [Ar] 4s2 3d4 C C Ag Mn Ni Cl Sc B Na S V P Kr Cr C 1s2 2s2 2px1 2py1 2pz1 2 1 -1 +½ Quantum Numbers Fill in the table with the appropriate quantum number for the last electron added to the shell for each atom listed. n H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge Se Br Kr Rb Sr Y Zr l ml ms Periodic Table Worksheet 1. Where are the most active metals located? _______________________________ 2. Where are the most active nonmetals located? ____________________________ 3. As you go from left to right across a period, the atomic size (decreases/increases). Why? ____________________________________________________________ 4. As you travel down a group, the atomic size (decreases/increases) Why? __________________________________________________________________ 5. A negative ion is (larger/smaller) than its parent atom? _____________________ 6. A positive ion is (larger/smaller) than its parent atom? _____________________ 7. As you go from left to right across a period, the first ionization energy generally (decreases/increases). Why? _________________________________________ 8. As you go down a group, the first ionization energy generally (decreases/ increases). Why? __________________________________________________ 9. Where is the highest electronegativity found? ____________________________ 10. Where is the lowest electronegativity found? _____________________________ 11. Elements of group 1 are called? _______________________________________ 12. Elements of group 2 are called? _______________________________________ 13. Elements of groups 3 – 12 are called? ___________________________________ 14. As you go from left to right across the periodic table, the elements go from (metals/nonmetals) to (metal/nonmetals). 15. Group 17 elements are called? Group 16? Group 15? _____________________ 16. The most active elements in group 17 is? ________________________________ 17. Group 18 elements are called? ________________________________________ 18. What sublevels are filling across the transition elements? The Lanthanides and Actinides? The Main block elements? __________________________________ 19. Elements within a group have a similar number of ________________________. 20. Elements across a period have the same number of ________________________. 21. A colored ion generally indicated a ____________________________________. 22. As you go down a group, the elements generally become (more/less) metallic. 23. The majority of the elements in the periodic table are (metals/nonmetals). 24. Elements in the periodic table are arranged according to their ______________. 25. An element with both metallic and nonmetallic properties is called a _________.