Limiting Reactant Methods & Excess Calculation
advertisement

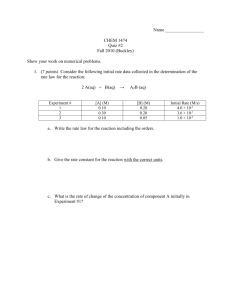
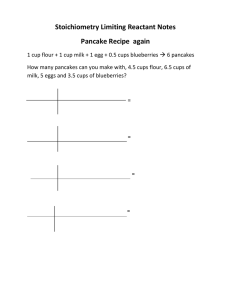
Finding the Limiting Reactant Method 1: 1. Write & Balance the equation 2. Change from grams to moles for all reactants (divide by the molar mass) 3. Use the mole ratio to change 1st reactant to 2nd reactant moles (moles 1st moles 2nd needed) 4. Compare moles of 2nd reactant from step 2 to the amount from step 3 5. If step 2 is smaller than step 3’s answer the 2nd reactant is the limiting reactant Finding the Limiting Reactant Method 2 1. Change from mass of the 1st reactant to grams of a product g r e a c t a n t1 m o lm o l p r o d u c t m . m . p r o d u c t . g p r o d u c t m . m . r e a c t a n tr m o l e a c t a n t .1 m o l 2. Change from mass of the 2nd reactant to grams of the same product g r e a c t a n t1 m o lm o l p r o d u c t m . m . p r o d u c t . g p r o d u c t m . m . r e a c t a n tr m o l e a c t a n t .1 m o l 3. The smallest answer comes from the limiting reactant Finding Excess Reactant mass from grams 1. Change from mass of the limiting reactant to grams of the excess reactant g L . R .1 m o lm o l E . R .. m m . E . R . g E . R . m . m . L . R . m o l L . R .1 m o l 2. Subtract the answer from step 1 from the starting mass of the excess reactant Starting mass of E. R. – g E.R. = grams of E.R. left over