Yeast Fermentation Lab: Bioengineering Design Challenge
advertisement

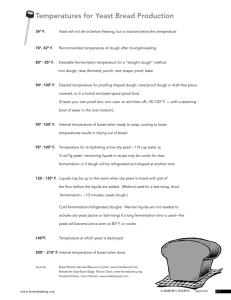
Alcoholic Fermentation in Yeast – A Bioengineering Design Challenge1 I. Introduction Anaerobic energy production in yeast will be studied in this lab investigation. Cultures around the world have for millennia used yeast fermentation to produce bread and alcoholic beverages. Yeast is able to metabolize some foods, but not others. In order for an organism to make use of a potential source of food, it must be capable of transporting the food into its cells. It must also have the proper enzymes capable of breaking the food’s chemical bonds in a useful way. Sugars are vital to all living organisms. Yeast is capable of using some, but not all sugars as a food source. Yeast can metabolize sugar in two ways, aerobically, with the aid of oxygen, or anaerobically, without oxygen. Although the aerobic fermentation of sugars is energetically much more efficient, in this experiment we will set the conditions so that yeast carries out anaerobic respiration—i.e. Alcoholic fermentation. When the yeast respire glucose aerobically, oxygen gas is consumed at the same rate that CO2 gas is produced—there would be no change in the gas pressure in the test tube. The net equation for the more than two dozen steps involved in the aerobic respiration of glucose is: C6H12O6 + 6 O2 6 H2O + 6 CO2 + energy (36-38 ATP + Heat) When yeast ferments the sugars anaerobically, however, CO2 production will cause a change in the pressure of a closed test tube, since little oxygen is being consumed. The alcoholic fermentation of glucose is described by the following net equation: C6H12O6 Ethyl Alcohol+ 2 CO2 (g) + energy (2 ATP + heat) Both alcoholic fermentation and aerobic respiration are multi-step processes that involve the transfer of energy stored in the chemical bonds of glucose to bonds in adenosine triphosphate, ATP. The energy stored in ATP can then be used to perform cellular work: provide energy for biosynthetic reactions (e.g. growth and repair processes, active transport, etc.). All organisms (i.e. monerans, protists, fungi, plants, and animals) utilize aerobic respiration and/or fermentation (anaerobic respiration) to produce ATP to power their cellular processes. Since ethanol is harmful to cellular membranes yeast cells will die if ethanol concentrations reach a critical level. 1. The ingredients for bread include flour, yeast, sugar and water. What makes the dough rise, so the bread will be fluffy instead of flat? 2. Examine the little dry grains of yeast that are used to make bread dough. Do you think that these grains of yeast are alive? Explain why or why not. One way to test whether these little dry grains of yeast are alive is to test whether they can carry out alcoholic fermentation. Alcoholic fermentation is the main process that produces ATP in yeast cells. This figure shows that during alcoholic fermentation: • The sugar glucose is broken down to the alcohol ethanol plus carbon dioxide. • ATP is synthesized from ADP and P. • Energy released by the first reaction provides the energy needed for the second reaction. The pair of curved arrows represents coupled chemical reactions; the top reaction provides energy for the bottom reaction. 3a. Why is this type of fermentation called alcoholic fermentation? 3b. Why do cells need ATP? 3c. How can alcoholic fermentation result in the production of gas bubbles? Alcoholic fermentation is a complex process that includes twelve different chemical reactions. Each of the twelve chemical reactions in alcoholic fermentation of glucose requires an enzyme. 4. What are enzymes? What does it mean to say that "a chemical reaction requires an enzyme"? The sugar you will use is sucrose, the common sugar that people put in their coffee and use for baking. Sucrose is a disaccharide that yeast cells convert to glucose for alcoholic fermentation. When the yeast cells produce CO2, this gas is trapped in bubbles in a layer of foam. You will complete one experiment. You only need to collect data for your experiment and then we will collectively discuss class results. Some more information about yeast to consider. Ideal temperature is 40˚-45˚ Celsius (105- 110˚ F.) 3-5% sucrose seems to be an ideal sucrose concentration for yeast to ferment. You will use 1/4 tsp of yeast. You will also use 10 mL of liquid—sucrose water, milk etc. Other supplies/ideas to test: various concentrations of sucrose (3%, 10%, 20%, 40%) a different type of sugar (glucose—corn syrup, lactose-milk) Possibly other types of sugar (brown sugar and powdered sugar) different temperatures of water/milk (0˚ C, 40˚C, 100˚C (212˚F) and boiling?? You will need to make sure you have both O2 and CO2 sensors in your bottle—see picture below. You also need to make sure you have changed your Mode: Time Based to 600 seconds instead of the 300 seconds it is normally. 02% C02 ppm Bioengineering Design Challenge When bakers make bread, they include flour with the yeast, sugar and water. The gluten protein in the flour gives elasticity to the dough and traps the CO2 bubbles produced by the yeast so the bread dough rises and the bread becomes fluffy. The fluffiness of the bread can be influenced by the relative amounts of yeast, sugar, water and flour, as well as other ingredients in the dough. The fluffiness of the bread can also be influenced by the temperature of the dough as it rises and how long the dough rises. Design Challenge. Jim Baker wants to make his bread as fluffy as possible without spending too much time waiting for the dough to rise. He has asked your class to find the amount of sucrose and temperature that produces the most CO2 in 10 minutes. He does not want his bread to be too sweet, so he doesn't want to use any more sucrose than needed for maximum CO2 production. To maintain good flavor and texture of the bread, he wants to keep the amount of yeast the same as in your experiment in Part II. Scientific Background. CO2 is produced by alcoholic fermentation, which includes multiple chemical reactions, each catalyzed by a different enzyme. Fortunately, you do not need to think about all of these chemical reactions and enzymes to predict the effects of changes in the amount of sucrose or temperature on the rate of CO2 production; you can get sufficiently accurate predictions by thinking about the expected effect for a single reaction catalyzed by a single enzyme. 11a. What happens to the rate of a chemical reaction catalyzed by an enzyme when the concentration of substrate increases? In the graph, draw a curve to show the expected change in the rate of CO2 production as the amount of sucrose increases. 11b. What happens to the rate of a chemical reaction catalyzed by an enzyme when temperature increases? In the graph, draw a curve to show the expected change in the rate of CO2 production as the temperature increases. Data Table 1: Starting O2%, CO2 ppm and Ending O2%, CO2 ppm for various concentrations of yeast, sucrose, different types of sugar, temperatures etc. You will possibly complete two trials. What is in your solution in your biochamber?—Be specific Example: 3% yeast, 10mL of 10% sucrose at 40˚C Starting O2% Concentration Ending O2% Concenration 19.91% 19.59% Starting CO2 ppm 3485 ppm Ending CO2 ppm 11702 ppm 1). According to our experiments, what was the ideal situation for yeast? 2). Explain yeast fermentation using the following words: Glucose, alcohol and CO2 cytoplasm, glycolysis, 2 ATP and pyruvate. 3). What is the difference between aerobic and anaerobic respiration? 4). If humans undergo fermentation, what molecule do we produce instead of ethyl alcohol?