Bio practise Question
advertisement
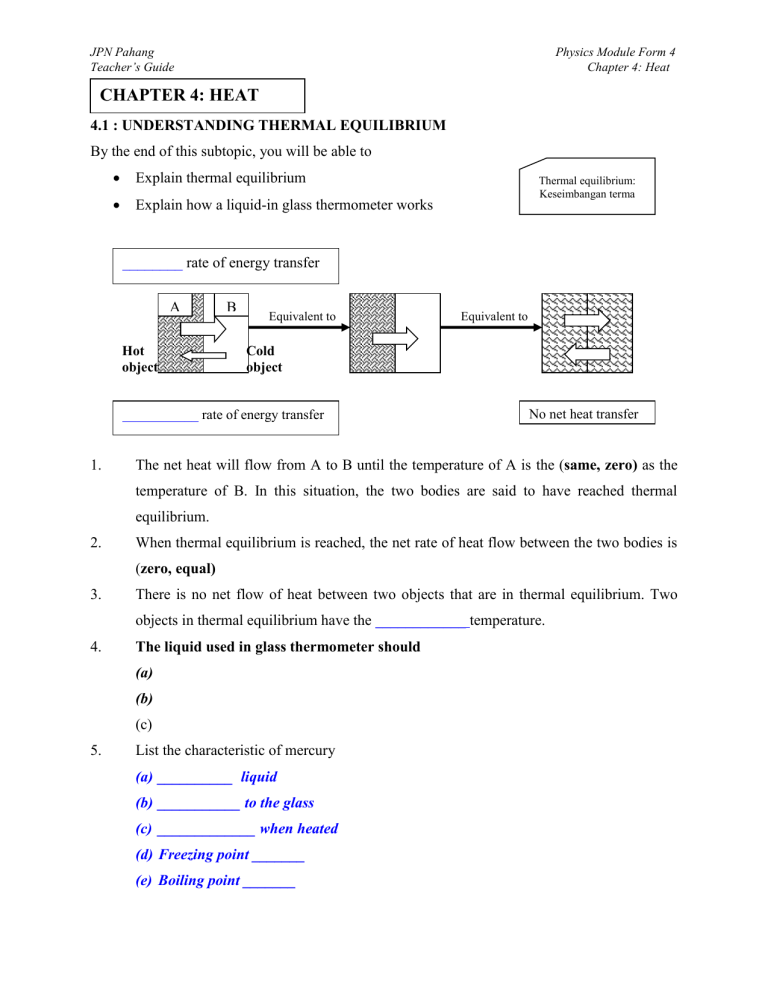
JPN Pahang Teacher’s Guide Physics Module Form 4 Chapter 4: Heat CHAPTER 4: HEAT 4.1 : UNDERSTANDING THERMAL EQUILIBRIUM By the end of this subtopic, you will be able to Explain thermal equilibrium Explain how a liquid-in glass thermometer works Thermal equilibrium: Keseimbangan terma ________ rate of energy transfer A Hot object B Equivalent to Cold object ___________ rate of energy transfer 1. Equivalent to No net heat transfer The net heat will flow from A to B until the temperature of A is the (same, zero) as the temperature of B. In this situation, the two bodies are said to have reached thermal equilibrium. 2. When thermal equilibrium is reached, the net rate of heat flow between the two bodies is (zero, equal) 3. There is no net flow of heat between two objects that are in thermal equilibrium. Two objects in thermal equilibrium have the ____________ temperature. 4. The liquid used in glass thermometer should (a) (b) (c) 5. List the characteristic of mercury (a) __________ liquid (b) ___________ to the glass (c) _____________ when heated (d) Freezing point _______ (e) Boiling point _______ JPN Pahang Teacher’s Guide Physics Module Form 4 Chapter 4: Heat 6. (Heat, Temperature) is a form of energy. It flows from a hot body to a cold body. 7. The SI unit for (heat, temperature) is Joule, J. 8. ( Heat , Temperature ) is the degree of hotness of a body 9. The SI unit for (heat, temperature) is Kelvin, K. 10. ___________ fixed point (l 0 )/ ice point 11. ____________ fixed point( l 100)/steam point: the temperature of steam from water that is : the temperature of pure melting ice/00C boiling under standard atmospheric pressure /1000C Temperature, θ = l0 l100 lθ lθ - l0 x 1000C l100 - l0 : length of mercury at ice point : length of mercury at steam point : length of mercury at θ point Exercise 4.1 Section A: Choose the best answer 1. The figure shows two metal blocks. Which the following statement is false? A. B. C. D. 2. B. It warms the water of the tea C. It turns into heat energy and disappears. 3. Which of the following temperature corresponds to zero on the Kelvin scale? A. 2730 C B. 00C C. -2730 C D. 1000 C 4. How can the sensitivity of a liquid- in – glass thermometer be increased? A. Using a liquid which is a better conductor of heat P and Q are in thermal contact P and Q are in thermal equilibrium Energy is transferred from P to Q Energy is transferred from Q to P When does the energy go when a cup of hot tea cools? A. It warms the surroundings JPN Pahang Teacher’s Guide B. Using a capillary tube with a narrower bore. C. Using a longer capillary tube D. Using a thinner-walked bulb 5. Which instrument is most suitable for measuring a rapidly changing temperature? A. Alcohol-in –glass thermometer B. Thermocouple C. Mercury-in-glass thermometer D. Platinum resistance thermometer Physics Module Form 4 Chapter 4: Heat 6. When shaking hands with Anwar, Kent Hui noticed that Anwar’s hand was cold. However, Anwar felt that Kent Hui hand was warm. Why did Anwar and Kent Hui not feel the same sensation? A. Both hands in contact are in thermal equilibrium. B. Heat is flowing from Kent Hui’s hand to Anawr’s hand C. Heat is following from Anwar’s hand to Kent Hui hand. Section B: Answer all the questions by showing the calculation 1. The length of the mercury column at the ice point and steam point are 5.0 cm and 40.0cm respectively. When the thermometer is immersed in the liquid P, the length of the mercury column is 23.0 cm. What is the temperature of the liquid P? 2. The length of the mercury column at the steam point and ice point and are 65.0 cm and 5.0cm respectively. When the thermometer is immersed in the liquid Q, the length of the mercury column is 27.0 cm. What is the temperature of the liquid Q? JPN Pahang Teacher’s Guide 3. Physics Module Form 4 Chapter 4: Heat The distance between 00C and 1000C is 28.0 cm. When the thermometer is put into a beaker of water, the length of mercury column is 24.5cm above the lower fixed point. What is the temperature of the water? 4. The distance between 00C and 1000C is 25 cm. When the thermometer is put into a beaker of water, the length of mercury column is 16cm above the lower fixed point. What is the temperature of the water? What is the length of mercury column from the bulb at temperatures i) 300C JPN Pahang Teacher’s Guide Physics Module Form 4 Chapter 4: Heat SECTION C: Structured Questions 1. Luqman uses an aluminium can, a drinking straw and some plasticine to make a simple thermometer as shown in figure below. He pours a liquid with linear expansion into the can. (a) Suggest a kind of liquid that expands linearly. (1m) ……………………………………………………………………………………………. (b) He chooses two fixed points of Celsius scale to calibrate his thermometer. State them (2m) ……………………………………………………………………………………………… ……………………………………………………………………………………………… (c) If the measurement length of the liquid inside the straw at the temperature of the lower fixed point and the upper fixed point are 5cm and 16 cm respectively, find the length of the liquid at 82.50C. (d) Why should he use a drinking straw of small diameter? ……………………………………………………………………………………………… (e) What kind of action should he take if he wants to increase the sensitivity of his thermometer? ……………………………………………………………………………………………… ……………………………………………………………………………………………… JPN Pahang Teacher’s Guide 2. Physics Module Form 4 Chapter 4: Heat What do you mean by heat and temperature? …………………………………………………………………………………………….... ……………………………………………………………………………………………… ……………………………………………………………………………………………… 4.2 : UNDERSTANDING SPECIFIC HEAT CAPACITY By the end of this subtopic, you will be able to 1. Define specific heat capacity State that c = Q/MCθ Determine the specific heat capacity of a liquid Determine the specific heat capacity of a solid Describe applications of specific heat capacity Solve problems involving specific heat capacity Heat capacity Muatan haba Specific heat capacity Muatan haba tentu The ____________ of a body is the ______________ that must be supplied to the body to increase its temperature by _________. 2. 3. The heat capacity of an object depends on the (a) ………………………………………………………………………………………. (b) ………………………………………………………………………………………. (c) ……………………………………………………………………………………… The ____________ of a substance is the amount of heat that must be supplied to increase the temperature by 1 0C for a mass of 1 kg of the substance. Unit ________ Specific heat capacity, c = Q__ m∆θ 4. The heat energy absorbed or given out by an object is given by Q = _________ 5. High specific heat capacity absorb a large amount of heat with only a ________ temperature increase such as plastics. JPN Pahang Teacher’s Guide 6. Physics Module Form 4 Chapter 4: Heat Conversion of energy Heater Electrical energy Power = P _________ energy __________energ y Object falls from A high position Moving object stopped due to friction Heat energy _________ Heat energy ___________ Heat energy __________ Power = P 7. Applications of Specific Heat Capacity _____________ increase in temperature Small value of c _________ Increase in temperature Two object of equal mass Big value of c Equal rate of heat supplied Explain the meaning of above application of specific heat capacity: (a) (i) Water as a coolant in a car engine __________ is a good example of substance with a high specific capacity. It is used as a ___________ to prevent overheating of the engine .Therefore, water acts as a ____________ as it can absorb a great amount of heat before it boils. JPN Pahang Teacher’s Guide (b) Physics Module Form 4 Chapter 4: Heat Household apparatus and utensils ………………………………………………………………………………………... ………………………………………………………………………………………... ………………………………………………………………………………………... ………………………………………………………………………………………... (c) Sea breeze ……………………………………………… ……………………………………………… ……………………………………………… ……………………………………………… ……………………………………………… ……………………………………………… ……………………………………………… (d) Land breeze ……………………………………………… ……………………………………………… ……………………………………………… ……………………………………………… ……………………………………………… ……………………………………………… JPN Pahang Teacher’s Guide Physics Module Form 4 Chapter 4: Heat Exercise 4.2 SECTION A : Choose the best answer 1. The change in the temperature of an object does not depend on A. the mass of the object B. the type of substance the object is made of C. the shape of the object D. the quantity of heat received 2. Which of the following defines the specific heat capacity of a substance correctly? A. The amount of heat energy required to raise the temperature of 1kg of the substance B. The amount of heat energy required to raise 1kg of the substance by 10C. C. The amount of heat energy required to change 1kg of the substance from the solid state to the liquid state. 3. Heat energy is supplied at the same rate to 250g of water and 250g of ethanol. The temperature of the ethanol rises faster. This is because the ethanol.. A. is denser than water B. is less dense than water C. has a larger specific heat capacity than water D. has a smaller specific heat capacity than water 4. In the experiment to determine the specific heat capacity of a metal block, some oil is poured into the hole containing thermometer. Why is this done? A. To ensure a better conduction of heat B. To reduce the consumption of electrical energy C. To ensure the thermometer is in an upright position. D. To reduce the friction between the thermometer and the wall of the block. JPN Pahang Teacher’s Guide Physics Module Form 4 Chapter 4: Heat SECTION B: Answer all questions by showing the calculation 1. How much heat energy is required to raise the temperature of a 4kg iron bar from 320C to 520C? (Specific heat capacity of iron = 452 Jkg-1 0C-1). 2. Calculate the amount of heat required to raise the temperature of 0.8 kg of copper from 350C to 600C. (Specific heat capacity of copper = 400 J kg-1 C-1). 3. Calculate the amount of heat required to raise the temperature of 2.5 kg of water from 320C to 820C. (Specific heat capacity of water = 4200 J kg-1 C-1). 4. 750g block of an aluminium at 1200C is cooled until 450C. Find the amount of heat is released. . (Specific heat capacity of aluminium = 900 J kg-1 C-1). JPN Pahang Teacher’s Guide 5. Physics Module Form 4 Chapter 4: Heat 0.2 kg of water at 700C is mixed with 0.6 kg of water at 300C. Assuming that no heat is lost, find the final temperature of the mixture. (Specific heat capacity of water = 4200 J kg1 C-1) SECTION C: Structured questions 1. In figure below, block A of mass 5kg at temperature 1000C is in contact with another block B of mass 2.25kg at temperature 200C. 5kg 2.25kg A B 1000C 200C Assume that there is no energy loss to the surroundings. (a) Find the final temperature of A and B if they are in thermal equilibrium. Given the specific heat capacity of A and B are 900 Jkg-1 C-1 and 400 Jkg-1 C-1 respectively. JPN Pahang Teacher’s Guide Physics Module Form 4 Chapter 4: Heat (b) Find the energy given by A during the process. (c) Suggest one method to reduce the energy loss to the surroundings. …………………………………………………………………………………………..