18 Small Web-like carbon networks from ZIF-8 for ORR
advertisement

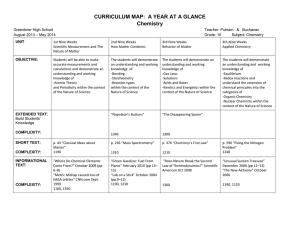
small NANO FULL PAPER Zn–Air Batteries MICRO www.small-journal.com Web-Like Interconnected Carbon Networks from NaCl-Assisted Pyrolysis of ZIF-8 for Highly Efficient Oxygen Reduction Catalysis Yuhong Qian, Tao An, Karl Erik Birgersson, Zhaolin Liu, and Dan Zhao* oxygen reduction reaction (ORR) on their cathodes is well-known for its sluggish kinetics, limiting the output power of the mentioned systems.[3] Electrocatalysts thus become necessary to accelerate ORR and promote device efficiency. Platinum (Pt) is regarded as the benchmark ORR catalyst owing to its outstanding activity.[4] However, it has been predicted that the global reserve of Pt is difficult to meet the longterm needs, even by alloying Pt with other cheap metals to reduce its consumption.[5] As a result, developing nonprecious metal catalysts and even metal-free catalysts to replace Pt has become an alternative approach.[6] Due to the heterogeneous reaction conditions, the catalytic performance of ORR catalysts relies not only on their intrinsic activity but also on the mass/electron transport properties.[7] In either half-cell measurements or full-cell assembly tests, O2 has to diffuse to the active sites before ORR takes place. A micropore-dominant catalyst may be abundant in active sites owing to the high specific surface area, but the lack of macropores may lead to difficulty in mass transfer of reactants.[8] The mass transfer resistance can delay O2 from reaching the active sites and reduce the electrochemical response of the catalysts, resulting in increased overpotential and reduced device efficiency. An improved design of catalyst morphology by enriching macropores can significantly improve the performance of electrocatalysts. For example, Kim et al. prepared a macroporous Pt electrode with inverse opal (IO) structure by electrodeposition of Pt on polystyrene beads.[9] The IO electrode shows a 17% increase in maximum power density compared with conventional electrode, and a reduced voltage loss from both Ohmic resistance and mass transport.[10] Shui et al. prepared a continuous carbon network from electrospinned polyacrylonitrile nanofibers.[11] In PEMFC tests, the network catalyst has a 200% increase in maximum power density compared with the one prepared from activated carbon (0.9 W cm−2 vs 0.3 W cm−2). The easy access to active sites is believed to be the key for the significant performance enhancement. Besides porosity, the connectivity in electrocatalysts is also important as it affects the electron conductivity. It is common to prepare ORR catalysts in granular form. The missing of contact between each particle may lead to an increase in electrical resistance resulting in extra hindrance for applications.[12] In the study of The oxygen reduction reaction (ORR) is under intense research due to its significance in energy storage and conversion processes. Recent studies show that interconnected and hierarchically porous structures can further enhance ORR kinetics as well as catalyst durability, but their preparation can be quite time and/or chemical consuming. Here, a simple approach is reported to prepare such complex structures by pyrolyzing composites containing NaCl and ZIF-8. The templating effect of molten NaCl connects ZIF-8 particles into web-like carbon networks. During ORR activity measurements, it achieves a 0.964 V onset potential and a 38 mV dec−1 Tafel slope, which are comparable to those of the benchmark Pt/C (0.979 V and 40 mV dec−1). Due to the metal-free feature, this catalyst exhibits a 16 mV shift in half-wave potential after a 10 000-cycle durability test, which is only 60% of that of Pt/C. The catalyst is also tested in Zn–air batteries and the assemblies are able to work at above 1.2 V for 140 h, which triples the life held by those with Pt/C. This study demonstrates a facile strategy to prepare metal-free ORR catalysts with interconnectivity and hierarchical porosity, and proves their great potentials in ORR catalysis and Zn–air batteries. 1. Introduction Electricity has been playing a significant role in the portfolio of clean energy due to its low environmental impact, wellbuilt transmission grid, and versatility in different applications.[1] Currently, devices that can convert chemical fuels into electricity are experiencing a surge of development, such as proton exchange membrane fuel cells (PEMFCs), direct alcohol fuel cells, and zinc/lithium/aluminum air batteries.[2] The Y. Qian, Prof. D. Zhao Department of Chemical and Biomolecular Engineering National University of Singapore 4 Engineering Drive 4, 117585, Singapore E-mail: chezhao@nus.edu.sg Dr. T. An, Dr. Z. Liu Institute of Materials Research and Engineering (IMRE) A*STAR (Agency for Science, Technology and Research) Innovis, 2 Fusionopolis Way, 138634, Singapore Prof. K. E. Birgersson Department of Mechanical Engineering National University of Singapore 9 Engineering Drive 1, 117575, Singapore The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/smll.201704169. DOI: 10.1002/smll.201704169 Small 2018, 1704169 1704169 (1 of 10) © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim small NANO MICRO www.small-journal.com www.advancedsciencenews.com Scheme 1. Schematic illustration of the preparation of interconnected hierarchically porous carbon by pyrolyzing the NaCl/ZIF-8 composite. Zhang et al., they added carbon nanotubes to connect the ORR active particles together.[13] The analysis on polarization curves reveals that the electron transport is improved. Based on the above discussion, the optimized morphology of electrocatalysts should be an interconnected carbon network with high electron conductivity, with abundant micropores to hold the active sites and macropores for low-resistance mass transfer. From the reported studies, the preparation of such interconnected structures often requires specific instruments such as electrospinning setups, or tedious wet chemistry processes. A facile strategy for preparing such structures should be helpful for the development of highly efficient Pt-free ORR catalysts. Metal–organic frameworks (MOFs) are emerging materials as precursors to prepare Pt-free electrocatalysts due to their porous structures, tunable compositions, and high carbon/ nitrogen contents.[14] Zeolitic imidazolate frameworks (ZIFs) are a subclass of MOFs composed of imidazolate or its derivatives with tetrahedrally coordinated metal cations (e.g., Zn2+, Co2+), and have been widely used as precursors for the preparation of electrocatalysts.[15] Inorganic salts such as NaCl and ZnCl2 have been used to prepare highly porous carbon materials owing to their template effects.[16] Here, we report a simple method for preparing interconnected carbon structures by pyrolyzing NaCl/ ZIF-8 composites (Scheme 1). During the pyrolysis, the molten NaCl medium activates the surface of ZIF-8 particles and connects them into carbon fibers embedded with ORR active sites. Meanwhile, the usage of ZIF-8 leads to evaporation of Zn and endows the carbon network with micropores.[17] The voids generated after NaCl removal become macropores for fast mass transfer. Our best metal-free catalyst can achieve a 0.964 V onset potential and a limiting current density close to 6 mA cm−2 in 0.1 m KOH, which are comparable to those of the Pt/C benchmark. The catalyst was further assembled into Zn–air batteries to test its performance under actual working conditions. At a current density of 5 mA cm−2, our catalyst is able to work at above 1.2 V for 140 h, which is two time longer than that of Pt/C. This study sheds light on the preparation of highly efficient ORR electrocatalysts with interconnected and macroporous features. 2. Results and Discussion 2.1. Preparation and Morphological Characterization We selected ZIF-8 as the precursor for carbon material preparation because of its high nitrogen content and easiness of Small 2018, 1704169 synthesis. In order to have a larger portion of void volume in the derived carbon, mesoporosity was further introduced into ZIF-8. Nano-sized ZIF-8 (Z8) and mesoporous ZIF-8 (MZ8) with particle sizes of around 70 nm (Figure 1a,b) were synthesized via previously reported solution-based approaches.[18] The products demonstrate perfect match of powder X-ray diffraction (PXRD) pattern with the simulated one (Figure S1, Supporting Information).[19] The PXRD peaks of MZ8 are slightly broadened due to its lower crystallinity resulting from the introduction of surfactant during synthesis.[18b] The pore size distribution calculated from N2 sorption isotherms shows that Z8 is only rich in micropores, while MZ8 has a secondary pore size distribution at >2 nm, confirming its mesoporosity (Figure S2, Supporting Information). The nanoparticles can form uniform composites with NaCl (Figure S3, Supporting Information), ensuring the reaction homogeneity during subsequent pyrolysis. In addition, the ZIF-8 crystal structure is preserved in those composites (Figure S4, Supporting Information). In previous studies, molten salt shows templating effect during the preparation of porous carbon using small molecular precursors.[16a,e,f ] We chose NaCl in this study because of its low cost and moderate volatility at high temperature. The NaCl/ ZIF-8 composites (Z8-S and MZ8-S) were pyrolyzed under N2 to prepare interconnected carbon networks named Z8-S-P or MZ8-S-P, respectively. We also prepared ZIF-8-derived carbon without the addition of NaCl (Z8-P and MZ8-P). In scanning electron microscopy (SEM) analysis, there is not much morphology change of pure ZIF-8 particles before and after pyrolysis (Figure 1c,d). In sharp contrast, Z8-S-P and MZ8-S-P derived from NaCl/ZIF-8 composites exhibit an interconnected, web-like morphology (Figure 1e,f; Figure S5, Supporting Information). Removal of NaCl vacates the occupied space and forms pores in macroporous range. Compared with a dense catalyst layer consisted of granules, the web-like morphology provides much more space for mass transfer. By this virtue, we suppose it would show better ORR activity compared with pyrolyzed ZIF-8 particles, especially in the catalytic kinetics. Transmission electron microscopy (TEM) analysis further reveals the morphological information of the derived carbon. Pure ZIF-8 precursors without the addition of NaCl exhibit similar morphology before and after pyrolysis (Figure 2a–d). On the contrary, the meso and macropores (20–80 nm) of the weblike interconnected carbon structures can be clearly seen from the Z8-S-P and MZ8-S-P (Figure 2e,f). These macropores are distributed evenly inside the body of Z8-S-P and MZ8-S-P, and can act as channels for the transfer of reactants and electrolyte 1704169 (2 of 10) © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim small NANO MICRO www.small-journal.com www.advancedsciencenews.com pyrolysis affording the morphology change. Thermogravimetric analysis and differential scanning calorimetry were performed on the composites to test this assumption. However, there is no change in the melting point (MP) of NaCl among all the samples with different NaCl/ZIF-8 ratios (Figure S8, Supporting Information). We further prepared carbon materials derived from the NaCl/ ZIF-8 composite under 700, 800, and 900 °C, respectively. Since the MP of NaCl is 801 °C, the chosen pyrolysis temperature can prove whether the molten salt is necessary for the formation of the web-like morphology. For each pyrolysis temperature, the pyrolysis duration was varied from 0.5 to 3 h to capture any possible intermediate morphology. There is no obvious morphology change of the particles at the pyrolysis temperature of 700 °C during the whole 3 h of pyrolysis (Figure S9a–f, Supporting Information). When the pyrolysis temperature was increased to 800 °C, the particles became more spherical after 1 h of pyrolysis (Figure 3a). With prolonged pyrolysis under 800 °C, we noticed that these granules started to connect with each other (Figure 3b; Figure S9h–l, Supporting Information) and formed corallike particulates (Figure 3c). Such morphology difference under different pyrolysis temperatures suggests an important role played by the molten NaCl in the morphology evolvement. The surface of the pyrolyzed ZIF-8 may be corroded and activated by the molten salt at higher pyrolysis temperatures, Figure 1. SEM images of nano-sized ZIF-8 precursors a) Z8, b) MZ8, and their derived carbon and form new CC bonds between particles c) Z8-P, d) MZ8-P, e) Z8-S-P, and f) MZ8-S-P. leading to morphology evolvement.[20] For pyrolysis performed at 900 °C, the web-like morphology can be quickly developed within 1 h (Figure 3d). during ORR catalysis. TEM images reveal that the four ZIFThe reason for this fast development could be the higher reac8-derived carbons are of low crystallinity without any crystal lattion rate at elevated temperatures, and an entirely molten NaCl tice (Figure S6, Supporting Information). Compared with Z8-P medium that facilitates the reaction. Due to the short reaction and MZ8-P, the carbon networks have a higher degree of intertime, there are still a number of unconverted ZIF-8 particles connectivity, which may help to reduce the Ohmic resistance.[11] (Figure S9n,o, Supporting Information). These detached partiInterestingly, thin carbon layers can be observed at the edges of cles can further react with the present carbon networks or other Z8-S-P and MZ8-S-P under high-resolution TEM (Figure 2e,f). particles, and turn into part of the carbon networks eventually We speculate that the molten NaCl may slightly delaminate the (Figure 3e,f, Figure S9n–s, Supporting Information). carbon during pyrolysis, and the sonication during TEM sample The next question we want to answer is: Can the NaClpreparation can further exfoliate bulk carbon into carbon assisted pyrolysis be applied in other MOFs to afford web-like sheets. Noteworthy, sonication is one of the key steps during structures? We extended this approach to other carboxylatethe preparation of electrocatalyst inks. Thus, we can expect a based MOFs including Zn-MOF-74, UiO-66(Zr), and HKUST-1. similar microstructure presented in the catalyst layer. In the Unfortunately, none of them can generate web-like structures atomic force microscopy analysis, Z8-P shows separated spots using the NaCl-assisted pyrolysis (Figure S10, Supporting Inforin the height profile; while MZ8-S-P is in chain-like shape with mation). We assume that the carboxylate ligands in these three thin sheets surrounding (Figure S7, Supporting Information). MOFs may not be favorable toward the formation of web-like These carbon sheets have a higher surface ratio than the carbon structure; while imidazolate ligands (such as 2-methylimidazole network, which can expose more active sites for ORR catalysis. in ZIF-8) may be more active during the pyrolytic decomposiIn order to understand how the web-like morphology was tion to form interconnected structures. In order to examine formed in Z8-S-P and MZ8-S-P, we initially proposed that NaCl this hypothesis, two other ZIFs containing imidazolate ligands, and ZIF-8 might form a eutectic mixture that can melt during Small 2018, 1704169 1704169 (3 of 10) © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim small NANO MICRO www.small-journal.com www.advancedsciencenews.com namely ZnIM (Zn-imidazolate) and ZnPhIM (Zn-benzimidazolate), were prepared and pyrolyzed under the assistance of NaCl (Figure S11a,d, Supporting Information).[21] After pyrolysis, these two ZIFs did exhibit morphology change from granules to sheets (Figure S11b,e, Supporting Information). The zoom-in images reveal a small portion of interconnectivity in those sheets, but not as obvious as the ones derived from nanosized ZIF-8 (Figure S11c,f, Supporting Information). This confirms that ZIFs composed of imidazolate ligands have a higher chance to undergo morphological evolution during NaClassisted pyrolysis than MOFs built by carboxylate ligands. The relatively low bond energy of CN bonds compared to that of CO bonds may be the origin of the high reactivity of ZIFs.[22] 2.2. Structural and Physical Properties Figure 2. TEM images of a) Z8, b) MZ8, c) Z8-P, d) MZ8-P, e) Z8-S-P, and f) MZ8-S-P. PXRD and Raman spectroscopy were used to investigate the chemical structures of the four carbon materials (Z8-P, MZ8-P, Z8-S-P, and MZ8-S-P). All the PXRD patterns show a broad (002) peak from carbon at around 26° (Figure 4a), indicating low degrees of graphitization. Raman spectra (Figure 4b) were deconvoluted into four parts, corresponding to signals from out-of-plane carbon atoms (1170 cm−1, purple), defects (1300 cm−1, green), heteroatoms (1480 cm−1, red), and graphitic carbon atoms (1560 cm−1, blue).[23] Both Z8-S-P and MZ8-S-P show lower D/G ratios than those of Z8-P and MZ8-P (Table S1, Supporting Information), suggesting that the NaCl-assisted pyrolysis can result in more ordered carbon with less defects. This result agrees with the previous studies that molten salt promotes graphitization during pyrolysis.[16b] Meanwhile, the decreased relative intensity from heteroatom part in the Raman spectra reveals lower N contents in NaCl-assisted pyrolyzed samples, which is also confirmed by elemental analysis (Table S2, Supporting Information). Figure 3. SEM images of a) Z8-S-P-800-1h, b) Z8-S-P-800-2h, c) Z8-S-P-800-3h, d) Z8-S-P-900-1h, e) Z8-S-P-900-2h, and f) Z8-S-P-900-3h. Small 2018, 1704169 1704169 (4 of 10) © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim small NANO MICRO www.small-journal.com www.advancedsciencenews.com Figure 4. a) PXRD patterns and b) Raman spectra of the ZIF-8-derived carbon. X-ray photon spectroscopy (XPS) was performed to further study the chemical states of carbon and nitrogen in the ZIF-8 derived carbon. As displayed in Figure 5a, the C 1s XPS spectra were deconvoluted into sp2 CC bonds (284.6 eV, blue), CN bonds (285.5 eV, red), and a small portion of CO bonds (286.6 eV, green).[24] Compared with carbon granules prepared by conventional pyrolysis, salt-assisted pyrolyzed samples have 5% more sp2 carbon and 3% less CN bonds, which is consistent with the elemental analysis results (Tables S2 and S3, Supporting Information). The N 1s XPS spectra can be deconvoluted into pyridinic N (398.4 eV, blue), pyrrolic N (399.6 eV, red), graphitic N (400.8 eV, green), and oxidized N (403.0 eV, purple).[25] The introduction of molten salt during pyrolysis has significantly reduced the pyridinic N content by at least 30% and increased graphitic N content by 20%. The oxidized N content has also been slightly increased (Table S3, Supporting Information). Pyridinic and graphitic N species are considered as the origin of ORR catalytic activity in N-doped carbon materials, and the absolute contents of them in our ZIF-8 carbon can be calculated (Table S4, Supporting Information). However, with limited quantitative studies, it is difficult to quantify if the conversion of nitrogen species is beneficial to ORR catalysis.[26] Electrochemical measurements are necessary to see if the catalytic performance is also affected by other factors, such as the possibly enhanced mass transport due to the web-like morphology. There are no discernable peaks in the Zn 2p XPS spectra, suggesting a thorough evaporation of Zn during pyrolysis (Figure S12, Supporting Information). Inductively coupled plasma confirms that the Zn content in the NaCl-assisted derived carbon is less than 0.005 wt%. The low Zn content suggests that it can barely contribute to the ORR acvitiy, and previous study has indicated that ZnNC is inferior to ORR catalysis.[27] Thus, the ORR activity in our ZIF-8 derived carbon should be mainly contributed by the nitrogen-containing sites. The surface area and pore size distribution of carbon materials were evaluated based on the N2 sorption isotherms at 77 K (Figure 6a), which all exhibit Type I shape with slight hysteresis indicating dominantly microporous texture (Table 1). Z8-P has a Brunauer–Emmett–Teller (BET) surface area of 1381 m2 g−1 and a pore volume of 1.12 cm3 g−1, which agrees well with the previous studies.[28] Although Z8-S-P has a 20% lower surface area, it shows a double total pore volume and 4-time macropore volume as those of Z8-P. The surface area loss may be caused by the framework decomposition during pyrolysis and enhanced graphitization with the NaCl-assisted pyrolysis. The significantly enhanced macropore volume indicates the successful introduction of mass transfer channels by the NaCl-assisted pyrolysis. MZ8-P and MZ8-S-P have 30% lower surface area and 20% higher pore volume compared with Z8-P and Z8-S-P, respectively. The inferior crystallinity of MZ8 to Z8 results in a lower BET surface area after pyrolysis, but the introduced mesopores endow the derived carbon with additional pore volume. Figure 5. a) C 1s and b) N 1s XPS spectra of the ZIF-8-derived carbon. Small 2018, 1704169 1704169 (5 of 10) © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim small NANO MICRO www.small-journal.com www.advancedsciencenews.com Figure 6. a) N2 sorption isotherms and b) DFT-calculated pore size distributions of the ZIF-8 derived carbon. When there is a series of catalysts with similar chemical structures, one with the highest surface area may show the best performance. However, recent studies indicate that it may not always be the case. For example, in a tortuous system, there may be inner surface which cannot be easily accessed by reactants. In addition, although micropore is the primary contributor to surface area, it also poses very high mass transfer resistance and leads to a large number of inaccessible active sites.[8,29] Therefore, the high surface areas of Z8-P and MZ8-P may not be fully utilized. On the other hand, active sites in Z8-S-P and MZ8-S-P are anchored along the carbon networks. The interconnected structure prevents them from stacking, and the carbon networks are in direct contact with the electrolyte, which can reduce the diffusion distance of reagent during electrochemical reactions. The macropores also provide enough space for the transfer of electrolyte and O2 with low resistance. As a result, the macroporous, web-like structure carbon may show outstanding performance in ORR catalysis. 2.3. Electrochemical Activity Measurements and Zn–Air Battery Tests Electrochemical behaviors of the ZIF-8 derived carbon materials were first investigated by cyclic voltammetry (CV). These materials show quasi-rectangular shape of CV curves in Ar-saturated 0.1 m KOH, indicating their inertness without the presence of O2 from 0.2 to 1.2 V versus reversible hydrogen electrode (RHE) (Figure 7a; Figure S14, Supporting Information). The area enclosed by the CV curves of Z8/MZ8-P is much larger than that of Z8/MZ8-S-P, suggesting the high surface area of pyrolyzed ZIF-8 particles.[30] In O2-saturated electrolyte, a peak at ≈0.8 V versus RHE appears and MZ8-S-P has the most pronounced peak for oxygen reduction. We further performed cathodic linear sweep voltammetry in O2-saturated 0.1 m KOH at 1600 rpm to reveal the ORR activity of the ZIF-8 derived carbon under steady state. Figure 7b shows that Z8-P has the worst ORR activity with a 0.869 V of onset potential and a 0.749 V of half-wave potential, and its poor mass transport is denoted by the slowly increasing current density at E < 0.7 V. MZ8-P shows similar ORR catalytic behavior with slightly enhanced mass transport, probably due to the extra pore volume brought by the mesopores in the precursor. With the web-like morphology introduced by the NaCl-assisted pyrolysis, Z8-S-P and MZ8S-P are able to reach the limiting current plateau at ≈0.75 V. This proves that the web-like morphology greatly reduces the mass transport resistance. On the other hand, the ORR onset potential is boosted to 0.964 V. Based on the XPS deconvolution and elemental analysis, the increased content of graphitic N may be one of the possible reasons of this 95 mV enhancement. Notably, our best catalyst MZ8-S-P has only a 16 mV lower onset potential compared with the benchmark ORR catalyst Pt/C, suggesting a comparable ORR activity between them. The electrochemical double-layer capacitance (EDLC) was measured to estimate the electrochemical active surface area of the ZIF-derived carbon (Figure S15, Supporting Information), and a strong correlation was found between the EDLC and the micropore surface area of the samples (Figure S16, Supporting Table 1. Porosity features and ORR catalytic activities of ZIF-8 derived carbon and Pt/C. ORR catalytic activityd) Surface area and pore volume BET SAa) and VTotalPb,c) Micropore SAa) and VMicroPb) Vd> 40 nmb) Vd> 40 nm/VTotalP [%] Eonsete) Ehalf-wavef) Z8-P 1381, 1.12 1225, 0.42 0.16 14 0.869 0.749 4.71 Z8-S-P 1086, 2.00 668, 0.25 0.60 30 0.964 0.862 5.35 MZ8-P 899, 1.34 763, 0.25 0.20 15 0.930 0.813 5.11 jmax MZ8-S-P 713, 2.34 414, 0.17 0.98 42 0.964 0.855 5.89 Pt/C 218, 0.26 120, 0.04 0.05 23 0.979 0.834 5.69 a)m2 g−1; b)cm3 g−1; c)Determined at P/P0 = 0.99; d)In 0.1 m KOH; e)Determined when j = 0.1 mA cm−2; f)The potential when j = ½ jmax. Small 2018, 1704169 1704169 (6 of 10) © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim small NANO MICRO www.small-journal.com www.advancedsciencenews.com Figure 7. a) CVs of MZ8-S-P and Pt/C in Ar-saturated and O2-saturated 0.1 m KOH; b) CLSV, c) number of electrons transferred and H2O2 yields, and d) Tafel plots of ZIF-8 derived carbon and Pt/C at 1600 rpm in O2-saturated 0.1 m KOH. e) CLSVs of MZ8-S-P and Pt/C before and after 10 000 cycles of accelerated degradation test (the inset shows the negative shift in half-wave potential). f) CLSVs of MZ8-S-P prepared from precursors with different NaCl/MZ8 mass ratio. Information). Interestingly, Z8-P shows the highest EDLC (22.3 mF cm−2) but the worst ORR activity among them. This suggests that the surface area brought by the micropores is not always beneficial to ORR catalysis if macropores are missing. We also collected the electrochemical impedance spectra of the ZIF-8 derived carbon and performed an equivalent circuit fitting (Figure S17 and Table S5, Supporting Information). The result shows that Z8-S-P has much lower resistance of both diffusion and electron transfer than those of Z8-P. The low resistance for electron transfer may be another reason for the high ORR onset potential of Z8-S-P and MZ8-S-P.[31] The ORR performance of MZ8-S-P in 0.5 m H2SO4 was also measured (Figure S18, Supporting Information). Similar to most N-doped metal-free ORR catalysts, MZ8-S-P shows a mediocre activity in acidic media. The number of electrons transferred (n) is another critical parameter for ORR catalysts. Generally speaking, oxygen molecule is reduced either by a 2-e− process to peroxide, or by a 4-e− process to water.[32] However, the peroxide generated in the 2-e− process is harmful to the cell assembly and reduces the cell life.[33] Therefore, ORR catalysts with high selectivity toward the 4-e− process are preferred. Figure 7c shows the n and peroxide yield determined by rotating ring-disk electrode tests. By preparing the catalysts through NaCl-assisted pyrolysis, the n was increased from 3.5 to 3.9, and peroxide yield was reduced from >20% to <5% between 0.2 and 0.8 V versus RHE. Koutecky– levich (KL) equation is another method to determine n by electrode kinetics calculation.[34] The n of the carbon materials from Small 2018, 1704169 the KL equation is shown in Figure S19 (Supporting Information). The two sets of results are close to each other, confirming the 4-e− process selectivity of the carbon materials from saltassisted pyrolysis. Tafel slope is another critical parameter to describe the kinetics of ORR catalysts. Figure 7d shows the Tafel plots of our carbon materials and the commercial Pt/C. Among them, Z8-P has the highest slope of 60 mV dec−1, meaning a slow current development as overpotential increases. The Tafel slope is gradually reduced by introducing mesoporosity in the precursors and the NaCl-assisted pyrolysis, and the value finally reaches only 38 mV dec−1. Again, the NaCl-assisted pyrolysis shows great benefit in ORR kinetics as both Z8-S-P and MZ8-S-P share the same Tafel slope. In comparison, the Tafel slope of Pt/C is slightly higher (40 mV dec−1). Thus, by simple precursor pretreatment to control the catalyst morphology, we are able to produce metal-free ORR catalysts that have comparable catalytic activity to that of Pt/C in catalyzing ORR in alkaline media. Durability is of great importance to ORR catalysts since they are often used under long-term working condition. A 10 000 cycle accelerating degradation test (ADT) was carried out to check the durability of the MZ8-S-P. As the benchmark reference, commercial Pt/C was tested as well. After cycling, Pt/C has a 27 mV negative shift in half-wave potential, which is twice as much as that of MZ8-S-P (Figure 7e). In addition, the limiting current density of Pt/C decreases by 4.8%, which is higher than that of MZ8-S-P (2.8%). The results suggest that our carbonfree catalyst is more robust than Pt/C to retain the ORR activity 1704169 (7 of 10) © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim small NANO MICRO www.small-journal.com www.advancedsciencenews.com during long-term usage. Since Pt/C contains Pt nanoparticles supported on porous carbon, the most possible reason for its performance loss after ADT is Pt particle aggregation or detachment.[35] On the other hand, the active sites of MZ8-S-P are not metal nanoparticles but immobile N species embedding inside the carbon, resulting in improved catalyst stability. The interconnected morphology stays intact after the ADT, suggesting its robustness during long-term working condition (Figure S20, Supporting Information). The mass ratio between NaCl and ZIF-8 was also varied to find the optimized composite for catalyst preparation (Figure 7f). For the mass ratios of NaCl/MZ8 from 1 to 20, the ORR performance improves with increased amount of NaCl. However, further increase of NaCl amount leads to worse ORR kinetics. Therefore, the optimized weight ratio between NaCl and ZIF-8 nanocrystals in this study is 20. Additionally, we prepared another two ZIF-8 derived carbon with CsCl (MP 645 °C) and BaCl2 (MP 962 °C) through the same process. The SEM images and electrochemical measurements again reveal the relationship between the molten salt and the macroporous morphology/enhanced ORR kinetics, regardless of the salt composition (Figures S21 and S22, Supporting Information). Therefore, an economical compound such as NaCl would suffice for the preparation of macroporous morphology. With both outstanding ORR activity and durability in alkaline media, the performance of MZ8-S-P was further tested in a primary Zn–air battery assembly with a 4 cm2 air electrode. The battery using MZ8-S-P exhibits a comparable polarization curve to that of the battery using Pt/C (Figure 8a), showing a slightly higher discharge voltage at current densities >40 mA cm−2 with 6.6% higher maximum output power (55.0 mW vs 51.6 mW). The improvement agrees with the previous studies that the benefit from macropores only appears at elevated current densities.[9,11] On the other hand, the battery life has been substantially improved. At a discharge current density of 5 mA cm−2, the MZ8-S-P can discharge above 1.2 V for more than 140 h, comparing with 50 h of Pt/C (Figure 8b). In addition, MZ8S-P can maintain a stable discharge voltage, but Pt/C undergoes fast deterioration even at the beginning of discharge. The difference in the voltage profiles suggests that the active sites inside MZ8-S-P are well preserved during the working condition.[36] The discharge failure of both batteries, however, is not a result of deterioration of ORR active site or depletion of Zn anode. Similar to fuel cells, Zn–air batteries also suffer from the flooding issue, which greatly hinders and even cuts off the O2 transport.[37] Based on an in situ X-ray tomography study in Zn–air batteries, the total blockage of O2 transport channels can happen when only 30% of Zn is consumed.[38] The accumulation of electrolyte inside the gas diffusion channels is because of the additional volume taken by the anodic discharge products.[39] Therefore, providing extra space at the cathode side may increase the battery life hour. In this study, MZ8-S-P has a pore volume of 13.92 cm3 g−1 and a 40 darcy liquid permeability, which are 6-time and 40-time higher than those of Pt/C (Figure S23 and Table S6, Supporting Information), leading to a 30% longer working life time. Because of similar porosity, the battery containing Z8-P fails at around the same life time as that of Pt/C (Figure S24, Supporting Information). Combining ultrahigh pore volume with fast liquid permeation property, the unique web-like interconnect carbon networks obtained from NaCl-assisted pyrolysis of ZIF-8 provide low-resistance paths and abundant space for electrolyte transport, which can mitigate the negative effect from flooding during battery test and prolong the battery life accordingly. 3. Conclusions The development of Pt alternatives for ORR catalysis has been ongoing for decades. It has been gradually realized that the catalyst morphology which is rich in both micropores and macropores can be highly beneficial to both ORR activity and kinetics. In this study, we report a simple method for preparing interconnected, web-like, N-doped carbon networks by the NaCl-assisted pyrolysis of ZIF-8 nanocrystals. The molten salt medium turns separate nanocrystals into a continuous framework and promotes graphitization of the derived carbon materials. After removing NaCl, the generated macropores serve as channels for low-resistance mass transfer, endowing the carbon networks with improved ORR kinetics. Our best ORR catalyst shows a 0.964 V onset potential, a limiting current density of 5.89 mA cm−2, and a Tafel slope of 38 mV dec−1, which are comparable to those of Pt/C. Additionally, it shows significantly improved working lifetime in battery tests. This study demonstrates a simple and efficient method to prepare highly active, stable, metal-free ORR catalysts with interconnected network Figure 8. a) Battery LSVs and b) 5 mA cm−2 continuous discharge voltage records of air cathodes using MZ8-S-P and Pt/C. Small 2018, 1704169 1704169 (8 of 10) © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim small NANO www.small-journal.com www.advancedsciencenews.com morphology, which is highly favorable for Zn–air batteries and other possible electrochemical devices with long hours of use. Keywords carbon networks, interconnectivity, metal–organic frameworks, oxygen reduction reaction, Zn–air batteries 4. Experimental Section Preparation of ZIF-8 Nanoparticles and NaCl/ZIF-8 Composites: ZIF-8 nanocrystals (Z8) were prepared according to a previous study.[18a] Briefly, zinc nitrate tetrahydrate (0.261 g, 1 mmol) and 2-methylimidazaole (0.657 g, 8 mmol) were dissolved in 20 mL of methanol, respectively, and then mixed and stirred for 24 h. The product was collected by 10 000 rpm centrifuge and washed with pure methanol for three times. MZ8 was prepared based on the reported method.[18b] NaCl/ZIF-8 composites were prepared by dispersing ZIF-8 (0.1 g) with NaCl (2.0 g) in a mixed solvent containing methanol (1 mL) and water (9 mL), and collected by vacuum drying affording the products named Z8-S and MZ8-S. Preparation of Interconnected Carbon Networks: For the ZIF-8-derived carbon in granular form, 0.15 g of ZIF-8 was pyrolyzed at 900 °C under N2 atmosphere. The obtained carbon was collected and used for later experiments (Z8-P and MZ8-P). Interconnected carbon networks (Z8-S-P and MZ8-S-P) were prepared in a similar way, except that 2.1 g of NaCl/ ZIF-8 composite was used for pyrolysis. The products were collected and soaked in deionized water (DIW, 20 mL) at 80 °C overnight to remove NaCl, washed with DIW, collected by filtration, and dried under vacuum for further measurements. Electrochemical Characterization: Electrochemical properties of the obtained samples were studied using a PINE rotating-ring disk electrode setup (AFMSRCE, PINE Research) and an electrochemical workstation (760E, CH Instruments). 5 mg of catalyst was dispersed in 1 mL of solution containing DIW (0.4 mL), ethanol (0.58 mL), and 5 wt% Nafion solution (0.02 mL). The dispersion was sonicated for 1 h before use. For each measurement, 20 µL of ink was pipetted onto the working electrode (AFE7R9GCPT, PINE Research), resulting in a catalyst loading of ≈0.4 mg cm−2. The loading of 20% Pt/C (35849, Alfa Aesar) is 0.04 mgPt cm−2. The electrolyte was bubbled with Ar or O2 for at least 30 min before each measurement. Primary Zn–Air Battery Tests: 24 mg of catalyst was dispersed in 12 mL of ethanol and sonicated for 1 h. The slurry was brushed onto a piece of 6 cm × 2 cm carbon paper (10 BC, SGL Carbon) and dried naturally. The carbon paper was cut into 3 uniform pieces of 4 cm2 with a catalyst loading of 2 mg cm−2 for each piece. The loading of 20% Pt/C is 0.2 mgPt cm−2. A zinc plate was used as anode and the assembly was tested in ambient air atmosphere. The battery contains 25 mL of aqueous electrolyte (6 m KOH and 0.2 m ZnCl2). Linear scan voltammetry of batteries was conducted at a rate of 0.1 V s−1. Catalyst durability was evaluated by discharging the batteries at 5 mA cm−2 until the cell voltage was below 0.8 V. Supporting Information Supporting Information is available from the Wiley Online Library or from the author. Acknowledgements This work was supported by the National University of Singapore (CENGas R-261-508-001-646), Ministry of Education – Singapore (MOE AcRF Tier 1 R-279-000-472-112), and the Agency for Science, Technology, and Research (Grant Nos. PSF R-279-000-475-305 and IRG R-279-000-510-305). Conflict of Interest The authors declare no conflict of interest. Small 2018, 1704169 MICRO Received: November 29, 2017 Revised: January 26, 2018 Published online: [1] a) M. Granovskii, I. Dincer, M. A. Rosen, J. Power Sources 2006, 159, 1186; b) H. Khatib, IEE Proc.-A: Sci., Meas. Technol. 1993, 140, 24. [2] a) X. Luo, J. Wang, M. Dooner, J. Clarke, Appl. Energy 2015, 137, 511; b) A. Jayakumar, S. P. Sethu, M. Ramos, J. Robertson, A. Al-Jumaily, Ionics 2015, 21, 1; c) S. S. Munjewar, S. B. Thombre, R. K. Mallick, Renewable Sustainable Energy Rev. 2017, 67, 1087; d) Y. Li, H. Dai, Chem. Soc. Rev. 2014, 43, 5257; e) Z. Ma, X. Yuan, L. Li, Z.-F. Ma, D. P. Wilkinson, L. Zhang, J. Zhang, Energy Environ. Sci. 2015, 8, 2144; f) M. Mokhtar, M. Z. M. Talib, E. H. Majlan, S. M. Tasirin, W. M. F. W. Ramli, W. R. W. Daud, J. Sahari, J. Ind. Eng. Chem. 2015, 32, 1. [3] L. Dai, Y. Xue, L. Qu, H.-J. Choi, J.-B. Baek, Chem. Rev. 2015, 115, 4823. [4] S. Sui, X. Wang, X. Zhou, Y. Su, S. Riffat, C.-j. Liu, J. Mater. Chem. A 2017, 5, 1808. [5] D. Banham, S. Ye, ACS Energy Lett. 2017, 2, 629. [6] a) M. Shao, Q. Chang, J. P. Dodelet, R. Chenitz, Chem. Rev. 2016, 116, 3594; b) C. Hu, L. Dai, Angew. Chem., Int. Ed. 2016, 55, 11736; c) Y. Gong, H. Fei, X. Zou, W. Zhou, S. Yang, G. Ye, Z. Liu, Z. Peng, J. Lou, R. Vajtai, B. I. Yakobson, J. M. Tour, P. M. Ajayan, Chem. Mater. 2015, 27, 1181. [7] C. Zhu, H. Li, S. Fu, D. Du, Y. Lin, Chem. Soc. Rev. 2016, 45, 517. [8] Y.-H. Cho, N. Jung, Y. S. Kang, D. Y. Chung, J. W. Lim, H. Choe, Y.-H. Cho, Y.-E. Sung, Int. J. Hydrogen Energy 2012, 37, 11969. [9] O. H. Kim, Y. H. Cho, S. H. Kang, H. Y. Park, M. Kim, J. W. Lim, D. Y. Chung, M. J. Lee, H. Choe, Y. E. Sung, Nat. Commun. 2013, 4, 2473. [10] X. Huang, Z. Zhang, J. Jiang, 2006 IEEE Int. Symp. on Industrial Electronics, Vol. 2, IEEE, Montreal, Quebec, Canada 2006, p. 1613. [11] J. Shui, C. Chen, L. Grabstanowicz, D. Zhao, D. J. Liu, Proc. Natl. Acad. Sci. USA 2015, 112, 10629. [12] H. X. Zhong, J. Wang, Y. W. Zhang, W. L. Xu, W. Xing, D. Xu, Y. F. Zhang, X. B. Zhang, Angew. Chem., Int. Ed. 2014, 53, 14235. [13] C. Zhang, Y. C. Wang, B. An, R. Huang, C. Wang, Z. Zhou, W. Lin, Adv. Mater. 2016, 29, 1604556. [14] a) W. Xia, R. Zou, L. An, D. Xia, S. Guo, Energy Environ. Sci. 2015, 8, 568; b) Y. Hou, T. Huang, Z. Wen, S. Mao, S. Cui, J. Chen, Adv. Energy Mater. 2014, 4, 1400337; c) Q. L. Zhu, W. Xia, T. Akita, R. Zou, Q. Xu, Adv. Mater. 2016, 28, 6391; d) H. Hu, L. Han, M. Yu, Z. Wang, X. W. Lou, Energy Environ. Sci. 2016, 9, 107; e) E. Proietti, F. Jaouen, M. Lefevre, N. Larouche, J. Tian, J. Herranz, J. P. Dodelet, Nat. Commun. 2011, 2, 416; f) D. Zhao, J.-L. Shui, L. R. Grabstanowicz, C. Chen, S. M. Commet, T. Xu, J. Lu, D.-J. Liu, Adv. Mater. 2014, 26, 1093; g) Y. Z. Chen, C. Wang, Z. Y. Wu, Y. Xiong, Q. Xu, S. H. Yu, H. L. Jiang, Adv. Mater. 2015, 27, 5010; h) P. Zhang, F. Sun, Z. Xiang, Z. Shen, J. Yun, D. Cao, Energy Environ. Sci. 2014, 7, 442; i) B. Volosskiy, H. Fei, Z. Zhao, S. Lee, M. Li, Z. Lin, B. Papandrea, C. Wang, Y. Huang, X. Duan, ACS Appl. Mater. Interfaces 2016, 8, 26769. [15] a) J. Li, Q.-L. Zhu, Q. Xu, Chem. Commun. 2015, 51, 10827; b) L. Shang, H. Yu, X. Huang, T. Bian, R. Shi, Y. Zhao, G. I. Waterhouse, L. Z. Wu, C. H. Tung, T. Zhang, Adv. Mater. 2016, 28, 1668; c) B. Y. Guan, L. Yu, X. W. Lou, Energy Environ. Sci. 2016, 1704169 (9 of 10) © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim small NANO www.small-journal.com www.advancedsciencenews.com 9, 3092; d) Q. Lai, Y. Zhao, Y. Liang, J. He, J. Chen, Adv. Funct. Mater. 2016, 26, 8334. [16] a) N. Fechler, T.-P. Fellinger, M. Antonietti, Adv. Mater. 2013, 25, 75; b) W. Ding, L. Li, K. Xiong, Y. Wang, W. Li, Y. Nie, S. Chen, X. Qi, Z. Wei, J. Am. Chem. Soc. 2015, 137, 5414; c) G. Fu, Z. Cui, Y. Chen, Y. Li, Y. Tang, J. B. Goodenough, Adv. Energy Mater. 2017, 7, 1601172; d) Y. Zhang, L.-B. Huang, W.-J. Jiang, X. Zhang, Y.-Y. Chen, Z. Wei, L.-J. Wan, J.-S. Hu, J. Mater. Chem. A 2016, 4, 7781; e) W. Wang, J. Luo, W. Chen, J. Li, W. Xing, S. Chen, J. Mater. Chem. A 2016, 4, 12768; f) X. Zheng, X. Cao, X. Li, J. Tian, C. Jin, R. Yang, Nanoscale 2017, 9, 1059. [17] H.-L. Jiang, B. Liu, Y.-Q. Lan, K. Kuratani, T. Akita, H. Shioyama, F. Zong, Q. Xu, J. Am. Chem. Soc. 2011, 133, 11854. [18] a) J. Cravillon, S. Münzer, S.-J. Lohmeier, A. Feldhoff, K. Huber, M. Wiebcke, Chem. Mater. 2009, 21, 1410; b) Y.-n. Wu, M. Zhou, B. Zhang, B. Wu, J. Li, J. Qiao, X. Guan, F. Li, Nanoscale 2014, 6, 1105. [19] K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O’Keeffe, O. M. Yaghi, Proc. Natl. Acad. Sci. USA 2006, 103, 10186. [20] A. R. Kamali, D. J. Fray, Carbon 2013, 56, 121. [21] a) E. C. Spencer, R. J. Angel, N. L. Ross, B. E. Hanson, J. A. Howard, J. Am. Chem. Soc. 2009, 131, 4022; b) M. J. Cliffe, C. Mottillo, R. S. Stein, D.-K. Bučar, T. Friščić, Chem. Sci. 2012, 3, 2495. [22] Y.-R. Luo, Comprehensive Handbook of Chemical Bond Energies, CRC Press, Boca Raton, Florida, USA 2007. [23] A. C. Ferrari, J. Robertson, Phys. Rev. B 2000, 61, 14095. [24] Y. Li, W. Zhou, H. Wang, L. Xie, Y. Liang, F. Wei, J.-C. Idrobo, S. J. Pennycook, H. Dai, Nat. Nanotechnol. 2012, 7, 394. [25] H. Wang, T. Maiyalagan, X. Wang, ACS Catal. 2012, 2, 781. [26] a) D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo, J. Nakamura, Science 2016, 351, 361; b) H. B. Yang, J. Miao, S.-F. Hung, J. Chen, H. B. Tao, X. Wang, L. Zhang, R. Chen, J. Gao, H. M. Chen, L. Dai, B. Liu, Sci. Adv. 2016, 2, e150112; c) L. Lai, J. R. Potts, D. Zhan, L. Wang, C. K. Poh, C. Tang, H. Gong, Small 2018, 1704169 MICRO Z. Shen, J. Lin, R. S. Ruoff, Energy Environ. Sci. 2012, 5, 7936. [27] a) B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li, T. Zhang, Nat. Chem. 2011, 3, 634; b) P. Liu, Y. Zhao, R. Qin, S. Mo, G. Chen, L. Gu, D. M. Chevrier, P. Zhang, Q. Guo, D. Zang, B. Wu, G. Fu, N. Zheng, Science 2016, 352, 797; c) N. Cheng, S. Stambula, D. Wang, M. N. Banis, J. Liu, A. Riese, B. Xiao, R. Li, T.-K. Sham, L.-M. Liu, G. A. Botton, X. Sun, Nat. Commun. 2016, 7, 13638; d) I. Martinaiou, A. Shahraei, F. Grimm, H. Zhang, C. Wittich, S. Klemenz, S. J. Dolique, H.-J. Kleebe, R. W. Stark, U. I. Kramm, Electrochim. Acta 2017, 243, 183. [28] L. Zhang, Z. Su, F. Jiang, L. Yang, J. Qian, Y. Zhou, W. Li, M. Hong, Nanoscale 2014, 6, 6590. [29] M. Uchida, J. Electrochem. Soc. 1996, 143, 2245. [30] B. Li, F. Dai, Q. Xiao, L. Yang, J. Shen, C. Zhang, M. Cai, Energy Environ. Sci. 2016, 9, 102. [31] H. Yin, C. Zhang, F. Liu, Y. Hou, Adv. Funct. Mater. 2014, 24, 2930. [32] L. Zhang, Z. Xia, J. Phys. Chem. C 2011, 115, 11170. [33] N. Macauley, M. Watson, M. Lauritzen, S. Knights, G. G. Wang, E. Kjeang, J. Power Sources 2016, 336, 240. [34] Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier, H. Dai, Nat. Mater. 2011, 10, 780. [35] A. Mehmood, M. A. Scibioh, J. Prabhuram, M.-G. An, H. Y. Ha, J. Power Sources 2015, 297, 224. [36] A. Zadick, L. Dubau, N. Sergent, G. Berthomé, M. Chatenet, ACS Catal. 2015, 5, 4819. [37] a) H. Li, Y. Tang, Z. Wang, Z. Shi, S. Wu, D. Song, J. Zhang, K. Fatih, J. Zhang, H. Wang, Z. Liu, R. Abouatallah, A. Mazza, J. Power Sources 2008, 178, 103; b) P. Pei, K. Wang, Z. Ma, Appl. Energy 2014, 128, 315. [38] D. Schröder, T. Arlt, U. Krewer, I. Manke, Electrochem. Commun. 2014, 40, 88. [39] a) T. Arlt, D. Schroder, U. Krewer, I. Manke, Phys. Chem. Chem. Phys. 2014, 16, 22273; b) J. Stamm, A. Varzi, A. Latz, B. Horstmann, J. Power Sources 2017, 360, 136. 1704169 (10 of 10) © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim