lecture 2
advertisement

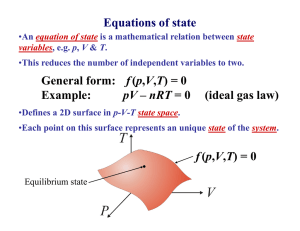
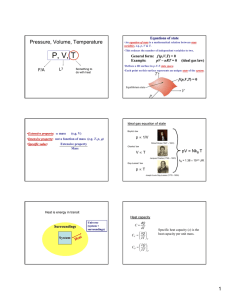
Thermal Physics • Too many particles… can’t keep track! • Use pressure (p) and volume (V) instead. Pressure, Volume, Temperature P, V, T F/A L3 Something to do with heat Before figuring out what is temperature… What is heat? Heat is energy in transit Surroundings System Universe (system + surroundings) Empirical relation between temperature and heat Heat capacity dQ C dT Q CV T V Q CP T P Specific heat capacity (c) is the heat capacity per unit mass. Equations of state •An equation of state is a mathematical relation between state variables, e.g. p, V & T. •This reduces the number of independent variables to two. General form: f (p,V,T) = 0 Example: pV – nRT = 0 (ideal gas law) •Defines a 2D surface in p-V-T state space. •Each point on this surface represents an unique state of the system. f (p,V,T) = 0 Equilibrium state State variables •Extensive property: mass (e.g. V) •Intensive property: not a function of mass (e.g. T, p, ) •Specific value: Extensive property Mass Heat is NOT a state variable Ideal gas equation of state Boyle’s law p 1/V Robert Boyle (1627 – 1691) Charles’ law pV = NkB T VT Jacques Charles (1746 – 1823) Gay-Lussac’ law pT Joseph Louis Gay-Lussac (1778 - 1850) kB = 1.38 10-23 J/K What is temperature? Temperature is what you measure with a thermometer Temperature is the thing that’s the same for two objects, after they’ve been in contact long enough. Long enough so that the two objects are in thermal equilibrium. Time required to reach thermal equilibrium is the relaxation time. Temperature is a measure of the tendency of an object to spontaneously give up/absorb energy to/from its surroundings. Diathermal wall is a boundary that freely allows heat to be exchanged i.e. very short relaxation time for systems separated by a diathermal wall. T 1- T 2 T 1- T 2 Adiabatic wall time time Zeroth law of thermodynamics A C If two systems are separately in thermal equilibrium with a third system, they are in thermal equilibrium with each other. Diathermal wall B C C can be considered the thermometer. If C is at a certain temperature then A and B are also at the same temperature. Temperature scales • Assign arbitrary numbers to two convenient temperatures such as melting and boiling points of water. 0 and 100 for the celsius scale. • Take a certain property of a material and say that it varies linearly with temperature. X = aT + b • For a gas thermometer: P = aT + b Platinum resistance thermometer 150 R () 100 50 0 0 50 100 150 200 T (K) 250 300 350 400 CERNOX thermometer R ( ) 10000 1000 100 0 100 200 T (K) 300 400 Thermometer needs to have a much lower heat capacity than the sample T T0 + T T0 + T T0 Heater T0 Sample Thermometer Relaxation calorimetry t Heat capacity dQ C dT Q CV T V Specific heat capacity (c) is the heat capacity per unit mass. Q CP T P What is heat doing at the microscopic level to change the temperature?