ChemQuestsanswers
advertisement
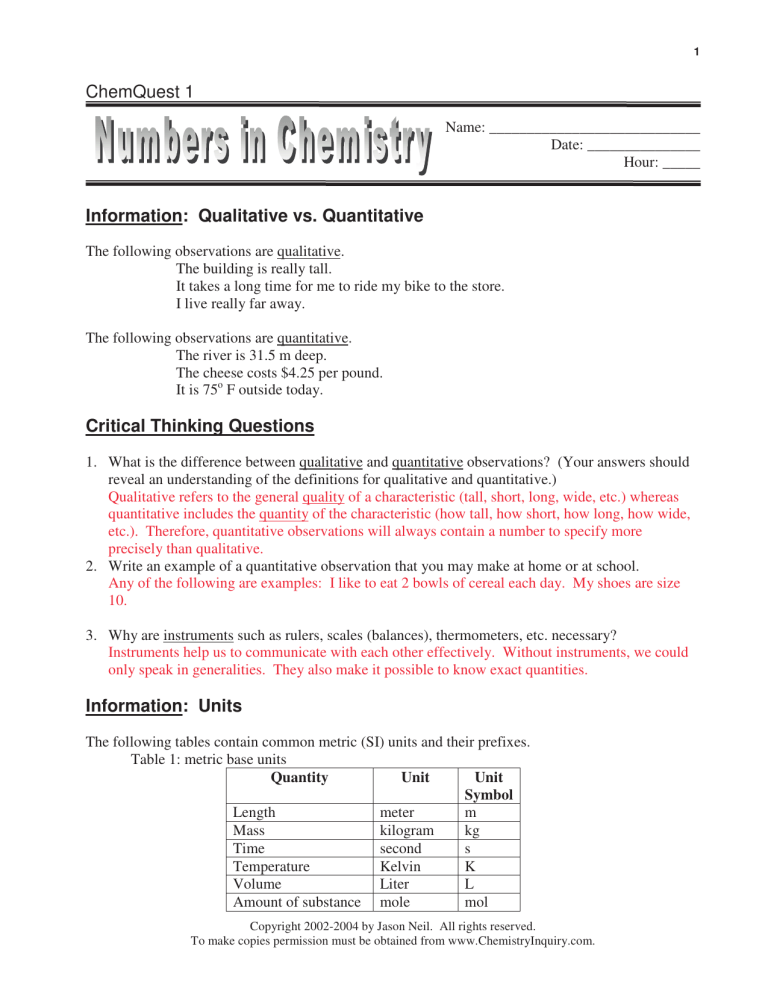
1 ChemQuest 1 Name: ____________________________ Date: _______________ Hour: _____ Information: Qualitative vs. Quantitative The following observations are qualitative. The building is really tall. It takes a long time for me to ride my bike to the store. I live really far away. The following observations are quantitative. The river is 31.5 m deep. The cheese costs $4.25 per pound. It is 75o F outside today. Critical Thinking Questions 1. What is the difference between qualitative and quantitative observations? (Your answers should reveal an understanding of the definitions for qualitative and quantitative.) Qualitative refers to the general quality of a characteristic (tall, short, long, wide, etc.) whereas quantitative includes the quantity of the characteristic (how tall, how short, how long, how wide, etc.). Therefore, quantitative observations will always contain a number to specify more precisely than qualitative. 2. Write an example of a quantitative observation that you may make at home or at school. Any of the following are examples: I like to eat 2 bowls of cereal each day. My shoes are size 10. 3. Why are instruments such as rulers, scales (balances), thermometers, etc. necessary? Instruments help us to communicate with each other effectively. Without instruments, we could only speak in generalities. They also make it possible to know exact quantities. Information: Units The following tables contain common metric (SI) units and their prefixes. Table 1: metric base units Quantity Unit Unit Symbol Length meter m Mass kilogram kg Time second s Temperature Kelvin K Volume Liter L Amount of substance mole mol Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 2 Table 2: prefixes for metric base units. Prefix Mega M kilo k deci d centi c milli m micro µ nano n pico p Symbol Meaning million thousand tenth hundredth thousandth millionth billionth trillionth Note the following examples: • “milli” means thousandth so a milliliter (symbol: mL) is one thousandth of a Liter and it takes one thousand mL to make one L. • “Mega” means million so “Megagram” (Mg) means one million grams NOT one millionth of a gram. One millionth of a gram would be represented by the microgram (µg). It takes one million micrograms to equal one gram and it takes one million grams to equal one Megagram. • One cm is equal to 0.01 m because one cm is “one hundredth of a meter” and 0.01 m is the expression for “one hundredth of a meter” Critical Thinking Questions 4. How many milligrams are there in one kilogram? 1,000,000 mg 5. 21.5 km is how many meters? 21,500 m 6. Is it possible to answer this question: How many mg are in one km? Explain. No, because milligrams and kilometers are different units of measure. 7. What is the difference between a Mm and a mm? Which is larger one Mm or one mm? A Mm is a megameter (1,000,000 meters) and a mm is a millimeter (1/1000 of a meter). Information: Scientific Notation “Scientific notation” is used to make very large or very small numbers easier to handle. For example the number 45,000,000,000,000,000 can be written as “4.5 x 1016 ”. The “16” tells you that there are sixteen decimal places between the right side of the four and the end of the number. Another example: 2.641 x 1012 = 2,641,000,000,000 the “12” tells you that there are 12 decimal places between the right side of the 2 and the end of the number. Very small numbers are written with negative exponents. For example, 0.00000000000000378 can be written as 3.78 x 10-15. The “-15” tells you that there are 15 decimal places between the right side of the 3 and the end of the number. 3 Another example: 7.45 x 10-8 = 0.0000000745 the “-8” tells you that there are 8 decimal places between the right side of the 7 and the end of the number. In both very large and very small numbers, the exponent tells you how many decimal points are between the right side of the first digit and the end of the number. If the exponent is positive, the decimal places are to the right of the number. If the exponent is negative, the decimal places are to the left of the number. Critical Thinking Questions 8. Two of the following six numbers are written incorrectly. Circle the two that are incorrect. a) 3.57 x 10-8 b) 4.23 x 10-2 c) 75.3 x 102 d) 2.92 x 109 e) 0.000354 x 1014 f) 9.1 x 104 What do you think is wrong about the two numbers you circled? C should be written 7.53x103 and E should be written 3.54x1010. The correct format for writing numbers in scientific notation is with only one digit to the left of the decimal place. Also, the number should not begin with zeros. 9. Write the following numbers in scientific notation: a) 25,310,000,000,000,000 = 2.531x1016 b) 0.000000003018 = 3.018x10-9 10. Write the following scientific numbers in regular notation: a) 8.41 x 10-7 = 0.000000841 b) 3.215 x 108 = 321,500,000 Information: Multiplying and Dividing Using Scientific Notation When you multiply two numbers in scientific notation, you must add their exponents. Here are two examples. Make sure you understand each step: (4.5x1012) x (3.2x1036) = (4.5)(3.2) x 1012+36 = 14.4x1048 1.44x1049 (5.9x109) x (6.3x10-5) = (5.9)(6.3) x 109+(-5) = 37.17x104 3.717x105 When you divide two numbers, you must subtract denominator’s exponent from the numerator’s exponent. Here are two examples. Make sure you understand each step: 2.8 x1014 2.8 = x1014−7 = 0.875x107 = 8.75 x106 7 3.2 x10 3.2 5.7 x1019 5.7 = x1019−( −9) = 1.84 x1019+9 = 1.84 x10 28 −9 3.1 3.1x10 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 4 Critical Thinking Questions 11. Solve the following problems. a) (4.6x1034)(7.9x10-21) = (4.6)(7.9) x 1034+(-21) = 36.34 x1013 3.634x1014 b) (1.24x1012)(3.31x1020) = (1.24)(3.31)x1012+20 = 4.1x1032 12. Solve the following problems. a) 8.4 x10 −5 8.4 = x10 − 5−17 = 2.05 x10 − 22 4.1x10 17 4.1 b) 5.4 x10 32 5.4 = x10 32 −14 = .74 x1018 = 7.4 x1017 7.3 x10 14 7.3 Information: Adding and Subtracting Using Scientific Notation Whenever you add or subtract two numbers in scientific notation, you must make sure that they have the same exponents. Your answer will them have the same exponent as the numbers you add or subtract. Here are some examples. Make sure you understand each step: 4.2x106 + 3.1x105 make exponents the same, either a 5 or 6 42x105 + 3.1x105 = 45.1x105 = 4.51x106 7.3x10-7 - 2.0x10-8 make exponents the same, either -7 or -8 73x10-8 – 2.0x10-8 = 71x10-8 = 7.1x10-7 Critical Thinking Questions 13. Solve the following problems. a) 4.25x1013 + 2.10x1014 = first, change exponents so both are either 13 or 14 4.25x1013 + 21.0x1013 = 25.25x1013 2.525 x 1014 b) 6.4x10-18 – 3x10-19 = first, change exponents so both are either -18 or -19 6.4x10-18 – 0.3x10-18 = 6.1 x 10-18 c) 3.1x10-34 + 2.2x10-33 = first, change exponents so both are either -34 or -33 3.1x10-34 + 22x10-34 = 25.1x10-34 2.51x10-33 5 ChemQuest 2 Name: ____________________________ Date: _______________ Hour: _____ Information: Significant Figures We saw in the last ChemQuest that scientific notation can be a very nice way of getting rid of unnecessary zeros in a number. For example, consider how convenient it is to write the following numbers: 32,450,000,000,000,000,000,000,000,000,000,000 = 3.245 x 1034 0.000000000000000127 = 1.27 x 10-16 There are a whole lot of zeros in the above numbers that are not really needed. As another example, consider the affect of changing units: 21,500 meters = 21.5 kilometers 0.00582 meters = 5.82 millimeters Notice that the zeros in “21,500 meters” and in “0.00582 meters” are not really needed when the units change. Taking these examples into account, we can introduce three general rules: 1. Zeros at the beginning of a number are never significant (important). 2. Zeros at the end of a number are not significant unless… (you’ll find out later) 3. Zeros that are between two nonzero numbers are always significant. Therefore, the number 21,500 has three significant figures: only three of the digits are important— the two, the one, and the five. The number 10,210 has four significant figures because only the zero at the end is considered not significant. All of the digits in the number 10,005 are significant because the zeros are in between two nonzero numbers (Rule #3). Critical Thinking Questions 1. Verify that each of the following numbers contains four significant figures. Circle the digits that are significant. Significant digits are in red italics type. a) 0.00004182 b) 494,100,00 c) 32,010,000,000 d) 0.00003002 2. How many significant figures are in each of the following numbers? __5__ a) 0.000015045 __2__ b) 4,600,000 __4__ c) 2406 __1__ d) 0.000005 __6__ e) 0.0300001 __2__ f) 12,000 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 6 Information: The Exception to Rule #2 There is one exception to the second rule. Consider the following measured values. It is 1200 miles from my town to Atlanta. It is 1200.0 miles from my town to Atlanta. The quantity “1200.0 miles” is more precise than “1200 miles”. The decimal point in the quantity “1200.0 miles” means that it was measured very precisely—right down to a tenth of a mile. Therefore, the complete version of Rule #2 is as follows: Rule #2: Zeros at the end of a number are not significant unless there is a decimal point in the number. A decimal point anywhere in the number makes zeros at the end of a number significant. Not significant because these are at the beginning of the number! 0.0000007290 This zero is significant because it is at the end of the number and there is a decimal point in the number. Critical Thinking Questions 3. Verify that each of the following numbers contains five significant figures. Circle the digits that are significant. Digits in red italics are significant. a) 0.00030200 b) 200.00 c) 2300.0 d) 0.000032000 4. How many significant figures are there in each of the following numbers? __6__ a) 0.000201000 __5__ b) 23,001,000 __3__ c) 0.0300 __7__ d) 24,000,410 __7__ e) 2400.100 __2__ f) 0.000021 Information: Rounding Numbers In numerical problems, it is often necessary to round numbers to the appropriate number of significant figures. Consider the following examples in which each number is rounded so that each of them contains 4 significant figures. Study each example and make sure you understand why they were rounded as they were: 42,008,000 42,010,000 12,562,425,217 12,560,000,000 0.00017837901 0.0001784 120 120.0 Critical Thinking Questions 5. Round the following numbers so that they contain 3 significant figures. a) 173,792 b) 0.0025021 c) 0.0003192 d) 30 __174,000___ __0.00250___ __0.000319__ __30.0______ 7 6. Round the following numbers so that they contain 4 significant figures. a) 249,441 b) 0.00250122 c) 12,049,002 d) 0.00200210 __249,400___ __0.002501__ __12,050,000__ ___0.002002__ Information: Multiplying and Dividing When you divide 456 by 13 you get 35.0769230769… How should we round such a number? The concept of significant figures has the answer. When multiplying and dividing numbers, you need to round your answers to the correct number of significant figures. To round correctly, follow these simple steps: 1) Count the number of significant figures in each number. 2) Round your answer to the least number of significant figures. Here’s an example: 3 significant figures 4560 = 325.714285714 = 330 14 2 significant figures Final rounded answer should have only 2 significant figures since 2 is the least number of significant figures in this problem. Here’s another example: 13.1× 1.2039 = 15.77109 = 15.8 3 significant figures 5 significant figures Final rounded answer should have 3 significant figures since 3 is the least number of significant figures in this problem. Critical Thinking Questions 7. Solve the following problems. Make sure your answers are in the correct number of significant figures. a) (12.470)(270) = ___3400________ b) 36,000/1245 = ___29_________ c) (310.0)(12) = ____3700_________ d) 129.6/3 = ____40____________ e) (125)(1.4452) = ___181_________ f) 6000/2.53 = ____2000________ Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 8 Information: Rounding to a Decimal Place As you will soon discover, sometimes it is necessary to round to a decimal place. Recall the names of the decimal places: The hundred The ten thousands thousands place place The thousands place The hundreds place The tens place The ones place The tenths place The hundredths place The thousandths place If we rounded the above number to the hundreds place, that means that there can be no significant figures to the right of the hundreds place. Thus, “175,400” is the above number rounded to the hundreds place. If we rounded to the tenths place we would get 175,398.4. If we rounded to the thousands place we would get 175,000. Critical Thinking Questions 8. Round the following numbers to the tens place. a) 134,123,018 = ___134,123,020__ b) 23,190.109 = __23,190__________ c) 439.1931 = ___440___________ d) 2948.2 = ___2950______________ Information: Adding and Subtracting Did you know that 30,000 plus 1 does not always equal 30,001? In fact, usually 30,000 + 1 = 30,000! I know you are finding this hard to believe, but let me explain… Recall that zeros in a number are not always important, or significant. Knowing this makes a big difference in how we add and subtract. For example, consider a swimming pool that can hold 30,000 gallons of water. If I fill the pool to the maximum fill line and then go and fill an empty one gallon milk jug with water and add it to the pool, do I then have exactly 30,001 gallons of water in the pool? Of course not. I had approximately 30,000 gallons before and after I added the additional gallon because “30,000 gallons” is not a very precise measurement. So we see that sometimes 30,000 + 1 = 30,000! Rounding numbers when adding and subtracting is different from multiplying and dividing. In adding and subtracting you round to the least specific decimal place of any number in the problem. 9 Example #1: Adding The tens place contains a significant figure. 350.04 +720 1070.04 1070 The hundredths place contains a significant figure. The answer gets rounded to the least specific place that has a significant figure. In this case, the tens place is less specific than the hundredths place, so the answer is rounded to the tens place. Example #2: Subtracting The thousands place contains a significant figure. 7000 - 1770 5230 The tens place contains a significant figure. The answer gets rounded to the least specific place that has a 5000 Critical Thinking Questions significant figure. In this case, the thousands place is less specific than the tens place, so the answer gets rounded to the thousands place. 9. a) 24.28 + 12.5 = ___36.8___________ b) 120,000 + 420 = ____120,000________ c) 140,100 – 1422 = __138,700_______ d) 2.24 – 0.4101 = ___1.83_____________ e) 12,470 + 2200.44 = __14,670______ f) 450 – 12.8 = _____440______________ 10. The following are problems involving multiplication, dividing, adding, and subtracting. Be careful of the different rules you need to follow! a) 245.4/120 = ___2.0______________ b) 12,310 + 23.5 = ___12,330___________ c) (31,900)(4) = ___100,000_________ d) (320.0)(145,712) = __46,000,000______ e) 1420 – 34 = ___1390____________ f) 4129 + 200 = ___4300_______________ Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 10 ChemQuest 3 Name: ____________________________ Date: _______________ Hour: _____ Information: Changes in Matter Books are made of matter. You are made of matter. “Matter” is a fancy word for the “stuff” of which all objects are made. Every day, matter is changed in different ways. For example, paper can be changed in many ways—it can be torn, folded, or burned. A chemical change is any alteration that changes the identity of matter. For example, by passing electricity through water it can be broken down into hydrogen and oxygen. Burning paper is a chemical change because after the change takes place, the paper has been changed into different substances (like ash, carbon dioxide, etc.). A physical change is any alteration that does not change the identity of the matter. Shredding paper does not change the paper into a different substance. Dissolving salt in water is a physical change because after the change, the salt and water are both still there. Critical Thinking Questions 1. Explain why each of the following is a physical change. a) boiling water until no water remains Boiling the water simply changes the state of matter, but it is still H2O before and after it is boiled. b) mixing sugar with coffee The sugar is still sugar before and after you mix it with coffee. Neither the sugar nor the coffee changes into something entirely different. 2. Explain why each of the following is a chemical change. a) a car rusting When a car rusts, the iron metal in the car changes into something else (iron oxide) which has completely different properties. b) food digesting The chemicals in the food are broken down into other chemicals during digestion. 3. Identify each of the following changes as chemical or physical by placing a C or P in each blank. __C__ a) acid rain corroding the statue of liberty __ P__ d) melting steel __P__ b) dissolving salt in water __C__ e) dissolving steel in acid __ P__ c) boiling salt water until just salt remains __ P__ f) cracking ice 11 Information: Elements, Compounds, Mixtures Examine the following tables. Following the name of each element or compound is the “chemical formula” of the element or compound; please see the periodic table for the meaning of some of the symbols (i.e. Na = sodium). Italics tell you that substance is organic. Elements Sodium (Na) Chlorine (Cl) Carbon (C) Oxygen (O) Hydrogen (H) Compounds Water (H2O) Methane (CH4) Sodium chloride, salt (NaCl) Carbon dioxide (CO2) Hydrogen Peroxide (H2O2) Pure Substances Salt (NaCl) Hydrogen (H) Carbon dioxide (CO2) Water (H2O) Aluminum (Al) Mixtures Salt water (NaCl and H2O) Sand Hydrogen (H) and Oxygen (O) Sodium (Na) and Chlorine (Cl) Kool-aid (sugar, water, etc.) Critical Thinking Questions 4. How are elements different from compounds? Elements are composed of only one type of atom, but compounds are composed of more than one. 5. How are compounds different from mixtures? Compounds are formed by a chemical change (i.e. two hydrogen and one oxygen atom bonding to form a water molecule), but mixtures are formed by a physical change (i.e. stirring salt and water together. 6. How are pure substances different from mixtures? Pure substances are not mixed with anything else, but mixtures are composed of two or more things physically (not chemically) combined. 7. Can something be both a mixture and a pure substance? Explain using examples from the tables. No, there is nothing from the table that is in both categories. 8. Is it always possible to identify something as an element, compound, pure substance or mixture just by looking at it? Explain using examples from the tables. No, some things look the same, but are not the same at the microscopic scale. For example, water (a compound) and salt water (a mixture) look exactly the same. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 12 9. Formulate a definition for each of the following terms. a) element: Matter that is composed of only one kind of atom. b) compound: Matter that is composed of two or more kinds of atoms chemically combined together (that is, it is made by a chemical change occurring between two or more atoms). c) mixture: Matter that is composed of two or more pure substances physically combined together. d) pure substance: Matter that is not mixed—either a pure element or a pure compound. 10. Categorize each of the following as an element, compound, mixture, or pure substance. If more than one label applies, then include both labels. (You will need more than one label sometimes.) a) ____Mixture_________ Popsicle c) __Element, Pure Substance_ Gold b) Compound, Pure Substance Sugar d) ______Mixture___________ Dishwater 11. If you have a container with hydrogen gas and oxygen gas in it do you have water? Why or why not? You do not have water because the hydrogen and oxygen atoms are simply mixed together; they are not bonded together. 12. Give an example of something that is an element. Your example should not already be on this sheet. Any substance listed on the periodic table. For example, phosphorus or nitrogen. 13. Give an example of something that is a compound. Your example should not already be on this sheet. Octane, hydrochloric acid, carbon monoxide, etc. 14. Give an example of something that is a mixture. Your example should not already be on this sheet. Soda pop, salad, bread and butter, etc. 15. What do all organic substances have in common? They all contain carbon. 13 Information: Homogeneous and Heterogeneous Mixtures Examine the following table. Example of Mixture Salt water Oil and water Sugar and salt (no water) Sugar and salt in water Sand and water Carbon dioxide, water, and ice 14 kt. gold (mixture of silver and gold) # of phases in mixture 1 2 2 1 2 3 1 How many kinds of states in mixture 2 1 1 2 2 3 1 Homogeneous or heterogeneous? Homogeneous Heterogeneous Heterogeneous Homogeneous Heterogeneous Heterogeneous Homogeneous Critical Thinking Questions 16. What is the difference between a "phase of matter" and a "state of matter"? Define each term as best you can. The “state” of matter tells us whether the substance is a solid, liquid, or gas. A “phase” of matter is a section of matter that is the same throughout. For example, in oil and water, there is one state—the liquid state—and there are two phases—the oil phase and the water phase. 17. What relationship exists between a homogeneous mixture and the number of phases in the mixture? A homogeneous mixture must have only one phase. 18. What is the difference between homogeneous and heterogeneous mixtures? A homogeneous mixture is the same throughout—one small sample of the mixture is an exact representation of the whole. A heterogeneous mixture has different parts—one small sample will not have the same composition as the rest of the mixture. 19. If you had to categorize elements as homogeneous or heterogeneous, what category would you put them in? All elements are homogeneous. For example, one small piece of gold should have the same composition as a larger piece of gold. 20. If you had to categorize compounds as homogeneous or heterogeneous, what category would you put them in? All compounds are homogeneous. For example, one drop of water will have the same composition as another drop—all H2O molecules. 21. Categorize each of the following as homogeneous or heterogeneous. heterogeneous a) salad heterogeneous b) ice water heterogeneous c) dishwater homogeneous d) 14 kt. Gold Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 14 ChemQuest 4 Name: ____________________________ Date: _______________ Hour: _____ Information: Phase diagrams Figure 1: Phase diagram for water. Note that the unit for pressure on the diagram is atmospheres (atm). Another unit is the kilopascal (kPa). Standard pressure is the pressure at sea level and it is equal to 1 atm, which is equivalent to 101.325 kPa. Standard temperature is 273 Kelvin (K), which equals 0oC. The abbreviation STP is used for "standard temperature and pressure" and it denotes a temperature of 0oC and 1 atm (or 273K and 101.325 kPa). Triple point A phase diagram is a graph that illustrates under what conditions the states of matter exist. For example, in the phase diagram of water above, it should be noted that at 1 atm (which equals 101.325 kPa) of pressure and 50 oC, H2O exists as a liquid. The dark solid lines represent the boundaries between different states. The term “vapor” is used instead of “gas” because vapor describes a substance that is normally a liquid at STP. The line that divides the liquid area from the vapor area has a special name: the vapor pressure curve. (The vapor pressure of water at any given temperature can be found looking at the vapor pressure curve.) To the left of the line, liquid exists. To the right of the line, vapor (gaseous H2O) exists. Right on the line, for example when the pressure is 1 atm and the temperature is 100oC, both liquid and vapor exist at the same time. When two or more things coexist at the same time it is called “equilibrium”. During equilibrium, liquid changes to vapor and vapor changes to liquid all at the same time and rate. Critical Thinking Questions 1. When a substance melts, what happens to the motion of the molecules of the substance? The molecules begin to move faster and get farther apart. 15 2. What is the freezing point and boiling point of water when the pressure on the water is 1 atm? (I.e. at what temperature will the water freeze and boil when the pressure is 1 atm?) Freezing point = 0oC; Boiling point = 100oC 3. How does lowering the pressure affect the boiling point? The boiling point also gets lower. 4. Estimate the boiling point of water when the pressure on the water is 0.1 atm. Approximately 73oC. 5. The triple point of a substance is the conditions under which a solid, liquid and gas all exist in equilibrium. On the phase diagram of water, label the location of the triple point. See diagram. 6. Dry ice sublimes; that is, it changes directly from a solid to a gas. Is it ever possible for ice to sublime? If so, describe the conditions necessary for sublimation to occur. Yes, ice will sublime at pressures below approximately 0.007 atm. 7. What is meant by the “vapor pressure” of water? The pressure that gaseous water exerts, or, the force that water molecules exert after the molecules have evaporated. 8. From the phase diagram, estimate the vapor pressure of water at 25 oC. Approximately 0.01 atm. 9. What is the vapor pressure of water when the temperature is 100 oC? Approximately 1 atm. 10. When the atmospheric pressure equals 1 atm, what is the boiling point of water? 100oC 11. From your answers to questions 9 and 10, formulate a definition for boiling point in terms of atmospheric pressure and vapor pressure of the liquid. The boiling point is the temperature at which the vapor pressure of a liquid equals the atmospheric pressure. As the atmospheric pressure decreases, so does the liquids vapor pressure and, thus, the boiling point will also decrease. 12. Is it ever possible for solid H2O to exist at a temperature above 0 oC? Yes, at lower pressures (pressures below 1 atm). 13. Is it ever possible for solid H2O to exist at a temperature above 25 oC? No, there is no solid region on the phase diagram at any temperature above roughly 5oC. 14. Describe the region where a solid-liquid equilibrium exists. A solid liquid equilibrium exists along the line that divides the liquid region from the vapor region. 15. Why might an equilibrium situation be described as a “reversible change”? Equilibrium describes a situation where two equal and opposite processes occur at the same rate. In this case, water evaporates and some vapor condenses, thereby “reversing” the change. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 16 ChemQuest 5 Name: ____________________________ Date: _______________ Hour: _____ Information: Heat and Temperature When a substance absorbs heat energy, the temperature of the substance increases. There are a number of factors (such as mass of the substance) that affect how much the temperature of a substance changes. Critical Thinking Questions 1. Consider two pots of water. Each pot has the same diameter, but pot A is deeper than pot B and so there is more water in pot A. If both of the pots are exposed to exactly the same amount of heat for five minutes on a stove, which pot will contain the hottest water after heating? The water in Pot B will be hotter. 2. Propose an explanation for the fact that even though both pots were exposed to the same amount of heat, one got hotter. Since Pot A has more water in it, it will take more heat to increase the temperature. 3. Fill in the blank: The greater the mass of a substance (like water in questions 1 and 2), the _______lower_________ the temperature change when heat is applied. greater or lower 4. Consider the metal hood of a car on a warm sunny day. Next to the car is a large puddle of water. The puddle of water is so large that it has the same mass as the hood of the car. Assume that the hood of the car and the puddle are exposed to the same amount of sunlight. Which will be hotter after two hours—the hood of the car or the puddle? The hood of the car will be hotter. 5. Propose an explanation for the fact that even though both the hood and the puddle were exposed to the same amount of heat energy and their masses were the same, one still got much warmer than the other. The metal must heat up faster than water does. There must be something about metal that allows it to heat up faster than water. 6. In general is it harder to change the temperature of metal (like on a car hood) or of water? In other words, would it require more heat energy to change the temperature of metal or of water? It would require more heat energy to change the temperature of water. 17 7. In one of the following blanks you will need to write “400” and in the other blank you will need to write “200”. If we wanted to change the temperature of water by 4 oC, then _400___ Joules of heat energy are required, but to change the temperature of metal by 4oC, then __200___ Joules of heat energy are required. Information: Specific Heat In questions 1 and 2 above you probably recognized that the temperature change of a substance depends on the mass of the substance. You also have probably experienced the fact that different substances heat at different rates as was discussed in questions 3 and 4 above. Each substance has its own specific heat capacity. The specific heat capacity is a measure of the amount of energy needed to change the temperature of the substance. The higher the specific heat, the more energy is required to change the temperature of the substance. Critical Thinking Questions 8. Which substance has a higher specific heat—water or a metal like the aluminum in a car hood? Since it requires more heat energy change the temperature of water and since specific heat is a measure of how much energy is needed to change the temperature of a substance, water must have a higher specific heat. 9. Consider 200 Joules (J) of heat energy applied to several objects and fill in the blank: The higher the specific heat of the object, the __lower___________ the temperature change of the object. lower or higher 10. Given the following symbols and your answers to the above questions, which of the following equations is correct. Make sure you have the correct answer before proceeding to the next questions! ∆T = temperature change of a substance Cp = specific heat capacity m = mass of the substance q = amount of heat energy applied to the substance (C p )(m) q (q)(m) A) ∆T = qmCp B) ∆T = C) ∆T = D) ∆T = (m)(C p ) Cp q HINT: equation D is not correct because according to that equation, a large mass (m) will lead to a large temperature change (∆T), but this is not consistent with question 3. 11. If ∆T is measured in oC, m is measured in grams (g) and q is measured in Joules (J), what are the units for specific heat capacity? q q J ∆T = Cp = Cp = (m)(C p ) (m)(∆T ) (g)( o C) Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 18 12. What is the temperature change of a 120 g piece of aluminum whose specific heat is 1.89 J/goC after 1800 J of heat energy is applied? 1800 J q ∆T = ∆T = = 7.936508 o C = 7.9 o C J (m)(C p ) (120 g)(1.89 o ) g C 13. A beaker containing 250.0 g of water is heated with 1500.0 J of heat energy. If the temperature of the water changed from 22.0000oC to 23.4354oC, what is the specific heat of water? ∆T = 23.4354 oC – 22.0000 oC = 1.4354 oC q 1500 J J q ∆T = Cp = Cp = = 4.180 o o (m)(C p ) (m)(∆T ) (250 g)(1.4354 C) g C 14. Heat energy equal to 25,000 J is applied to a 1200 g brick whose specific heat is 2.45 J/goC. a) What is the change in temperature of the brick? ∆T = q 25,000 = = 8.503401 = 8.5 o C (m)(C p ) (1200)(2.45) b) If the brick was initially at a temperature of 25.0oC, what is the final temperature of the brick? 25.0 + 8.5 = 33.5 oC 19 ChemQuest 6 Name: ____________________________ Date: _______________ Hour: _____ Information: Specific Heat Constants Depend on the State of Matter Recall that specific heat is the energy necessary to raise the temperature of one gram of a substance by one degree Celsius. The specific heat of liquid water is a constant equal to 4.18 J/(g-oC). This means that it takes 4.18 Joules (J) of heat energy to raise the temperature of each gram of water by one degree Celsius. For ice, the specific heat is 2.06 J/goC. Steam’s specific heat is 2.02 J/goC. You will want to remember the three different specific heat values for H2O. Critical Thinking Questions 1. How much energy is required to heat 32.5g of water from 34oC to 75oC? ∆T = 75-34 = 41 q = (m)(Cp)(∆T) = (32.5)(4.18)(41) = 5569.9 J 2. How many J of energy are needed to heat 45.0g of steam from 130oC to 245oC? Why don’t you use 4.18 J/goC in this calculation? ∆T = 245-130 = 115 q = (m)(Cp)(∆T) = (45.0)(2.02)(115) = 10,453.5 J You don’t use 4.18 because 4.18 is the specific heat for liquid water; 2.02 is for steam. 3. Why is it impossible for you to answer the following question right now: How much energy is required to heat 35g of H2O from 25oC to 150oC? The problem involves both liquid water and steam and we don’t know how to combine them in the same calculation….YET Information: Energy Involved in Changing State Obviously, it takes energy to heat a substance such as water. There is also an energy change when a substance changes state. For example, when water freezes, energy is taken away from the water and when water boils, energy is being added to the water. When water is heated up to 100oC, it will NOT automatically boil unless more energy is added. Once water is at the correct temperature (100oC), it will boil IF you add energy to change it from a liquid to a gas. The extra energy is needed to separate the molecules from one another. Note: at the boiling point of a liquid energy is added to the liquid, but the temperature of the liquid does NOT change. The temperature of H2O will increase above 100oC only after all of the water has been changed to steam. After the phase change, if more energy is applied, the temperature will go up. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 20 Figure 1: Graph of the temperature of H2O vs. energy added to the water. 100oC Temperature of H2O (oC) 0 oC 0 25 50 75 100 125 150 175 200 225 250 275 300 Energy added to water in kilojoules (kJ) Critical Thinking Questions 4. What is the significance of the horizontal portions of the graph? What is going on during those times? The horizontal portions indicate a phase change such as melting or boiling. 5. Label the temperature scale on the graph. See graph. 6. Does it require more energy to “vaporize” water at the boiling point or to melt water at the melting point? Explain. It takes more energy to vaporize than to melt because the horizontal section of the graph is much longer during the vaporizing, or boiling. Information: Mathematics of Changes of State Recall the equation: q = (m)(Cp)(∆T) where q is the heat energy, Cp is the specific heat of the substance, m is the mass of the substance, and ∆T is the change in the temperature. This equation works ONLY when there is no phase change. You use it to find how much energy is required to change the temperature of a certain substance as long as you know the specific heat of the substance and as long as no change in state occurs. Now for when there is a change in state: The energy required for a change of state is given a special name called enthalpy. So the “enthalpy of vaporization” (symbolized by ∆Hvap) of water is the energy needed to vaporize (or boil) one gram of water when the water is already at its boiling point. The ∆Hvap of water is 2260 J/g. So for each gram of hot (100oC) water, 2260 J are required to vaporize it. Similarly, the enthalpy of fusion (∆Hfus) of H2O is 334 J/g. So each gram of ice at 0oC there are 334 J of energy required to melt it. (Fusion is melting.) 21 Critical Thinking Questions 7. Verify that it takes 4140.7 J of energy to heat 12.7 g of water from room temperature (22oC) to the boiling point (100oC). Recall that the specific heat (Cp) of water is 4.18 J/(g-oC). ∆T = 100-22 = 78 q = (m)(Cp)(∆T) = (12.7)(4.18)(78) = 4140.7 J 8. Given the fact that the ∆Hvap of water is 2260 J/g we know that 2260 J of energy is required to vaporize 1 gram of water. To vaporize 2 grams of water it requires 4520 J. For 3 grams, 6780 J are needed. Verify that it takes 28,702 J of energy to vaporize 12.7 g of water, when the water is already at its boiling point. (2260 J/g)(12.7g) = 28,702 J; note: a helpful equation for phase changes is q=(m)(∆H) 9. Verify that it takes 1513.6 J of energy to heat 12.7 g of steam from 100oC to 159oC. Note: the specific heat of steam (Cp) is 2.02 J/(g-oC). ∆T = 159-100 = 59 q = (m)(Cp)(∆T) = (12.7)(2.02)(59) = 1513.6 J 10. Using only your answers to questions 7-9, calculate how many Joules of energy it takes to change 12.7 g of water at 22oC to steam at 159oC. Questions 7-9 are each different “steps” for taking 12.7g of water from 22oC to 159oC so you can simply add the answers: 4140.7 + 28,702 + 1513.6 = 34,356.3 J 11. Calculate how many Joules of energy would be required to change 32.9 g of water at 35oC to steam at 120oC. You will need to break this problem into four steps. a) Find the Joules needed to heat the water to the boiling point. q = (m)(Cp)(∆T) = (32.9)(4.18)(65) = 8938.9 J b) Find the Joules needed to vaporize the water. q=(m)(∆H) = (32.9)(2260) = 74,354 J c) Find the Joules needed to heat the vapor (steam) from the boiling point to 120oC. q = (m)(Cp)(∆T) = (32.9)(2.02)(20) = 1329.2 J d) Add your answers to steps a, b, and c. 8938.9 + 74,354, + 1329.2 = 84,622 J 12. How much heat energy would be required to change the temperature of 125g of ice from -32.9oC to liquid water at 75oC? q = (m)(Cp)(∆T) = (125)(2.06)(32.9) = 8471.75 J q=(m)(∆H) = (125)(334) = 41,750 J q = (m)(Cp)(∆T) = (125)(4.18)(75) = 39,187.5 J Total: 8471.75 + 41,750 + 39,187.5 = 89,406 J Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 22 ChemQuest 7 Name: ____________________________ Date: _______________ Hour: _____ Information: Density Object 1 2 3 4 5 6 7 Mass of Object (g) 21.50 12.6 41.90 32.90 59.5 0.594 17.23 Volume of Object (mL) 18.40 14.7 31.60 12.85 61.7 0.574 21.67 Density of Object (g/mL) 1.168 0.857 1.326 2.560 0.964 1.035 0.7951 Critical Thinking Questions 1. Consider the data for objects 1, 2 and 3. Which of the following equations correctly show the relationship(s) between mass (M), volume (V) and density (D)? There may be more than one answer. A) D= V M F) V = DM B) M= V D G) M = DV C) D= M V D) V= D M E) V= M D H) D = MV 2. For objects 4, 5, 6 and 7 there are blanks in the table. Using your answers to question 1, fill in the blanks. See the table above. 3. What are the units for density if the mass of an object was measured in kilograms and the volume in liters? kg/L 4. In your own words, define “density”. Density: the amount of mass an object has per unit volume. Density includes the idea of how much mass something has in comparison to its size. 5. Calculate the density of an object that has a mass of 45.0 kg and a volume of 20.0 L. Include units. m 45.0kg 2.25 kg/L D= = = V 20.0 L 23 Information: Mass and Weight The following is a table that relates the mass of an object and the weight of the same object. The pull of gravity is a constant. As long as you stay in the same place, the pull of gravity (g) does not change. For example, at sea level g is always equal to 9.8 m/s2. Object 1 2 3 4 Place Earth, sea level Earth, sea level Earth, Mt. Everest Moon Pull of Gravity 9.8 m/s2 9.8 m/s2 9.1 m/s2 1.6 m/s2 Mass 42 kg 29 kg 38 kg 51 kg Weight 411.6 N 284.2 N 345.8 N 81.6 N Critical Thinking Questions 6. What is the relationship between mass (M), the pull of gravity (g) and weight (W)? A) M = gW B) g = MW C) W = Mg 7. What is the weight of a 42 kg object (like object 1) if the object was on the moon where g is always 1.6 m/s2? W = Mg = (42kg)(1.6 m/s2) = 67.2 N = 67 N 8. Mass measures how much matter an object has. It is an indication of how much “stuff” is in the object. It does not describe the size of the object or the weight of the object. What is the difference between mass and weight? Weight is affected by gravity, but mass is not. 9. In question 8, you calculated the weight of object 1 on the moon. How did that weight compare to the object’s weight on Earth? Object 1 and all other objects weigh less on the moon than they do on Earth. The mass, however, of any object will be the same on Earth or on the moon. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 24 ChemQuest 8 Name: ____________________________ Date: _______________ Hour: _____ Information: Structure of the Atom Note the following symbols: (they are not to scale) = proton (positive charge) = electron (negative charge) = neutron (no charge) The following three diagrams are hydrogen atoms: 1 1 2 1 H H 3 1 H The following three diagrams are carbon atoms: 12 6 13 6 C (6 protons, 6 neutrons) Notice the type of notation used for atoms: C (6 protons, 7 neutrons) A Z 14 6 C (6 protons, 8 neutrons) X X = chemical symbol of the element Z = “atomic number” A = “mass number” 12 6 1 1 C, 13 6 C, and 146 C are notations that represent isotopes of carbon. 3 H , 21 H and 1 H are notations that represent isotopes of hydrogen. The part of the atom where the protons and neutrons are is called the nucleus. 25 Critical Thinking Questions 1 1. How many protons are found in each of the following: 1 H ? in Each has one proton, given by the atomic number of one. 1 2 1 H ? in 31 H ? 2 3 2. How many neutrons are found in each of the following:1 H ? in 1 H? in 1 H ? 2 1 3 1 H has one neutron 1 H has zero neutrons 1 H has two neutrons and subtracting the atomic Calculate the number of neutrons by taking the mass number number. 3 1 2 3. How many electrons are found in each of the following: 1 H ? in 1 H ? in 1 H ? Each has one electron. Every atom has the same number of electrons as protons. 4. What structural characteristics do all hydrogen atoms have in common? Each hydrogen atom has the same number of protons (1) and the same number of electrons (1). 5. What structural characteristics do all carbon atoms have in common? Each carbon atom has the same number of protons (6) and the same number of electrons (6). 6. What does the mass number tell you? Can you find the mass number of an element on the periodic table? It tells you the total number of protons plus neutrons. The mass number is not found on the periodic table. 7. What does the atomic number tell you? Can you find the atomic number of an element on the periodic table? It tells you the number of protons that an atom has. The atomic number is found on the periodic table. 8. Define the term isotope. The term “isotope” refers to an atom that has the same number of protons as another atom, but a different number of neutrons. 9. How does one isotope of carbon differ from another isotope of carbon? The isotopes have different numbers of neutrons (but the same number of protons). Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 26 Information: Atoms, Ions, Masses of Subatomic Particles The atomic mass unit (amu) is a special unit for measuring the mass of very small particles such as atoms. The relationship between amu and grams is the following: 1.00 amu = 1.66 x 10-24g Note the following diagrams of a fluorine atom and a fluorine ion. atom ion 9 protons 10 neutrons 9 protons 10 neutrons 19 9 F mass = 18.9980 amu atom 19 9 F -1 mass = 18.9985 amu ion 12 protons 12 neutrons 12 protons 12 neutrons 24 12 Mg mass = 23.9978 amu 24 12 Mg + 2 mass = 23.9968 amu Critical Thinking Questions 10. What is structurally different between an atom and an ion? Note: This is the ONLY structural difference between an atom and an ion. An ion has a different number of electrons than protons. An atom always has the same number of protons as electrons. For example, the fluoride ion has one more electron than protons and the magnesium ion has two fewer electrons than protons. 11. In atomic mass units (amu), what is the mass of an electron? Since the fluoride ion has one extra electron than the fluorine atom, you can subtract their masses to find the mass of one electron. 18.9985 – 18.9980 = 0.0005 amu 12. Is most of the mass of an atom located in the nucleus or outside the nucleus? How do you know? Most of the mass is inside the nucleus. The electrons only have a mass of 0.0005 amu each. All of the rest of the mass is in the nucleus, where the protons and neutrons are located. 27 13. If protons and neutrons have the same mass, what is the approximate mass of a proton and neutron in atomic mass units (amu)? Almost all of the nearly 19 amu of a fluorine atom is located in the nucleus, where there are a total of 19 protons and neutrons. The 19 protons and neutrons add up to a mass of approximately 19 amu and thus each proton and each neutron has a mass of about 1 amu. 14. The mass of carbon-14 (mass number = 14) is about 14 amu. Does this agree with what you determined in questions 11 and 13? Yes, since each proton and each neutron has a mass of about 1 amu (question #13) and the electrons have almost no mass (question #11) the mass of an atom that has 14 protons and neutrons should be about 14 amu. 15. The charge (in the upper right hand corner of the element symbol) is –1 for a fluorine ion. Why isn’t it +1 or some other number? The fluoride ion has one more electron (negative charge) than protons (positive charge). 16. What is the charge on every atom? Why is this the charge? Every atom has a charge of zero (neutral) because of equal numbers of protons and electrons. 17. How do you determine the charge on an ion? Compare the number of protons and electrons. Take the number of protons and subtract the number of electrons. 18. An oxygen ion has a –2 charge. (Use your periodic table if necessary) a) How many protons does the oxygen ion have? From the periodic table we see that oxygen is atomic number 8 and therefore it has 8 protons. b) How many electrons does the oxygen ion have? 10 electrons because it must have two more electrons than protons. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 28 ChemQuest 9 Name: ____________________________ Date: _______________ Hour: _____ Information: Weighted Averages Examine the table of student test scores for five tests they have taken. Test 1 2 3 4 5 Average Grade Student A 95 74 82 92 81 84.8 Student B 76 88 90 81 72 81.4 Critical Thinking Questions 1. Calculate the average grade for students A and B and enter the average in the table above. See table above. 2. If you know a student’s average grade can you tell what the student’s individual test scores were? Explain. No, two students with different test scores could still end up with the same average grade. 3. Suppose student C had an average of 83%. On each of his five tests he scored either 65% or 95%. Which score occurred more often? Explain. The score of 95% occurred more often because 95% is closer to the average of 83%. 4. What if the teacher decided that test five would count for 40% of the final grade and test four would count for 30% of the final grade and each of the other tests would count for 10%. Calculate the new average for each student. Note: this is called the weighted average. Student A’s new average: ____85.1____ Student B’s new average: ____78.5_______ Student A= (0.40)(81) + (0.30)(92) + (0.10)(82) + (0.10)(74) + (0.10)(95) = 85.1 Student B = (0.40)(72) + (0.30)(81) + (0.10)(90) + (0.10)(88) + (0.10)(76) = 78.5 Note: when finding the weighted average you do not divide at the end. 29 Information: Average Atomic Mass On the periodic table you can find the average atomic mass for an element. This average is a weighted average and it tells you the average mass of all the isotopes of an element. The periodic table does not contain mass numbers for individual atoms, instead you can find the average mass of atoms. The average atomic mass is calculated just how you calculated the weighted average in question 4 above. Critical Thinking Questions 5. Neon has three different isotopes. 90.51% of neon atoms have a mass of 19.992 amu. 0.27% of neon atoms have a mass of 20.994 amu. 9.22% of neon atoms have a mass of 21.991 amu. What is the average atomic mass of neon? (0.9051)(19.992) + (0.0027)(20.994) + (0.0922)(21.991) = 20.179 amu 6. Chlorine-35 is one isotope of chlorine. (35 is the mass number.) Chlorine-37 is another isotope of chlorine. How many protons and how many neutrons are in each isotope of chlorine? Chlorine has an atomic number of 17, so each isotope has 17 protons. Neutrons are found by taking the mass number and subtracting the atomic number. Chlorine-35 has 18 neutrons (35-17) and Chlorine-37 has 20 neutrons (37-17). 7. Of all chlorine atoms, 75.771% are chlorine-35. Chlorine-35 atoms have a mass of 34.96885 amu. All other chlorine atoms are chlorine-37 and these have a mass of 36.96590. Calculate the average atomic mass of chlorine. Percent of chlorine atoms that are chlorine-37: 100% - 75.771% = 24.229% (0.75771)(34.96885) + (0.24229)(36.96590) = 35.4527 amu 8. Do your answers for questions 5 and 7 agree with the average atomic masses for neon and chlorine on the periodic table? Yes, both answers should agree with the periodic table. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 30 Skill Practice 9. Complete the following table. Symbol # of neutrons 16 # of protons 15 # of electrons 15 Atomic # 15 Mass # 31 15 13 10 13 28 42 38 38 38 80 69 50 50 50 119 Po 126 84 84 84 210 N −3 8 7 10 7 15 31 2815 13 P Al +3 80 38 119 50 Sr 210 84 15 7 Sn 10. A certain element has two isotopes. One isotope , which has an abundance of 72.15% has a mass of 84.9118 amu. The other has a mass of 86.9092 amu. Calculate the average atomic mass for this element. % of other isotope = 100% - 72.15% = 27.85% (0.7215)(84.9118) + (0.2785)(86.9092) = 85.468 amu 11. Given the following data, calculate the average atomic mass of magnesium. Isotope Magnesium-24 Magnesium-25 Magnesium-26 Mass of Isotope 23.985 24.986 25.983 (0.7870)(23.985) + (0.1013)(24.986) + (0.1117)(25.983) = 24.309 amu Abundance 78.70% 10.13% 11.17% 31 ChemQuest 10 Name: ____________________________ Date: _______________ Hour: _____ Information: Bohr’s Solar System Model of the Atom All the planets are attracted to the sun by gravity. The reason that the Earth doesn’t just float out into space is because it is constantly attracted to the sun. Similarly, the moon is attracted to the Earth by Earth’s gravitational pull. So all of the planets are attracted to the sun but they never collide with the sun. Negative charges are attracted to positive ones. Therefore the negative electrons in an atom are attracted to the positive protons in the nucleus. In the early 1900’s scientists were looking for an explanation to a curious problem with their model of the atom. Why don’t atoms collapse? The negative electrons should collapse into the nucleus due to the attraction between protons and electrons. Why doesn’t this happen? Scientists were at a loss to explain this until Neils Bohr proposed his “solar system” model of the atom. Critical Thinking Questions: Bohr’s reasoning 1. Consider swinging a rock on the end of a string in large circles. Even though you are constantly pulling on the string, the rock never collides with your hand. Why is this? The rock must move so quickly that the pull from your hand is not enough to pull the rock into your hand. If you were to let go, it would fly away from you in a straight line. The pull from your hand keeps the rock near you, but because of its initial velocity it doesn’t get closer to you. 2. Even though the Earth is attracted to the sun by a very strong gravitational pull, what keeps the Earth from striking the sun? The fact that the earth is moving keeps it from striking the sun. The earth would move away from the sun, but the gravity of the sun attracts it, causing a circular orbit for the earth. 3. Why doesn’t the moon strike the Earth? The reasoning is the same as that for questions 1 and 2. 4. How could it be possible for electrons to not “collapse” into the nucleus? The electrons must be moving in orbits so fast that the pull from the nucleus is not enough to attract them toward the nucleus—this is Bohr’s “solar system model” of the atom. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 32 Information: Light Recall that light is a wave. White light is composed of all the colors of light in the rainbow. All light travels at the same speed (c), 3.00 x 108 m/s. (The speed of all light in a vacuum is always equal to 3.0 x 108 m/s.) Different colors of light have different frequencies (f) and wavelengths (λ). The speed (c), frequency (f) and wavelength (λ) of light can be related by the following equation: c=f •λ It is important that the wavelength is always in meters (m), the speed is in m/s and the frequency is in hertz (Hz). Note: 1 Hz is the inverse of a second so that 1 Hz = 1/s. Critical Thinking Questions 5. As the frequency of light increases, what happens to the wavelength of the light? As the frequency increases, the wavelength must be smaller. This is because the frequency times the wavelength must always equal 3.00x108 m/s. 6. What is the frequency of light that has a wavelength of 4.25 x 10-8 m? 3.00 x10 8 m / s c = f •λ → f = = = 7.06 x1015 Hz −8 λ 4.25 x10 m 7. What is the wavelength of light that has a frequency of 3.85 x 1014 Hz? c 3.00 x108 m / s c = f •λ → λ = = = 7.79 x10 −7 m 14 f 3.85 x10 Hz c Information: Energy levels After Bohr proposed the Solar System Model (that electrons orbit a nucleus just like planets orbit the sun), he called the orbits “energy levels”. Consider the following Bohr model of a Boron atom: = proton (positive charge) = electron (negative charge) = neutron (no charge) 1st energy level 2nd energy level Higher energy levels are further from the nucleus. For an electron to go into a higher energy level it must gain more energy. Sometimes the electrons can absorb light energy. (Recall that different colors of light have different frequencies and wavelengths.) If the right color (and therefore, frequency) of light is absorbed, then the electron gets enough energy to go to a higher energy level. The amount of energy (E) and the frequency (f) of light required are related by the following equation: E=h•f E is the energy measured in Joules (J), f is the frequency measured in Hz, and h is Planck’s constant in units of J/Hz which is the same as J-second. Planck’s constant, h, always has a value of 6.63x10-34J-s. 33 An electron that absorbs energy and goes to a higher energy level is said to be “excited.” If an excited electron loses energy, it will give off light energy. The frequency and color of light depends on how much energy is released. Again, the frequency and energy are related by the above equation. When an excited electron loses energy, we say that it returns to its “ground state”. Since not all electrons start out in the first energy level, the first energy level isn’t always an electron’s ground state. Critical Thinking Questions 8. Does an electron need to absorb energy or give off energy to go from the 2nd to the 1st energy level? It needs to give off energy to go to a lower energy level. 9. How is it possible for an electron go from the 3rd to the 4th energy level? The electron must absorb energy to go to a higher energy level. 10. Red light of frequency 4.37 x 1014 Hz is required to excite a certain electron. What energy did the electron gain from the light? E = h • f = (6.63 x10 −34 )(4.37 x1014 ) = 2.90 x10 −19 J 11. The energy difference between the 1st and 2nd energy levels in a certain atom is 5.01 x 10-19 J. What frequency of light is necessary to excite an electron in the 1st energy level? E 5.01x10−19 E = h• f → f = = = 7.56 x1014 Hz −34 h 6.63x10 12. a) What is the frequency of light given off by an electron that loses 4.05 x 10-19 J of energy as it moves from the 2nd to the 1st energy level? E 4.05 x10 −19 E = h• f → f = = = 6.11x1014 Hz − 34 h 6.63x10 b) What wavelength of light does this correspond to (hint: use c = f λ)? c 3.00 x10 8 m / s c = f •λ → λ = = = 4.91x10 −7 m 14 f 6.11x10 Hz 13. Do atoms of different elements have different numbers of electrons? Yes, atoms of different elements have different numbers of electrons, just as they have different numbers of protons. 14. If all of the electrons in atom “A” get excited and then lose their energy and return to the ground state the electrons will let off a combination of frequencies and colors of light. Each frequency and color corresponds to a specific electron making a transition from an excited state to the ground state. Consider an atom from element “B”. Would you expect the excited electrons to let off the exact same color of light as atom “A”? Why or why not? A different color would be let off because atom B and atom A have different numbers of electrons. Therefore, we would expect at least a slight difference in colors that are let off. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 34 ChemQuest 11 Name: ____________________________ Date: _______________ Hour: _____ Information: Energy Levels and Sublevels As you know, in his solar system model Bohr proposed that electrons are located in energy levels. The current model of the atom isn’t as simple as that, however. Sublevels are located inside energy levels just like subdivisions are located inside cities. Each sublevel is given a name. Note the following table: TABLE 1 Energy Level 1st energy level 2nd energy level 3rd energy level 4th energy level Names of sublevels that exist in the energy level s s and p s, p, and d s, p, d, and f Note that there is no such thing as a “d sublevel” inside of the 2nd energy level because there are only s and p sublevels inside of the 2nd energy level. Critical Thinking Questions 1. How many sublevels exist in the 1st energy level? One: only the s sublevel exists. 2. How many sublevels exist in the 2nd energy level? Two: the s and the p sublevels exist. 3. How many sublevels exist in the 3rd energy level? Three: the s, p, and d sublevels exist. 4. How many sublevels would you expect to exist in the 5th energy level? The number of sublevels equals the energy level number, so in the 5th energy level we should expect 5 sublevels to exist. 5. Does the 3f sublevel exist? (Note: the “3” stands for the 3rd energy level.) No, in the 3rd energy level there are only s, p, and d sublevels. The following sublevels exist in the 3rd energy level: 3s, 3p, and 3d. 35 Information: Orbitals So far we have learned that inside energy levels there are different sublevels. Now we will look at orbitals. Orbitals are located inside sublevels just like streets are located inside subdivisions. Different sublevels have different numbers of orbitals. TABLE 2 # of Orbitals Sublevel Possible s 1 p 3 d 5 f 7 Here’s an important fact: only two electrons can fit in each orbital. So, in an s orbital you can have a maximum of 2 electrons; in a d orbital you can have a maximum of 2 electrons; in any orbital there can only be two electrons. Since a d sublevel has 5 orbitals (and each orbital can contain up to two electrons) then a d sublevel can contain 10 electrons (= 5 x 2). Pay attention to the difference between “sublevel” and “orbital”. Critical Thinking Questions 6. How many orbitals are there in a p sublevel? 3 7. How many orbitals are there in a d sublevel? 5 8. a) How many total sublevels would be found in the entire 2nd energy level? Two, since the 2nd energy level has s and p sublevels. b) How many orbitals would be found in the entire 2nd energy level? 4 orbitals: the 2s sublevel has 1 orbital and the 2p sublevel has 3 orbitals for a total of 4 orbitals. 9. a) How many electrons can fit in an f sublevel? (2 electrons per orbital) x (7 orbitals in an f sublevel) = 14 electrons b) How many electrons can fit in an f orbital? Any orbital can only hold 2 electrons. 10. How many electrons can fit in a d orbital? in a p orbital? in any kind of orbital? Each orbital can hold a maximum of 2 electrons. 11. In your own words, what is the difference between a sublevel and an orbital? Orbitals are contained within sublevels. A sublevel is a grouping of orbitals. 12. How many electrons can fit in each of the following energy levels: 1st energy level = 2 because only 2 can fit in an s sublevel and the first energy level only has an s sublevel. 2nd energy level = 8; 2 in the 2s sublevel and 6 in the 2p sublevel giving a total of 8. 3rd energy level = 18; 2 in the 3s sublevel, 6 in the 3p sublevel, and 10 in the 3d sublevel giving a total of 18. 4th energy level = 32; 2 in the 4f, 6 in the 4p, 10 in the 4d, and 14 in the 4f. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 36 Information: Representing the Most Probable Location of an Electron The following is an “address” for an electron—a sort of shorthand notation. The diagram below represents an electron located in an orbital inside of the p sublevel in the 3rd energy level. EXAMPLE #1: Some important facts about the above diagram: • The arrow represents an electron. • The upward direction means that the electron is spinning clockwise. • “3p” means that the electron is in the p sublevel of the 3rd energy level. • Each blank represents an orbital. Since there are three orbitals in a p sublevel, there are also three blanks written beside the p. • In the diagram, the electron is in the first of the three p orbitals. Here’s another example: EXAMPLE #2: Critical Thinking Questions 13. In example #2, why are there 5 lines drawn next to the d? In a d sublevel there are 5 orbitals and therefore there needs to be 5 lines drawn. 14. In example #2, what does it mean to have the arrow pointing down? A downward arrow indicates that the electron is spinning counterclockwise. 15. Write the notation for an electron in a 2s orbital spinning clockwise. 16. Write the notation for an electron in the first energy level spinning clockwise. Note: in the first energy level, the only sublevel is a 1s. 17. What is wrong with the following notation? You should find two things wrong. There is no such thing as a d sublevel in the 2nd energy level. Also, a d sublevel needs to have 5 lines drawn for the 5 orbitals in the d sublevel. 18. Write the notation for an electron in the 4th energy level in an f sublevel spinning clockwise. 37 ChemQuest 12 Name: ____________________________ Date: _______________ Hour: _____ Information: Quantum Numbers Quantum mechanics is a set of complex mathematics that is used to describe the most probable location of an electron. Shortly after Bohr, a man by the name of Heisenberg proposed an uncertainty principle, which stated that it is impossible to know both the exact position and the exact velocity of a small particle at the same time. The location of an electron in an atom is based on probability—the most likely location for an electron. To locate the most probable location of a person you need 4 things. If you know 4 things: state, city, street and house number then you know the most probable location of the person. You also need 4 things, called “quantum numbers”, to describe the most probable location for an electron. Each “number” is actually symbolized by a letter: TABLE 1 Quantum number Principal quantum number, n Azimuthal quantum number, l Magnetic quantum number, ml Spin quantum number, ms What the Quantum number tells us which energy level the electron is in which sub-level within the energy level the electron is in which orbital within the sub-level the electron is in direction of electron spin (clockwise or counterclockwise) The four quantum numbers—n, l, ml and ms—come from a very complex equation. Together all four of them (n, l, ml, ms) will describe the most probable location of an electron kind of like how (x, y, z) describes the location of a point on a graph. TABLE 2: Rules governing what values quantum numbers are allowed to have Quantum number Possible values n 1, 2, 3, 4, …integer values l 0, 1, 2, …integers up until n-1 ml -l, ..., 0, …, +l ms +½ or -½ Examples: • If n = 3, then the electron is in the 3rd energy level and l is allowed to have only a value of 0, 1, or 2. It cannot equal anything higher than 2 because n-1=2. • If l = 1, then ml is allowed to have a value of -1, 0, 1. • If l = 2, then ml is allowed to have a value of -2, -1, 0, 1, or 2. • ms can only be +½ meaning that the electron is spinning clockwise or -½ meaning that the electron is spinning counterclockwise. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 38 Critical Thinking Questions 1. Using the quantum numbers (n, l, ml, ms) explain why each of the following is not an allowed combination of quantum numbers. The first one is done for you. a) (3, 4, 1, +½) Here n = 3 and so l can only have values of 0, 1, or 2. Here, however, l has a value of 4, which is impossible. b) (2, 1, -2, -½) Here l = 1 and so ml can have values of -1, 0, or 1. Therefore an ml value of -2 is incorrect. c) (1, 0, 0, -1) ms = -1, but it can only have values of +½ or -½ 2. If n=4, what are the possible values of l? 0, 1, 2, or 3 3. If l = 3, what are the possible values for ml? -3, -2, -1, 0, 1, 2, or 3 4. Fill in the blanks in the following table. Principle Quantum Number (n) n=1 n=2 n=3 n=4 Sublevels that are possible Possible values for l s s and p s, p, and d s, p, d and f 0 0 or 1 0, 1, or 2 0, 1, 2, or 3 5. Given the above table and remembering that the quantum number l tells us which sublevel (s, p, d, or f), complete the following statements. The first is done for you. • For an s sublevel, l equals ____0____. • For a p sublevel, l equals ___1____. • For a d sublevel, l equals ____2___. • For an f sublevel, l equals ___3____. Information: Correlating quantum numbers and sublevels TABLE 3 Azimuthal Quantum Number (l) Sublevel 0 1 2 3 s p d f # of Orbitals Possible in the sublevel 1 3 5 7 Possible ml values 0 -1, 0, +1 -2, -1, 0, +1, +2 -4, -3, -2, -1, 0, 1, 2, 3, 4 The same as Table 2 from ChemQuest 11 39 Critical Thinking Questions 6. How many ml values are possible for a d sublevel? 5 values are possible. (Since l = 2 for the d sublevel, the possible ml values are -2, -1, 0, 1, and 2—a total of 5 values possible.) 7. How many orbitals are there for a d sublevel? 5 8. How many ml values are possible for an f sublevel? 7 values are possible. (Since l = 3 for the f sublevel, the possible ml values are -3, -2, -1, 0, 1, 2, and 3—a total of 7 values possible.) 9. How many orbitals are there for a d sublevel? 7 10. Comparing your answers for 6 and 7 and your answers for 8 and 9, what correlation exists between the number of orbitals and the number of ml values possible? The number of possible ml values equals the number of orbitals for a sublevel. Each value of ml corresponds to an orbital. Information: Correlating quantum numbers to what you already know ms = +½ for this electron ml = +1 for this orbital n = 3 for the 3rd energy level. l = 1 for the p sublevel. ml = 0 for this orbital ml = -1 for this orbital The quantum numbers (n, l, ml, ms) for the above diagram are: (3, 1, -1, +½). • n = 3 indicating the third energy level • l = 1 indicating the p sublevel • ml = -1 indicating that the electron is the first of the three orbitals. • ms = +½ indicating a spin in the clockwise direction. Critical Thinking Questions 11. Explain why the set of quantum numbers (n, l, ml, ms) is (4, 3, -2, -½) for the following electron. ms = -½ when an arrow is pointing down n=4 for the 4th energy level l=3 for the f sublevel ml = -2 for this orbital 12. Draw an orbital diagram for an electron whose quantum numbers are (5, 2, 0, +½). Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 40 ChemQuest 13 Name: ____________________________ Date: _______________ Hour: _____ Information: Energy of Sublevels Each sublevel has a different amount of energy. For example, orbitals in the 3p sublevel have more energy than orbitals in the 2p sublevel. The following is a list of the sublevels from lowest to highest energy: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d… To help you, here is the above list with the orbitals included. Recall that each blank represents an orbital: 1s _, 2s _, 2p _ _ _, 3s _, 3p _ _ _, 4s _, 3d _ _ _ _ _ , 4p _ _ _ , 5s _, 4d _ _ _ _ _ , 5p _ _ _ , 6s _, 4f _ _ _ _ _ _ _ Note that d and f sublevels appear to be out of place. This is because they have extra high energies. For example, the 3d sublevel has a higher energy than a 4s sublevel and the 4f sublevel has a higher energy than the 6s sublevel. When electrons occupy orbitals, they try to have the lowest amount of energy possible. (This is called the Aufbau Principle.) An electron will enter a 2s orbital only after the 1s sublevel is filled up and an electron will enter a 3d orbital only after the 4s sublevel is filled. Recall that only two electrons can fit in each orbital. (This is called the Pauli Exclusion Principle.) When two electrons occupy the same orbital they must spin in opposite directions—one clockwise and the other counterclockwise. Critical Thinking Questions 1. a) How many electrons would an atom need to have before it can begin filling the 3s sublevel? 10; Before the 3s can be filled, all these orbitals must be filled with two electrons in each orbital: 1s _2s _2p _ _ _ b) What is the first element that has enough electrons to have one in the 3s sublevel? (Use your periodic table.) The 11th electron goes into the 3s sublevel. The atom with 11 electrons is sodium. 2. a) How many electrons would an atom need to have before it can begin filling the 3d sublevel? 20 electrons because all of these orbitals need to be filled: 1s _2s _2p _ _ _3s _3p _ _ _4s _ b) What is the first element that has enough electrons to begin placing electrons in the 3d sublevel? The 21st electron goes into the 3d sublevel. The atom with 21 electrons is scandium. 41 Information: Hund’s Rule Electrons can be “paired” or “unpaired”. Paired electrons share an orbital with their spins parallel. Unpaired electrons are by themselves. For example, boron has one unpaired electron. Boron’s orbital diagram is below: If we added one more electron to boron’s orbital diagram we will get carbon’s orbital diagram. One important question is: where does the next electron go? The electron has a choice between two equal orbitals—which of the 2p orbitals will it go in? Hund’s rule tells us which of the above choices is correct. Hund’s rule states: when electrons have a choice of entering two equal orbitals they enter the orbitals so that a maximum number of unpaired electrons result. Also, the electrons will have parallel spins. Therefore Choice A is carbon’s actual orbital diagram because the p electrons are in separate orbitals and they have parallel spins! The following are the electron orbital diagrams for the next elements, nitrogen and oxygen. Notice that nitrogen’s 2p electrons are all unpaired to obey Hund’s Rule. The 2p electrons are forced to begin pairing up in oxygen’s configuration. Critical Thinking Questions 3. How many “unpaired” electrons are in a nitrogen atom? 3 4. Why does carbon’s sixth electron have to go into another p orbital? Why can’t it go into a 2s orbital? Why can’t it go into a 3s orbital? The 2s sublevel is already full. It can’t go into a 3s orbital until all of the 2p orbitals are filled. 5. Write the electron orbital diagram for phosphorus. 6. Write the electron orbital diagram for arsenic. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 42 7. Compare the orbital diagrams for nitrogen (given above), phosphorus and arsenic. What is similar about the electrons in the last sublevel for each of them? They all have 3 electrons in a p sublevel. Information: Electron Configurations vs. Orbital Diagrams The electron orbital diagram of an atom can be abbreviated by using what is called electron configurations. The following is the electron configuration for carbon: 1s2 2s2 2p2. The following is the electron configuration for several elements whose orbital diagrams are given above: Carbon: 1s2 2s2 2p2 nitrogen: 1s2 2s2 2p3 oxygen: 1s2 2s2 2p4 Critical Thinking Questions 8. What are the small superscripts (for example, the 4 in oxygen) representing in an electron configuration? The superscripts tell us how many electrons are in the sublevel. The 4 in oxygen tells us that there are 4 electrons in the 2p sublevel. 9. What information is lost when using electron configurations instead of orbital diagrams? When might it be more helpful to have an orbital diagram instead of an electron configuration? We cannot tell as easily if the electrons are unpaired or paired. It would be more helpful to have an orbital diagram when evaluating paired or unpaired electrons. 10. How many unpaired electrons are in a sulfur atom? What did you need to answer this question— an orbital diagram or an electron configuration? There are two unpaired electrons. The orbital diagram was necessary (below). 11. Write the electron configuration for zirconium (atomic # = 40). 1s22s22p63s23p64s23d104p65s24d2 12. Write the configuration for argon (atomic # = 18). 1s22s22p63s23p6 13. Write the electron configuration for calcium (atomic # = 20). Notice that calcium has all of argon’s electrons plus two additional ones in a 4s orbital. 1s22s22p63s23p64s2 43 ChemQuest 14 Name: ____________________________ Date: _______________ Hour: _____ Information: Valence Electrons The electrons in the highest energy level are called valence electrons. Valence electrons are the electrons located farthest from the nucleus. Valence electrons are always in the highest energy level. The valence electrons are the most important electrons in an atom because they are the electrons that are the most involved in chemical reactions and bonding. The electron configuration for thallium (#81) is: 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p1 The outermost energy level (not sublevel) is the 6th energy level. How many total electrons does thallium have in the sixth level? 3, they are in boldfaced type above. Therefore, thallium has 3 valence electrons. Critical Thinking Questions 1. Write the electron configurations for a) oxygen: 1s22s22p4 b) sulfur: 1s22s22p63s23p4 2. How many valence electrons does oxygen have? In its outer energy level (the 2nd level) oxygen has a total of 6 valence electrons (2 in the 2s and 4 in the 2p). 3. How many valence electrons does sulfur have? In its outer energy level (the 3rd level) sulfur has a total of 6 valence electrons (2 in the 3s and 4 in the 3p). 4. Verify that selenium (atomic number = 34) has six valence electrons by drawing an electron configuration and giving a brief explanation. The electron configuration for selenium = 1s22s22p63s23p64s23d104p4. Selenium’s outermost level is the 4th energy level. There are a total of 6 electrons in selenium’s 4th energy level: 2 are in the 4s sublevel and 4 are in the 4p sublevel. Information: Bohr Diagrams Below are seven “Bohr diagrams” for atoms #3-9. FIGURE 1: Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 44 Critical Thinking Questions 5. In each of the Bohr diagrams in Figure 1, the first energy level only has two electrons drawn in it. Why is this? No more than 2 electrons can fit in the first energy level of any atom. 6. What is the maximum number of electrons that the second energy level can have? How many electrons can the 3rd energy level have? The 2nd energy level can hold 8 electrons and the 3rd can hold 18. (See ChemQuest 9, question 12). 7. Draw Bohr diagrams for the following atoms. a) magnesium b) phosphorus c) argon Information: Electron Dot Diagrams Below are electron dot diagrams, also known as “Lewis Structures” for atoms #3-10. FIGURE 2: The position of the dots is important. For example, another atom that has three dots in its Lewis structure is aluminum. Aluminum’s three dots must be positioned the same way as boron’s. Thus, aluminum’s Lewis structure is: FIGURE 3: Critical Thinking Questions 8. What relationship exists between an atom’s valence electrons and the number of dots in the Lewis structure of the atom? The number of dots in the Lewis structure equals the number of valence electrons. 9. Why does nitrogen’s Lewis Structure has five dots around it while nitrogen’s Bohr diagram contains 7 dots around it. The Lewis structure only shows the valence electrons, but the Bohr diagram shows all of the electrons. Nitrogen has 7 total electrons, 5 of which are valence. 45 10. Recall from questions 1-4 that oxygen, sulfur and selenium all have the same number of valence electrons (6). They also are in the same column of the periodic table. Predict how many valence electrons tellurium (Te) will have. 6, since tellurium is also in the same column. 11. Comparing Figure 2 and Figure 3 we see that boron and aluminum have the same number of dots in their Lewis structures. Notice they are in the same column of the periodic table. Write the Lewis structure for gallium (Ga). 12. Write the Lewis structure for sulfur and selenium. Compare the structures you write with oxygen’s Lewis structure from Figure 2. a) Sulfur b) Selenium 13. In question seven, you drew the Bohr diagram for magnesium. Now write the Lewis Structure for magnesium. What similarities exist between the Lewis Structures for magnesium and beryllium? Both have the same number of valence electrons and therefore the same number of dots. 14. Complete this statement: If elements are in the same column of the periodic table, they must have Lewis Structures that are ___similar______. similar or different 15. Why does sodium have the same Lewis structure as lithium? Because sodium is in the same column of the periodic table as lithium. 16. Lewis structures are easier to draw than Bohr diagrams, but what information is lost by drawing a Lewis structure instead of a Bohr Diagram? In a Lewis structure, you have no information about inner level electrons. 17. Draw the Lewis structure for the following elements. a) germanium b) bromine c) xenon d) potassium e) arsenic 18. You should be able to tell how many valence electrons an atom has by which column of the periodic table the element is in. How many valence electrons are in each of the following atoms? a) bromine b) tin c) krypton d) rubidium 7 4 8 1 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 46 ChemQuest 15 Name: ____________________________ Date: _______________ Hour: _____ Information: Relating Electron Configurations to the Periodic Table In this section you will see how the periodic table serves as a road map for writing electron configurations. Get your periodic table out and get ready. Remember that a row on the periodic table goes horizontally from left to right. Columns are vertical (up and down). Critical Thinking Questions 1. Write the electron configurations for Li, Na and K. (Remember for electron configurations, arrows are not necessary.) Li = 1s22s1 Na = 1s22s22p63s1 K = 1s22s22p63s23p64s1 2. What is similar about the electron configurations of all of the elements in question 1? Look at the very end of their configurations. Each of them ends with s1. 3. Lithium (Li) is in row 2 of the periodic table, sodium (Na) is in row 3, and potassium is in row 4. How do their row numbers affect how their electron configurations end? Li in row 2 ends with 2s1. Na in row 3 ends in 3s1. K in row 4 ends in 4s1. The row number is the same as the ending number of the sublevel. 4. Write the electron configurations for Be, Mg, and Ca. Be = 1s22s2 Mg = 1s22s22p63s2 Ca = 1s22s22p63s23p64s2 5. What is similar about the ending of the electron configurations for all of the elements in question 4? Each of them ends with s2. 6. Beryllium (Be) is in row 2 of the periodic table, magnesium (Mg) is in row 3, and Calcium (Ca) is in row 4. How do their row numbers affect how their electron configurations end? Be in row 2 ends with 2s2. Mg in row 3 ends in 3s2. Ca in row 4 ends in 4s2. Again, the row number is the same as the ending number of the sublevel. 7. Given what you have done in questions 1-6, complete the following statement. Elements ending in s1 are in column number _1 or IA_ and those ending in s2 are in column number _2 or IIA_ of the periodic table. 47 8. Name the element that have an electron configuration ending with… (the first is done for you) c) 7s1 _Francium (Fr)__ a) 5s1 _Rubidium (Rb)__ b) 6s2 _Barium (Ba)___ 9. Write the electron configurations for B, Al, and Ga and note their similarities. B = 1s22s22p1 Al = 1s22s22p63s23p1 Ga = 1s22s22p63s23p64s23d104p1 10. Write the electron configurations for N, P and As and note their similarities. N = 1s22s22p3 P = 1s22s22p63s23p3 As = 1s22s22p63s23p64s23d104p3 11. Complete the following statement: Elements ending in p1 are in column number 13 or IIIA of the periodic table and elements ending in p3 are in column number 15 or VA of the periodic table. Therefore, elements ending in p2 must be in column 14 or IVA of the periodic table. 12. Name the elements that have an electron configuration ending with…(the first is done for you) a) 3p4 __Sulfur (S)__ b) 5p6 __Xenon (Xe)___ c) 6p5 __Astatine (As)_ 13. Write the electron configurations for Ti, Zr, and Hf and note their similarities. Ti = 1s22s22p63s23p64s23d2 Zr = 1s22s22p63s23p64s23d104p65s24d2 Hf = 1s22s22p63s23p64s23d104p65s24d105p66s24f145d2 14. Write the electron configurations for Cr, Mo, and W and note what is similar about them. Cr = 1s22s22p63s23p64s23d4 Mo = 1s22s22p63s23p64s23d104p65s24d4 W = 1s22s22p63s23p64s23d104p65s24d105p66s24f145d4 15. Complete the following: Elements ending in d2 are in column number 4 or IVB and those ending in d4 are in column number 6 or VIB. Therefore, elements ending in d3 are in column number 5 or VB. Elements ending in d7 must be in column number 9 or VIIIB. 16. Notice from question 6 that an element that ends in 3s is in the 3rd row. Also, from questions 9-12 it should be clear that an element that ends in 3p is in the 3rd row. However, notice that an element that ends in 3d is in the 4th row instead of the 3rd row. Offer an explanation for this. The d sublevels have extra high amounts of energy. Remember for example, that is why the 3d sublevel comes after the 4s sublevel. This same explanation accounts for the fact that the d sublevels are “misplaced” by one row on the periodic table. 17. Name the elements that have an electron configuration ending with…(the first is done for you) a) 4d3 _Niobium (Nb)_ b) 5d8 _Platinum (Pt)__ c) 3d6 _Iron (Fe)______ Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 48 18. There are three major divisions on the periodic table: the “s block”, the “d block” and the “p block”. Where are these blocks of elements located? Give the column numbers of their locations. (Yes, there is also an “f block” but we won’t use that much.) s block: columns __1-2__ d block: columns __3-12_ p block: columns _13-18_ Information: Abbreviating the Electron Configurations Electron configurations can be shortened using a special group of elements called the noble gases. They are found in the column furthest to the right on the periodic table: helium, neon, argon, krypton, xenon, and radon. These gases are very non-reactive. All of the noble gases have electron configurations that end in p6. Because of their unique nonreactivity, they are often used to abbreviate long electron configurations. Take note of krypton’s electron configuration: 1s22s22p63s23p64s23d104p6. Now notice that strontium’s electron configuration is the same as krypton’s except that strontium has 38 electrons instead of 36. Strontium’s electron configuration is 1s22s22p63s23p64s23d104p65s2. Strontium has the same electron configuration as krypton and then two additional electrons in the 5s orbital. Therefore strontium’s electron configuration can be abbreviated as [Kr]5s2. This notation means that strontium has all of krypton’s electrons plus two more in the 5s sublevel. As another example, consider iodine. Instead of writing a long electron configuration, we can abbreviate it. Follow these steps: 1. Going backward from iodine on the periodic table, find the previous noble gas. It is krypton. 2. Take note of what krypton’s electron configuration ends with. It ends with 4p6 since it is in the 4th row and in the p6 column. 3. Iodine has 17 more electrons than krypton and so you can begin by writing [Kr] followed by orbitals for 17 more electrons. After 4p6 comes 5s2… Iodine = [Kr]5s24d105p5 Any of the noble gases can be used for abbreviations. Here are a few more examples: iron = [Ar]4s23d6 cesium = [Xe]6s1 phosphorus = [Ne]3s23p3 Study the above examples and make sure you understand why they are written that way. Critical Thinking Questions 19. Write abbreviated electron configurations for the following elements: a) Ruthenium (Ru) Ru = [Kr]5s24d6 b) Arsenic (As) As = [Ar]4s23d104p3 c) Tellurium (Te) Te = [Kr]5s24d105p4 49 ChemQuest 16 Name: ____________________________ Date: _______________ Hour: _____ Information: Shielding FIGURE 1: “Bohr Diagrams” of boron, carbon and nitrogen nucleus charge = +5 Boron nucleus charge = +6 Carbon nucleus charge = +7 Nitrogen Because the nucleus is positively charged, it exerts an attractive force on the electrons. However, the three electrons in boron’s outer energy level do not feel the full +5 attraction from the 5 protons in boron’s nucleus. Before the +5 attraction gets to the outer energy level it gets partially cancelled (or “shielded”) by the two electrons in the first energy level. The two electrons in the first energy level weaken the attractive force by two. Therefore to the outer energy level it only “feels” like a +3 charge rather than a +5 charge from the nucleus. Consider the diagram of carbon. An electron in the outer energy level only “feels” a charge of +4 coming from the nucleus because the two electrons in the first energy level shield two of the positive charges from the nucleus. Critical Thinking Questions 1. How large is the charge that the second energy level of nitrogen “feels” from the nucleus? Nitrogen’s second energy level feels a charge of +5 from the nucleus. The total charge of +7 gets weakened by the two electrons in nitrogen’s first energy level. 2. Why does the first energy level in each of the three above diagrams only contain two electrons? The first energy level cannot hold more than two electrons. 3. How many electrons can fit in the second energy level of any atom? 8 4. How many electrons can fit in the third energy level? 18 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 50 5. How many energy levels does aluminum have? How many electrons should be in each energy level? 3 energy levels; 2 electrons in the first, 8 in the second, and that leaves 3 for the third. 6. Draw a Bohr diagram for aluminum similar to those above. Nucleus has a +13 charge 7. How large is the charge that the second energy level of an aluminum atom “feels” from the nucleus? It feels a +11 charge. The +13 charge of the nucleus gets weakened to a +11 by the 2 electrons in the first energy level. 8. How large is the charge that the third energy level of an aluminum atom “feels” from the nucleus? It feels a +3 charge. The +13 charge of the nucleus gets weakened by the 2 electrons in the first energy level and the eight electrons in the second energy level. Information: Charge and Distance As you know, opposite charges attract. Examine the following diagrams of charged metal spheres. FIGURE 2: Diagram A +3 Diagram B -3 +4 Diagram C -4 +5 -5 The attraction between the two charged metal spheres in each diagram is represented by an arrow. The metal spheres are pulled closer together in diagram C because of the +5 to -5 attraction is stronger than the +4 to -4 attraction in Diagram B and the +3 to -3 attraction in Diagram A. Electrons behave the same way as the metal spheres are depicted in Figure 2. Consider the Bohr diagrams of boron, carbon and nitrogen in Figure 1. Recall that Boron’s outer electrons feel a +3 attraction from the nucleus. Carbon’s outer electrons feel a +4 attraction. In question 1, you found out that nitrogen’s outer electrons feel a +5 attraction. This attraction between the nucleus and outer energy level determines the size of the atom. If the attraction is strong the atom is small; if the attraction is weak, the atom spreads out and is larger. Critical Thinking Questions 9. Which atom is larger: nitrogen or carbon? Why? Carbon, because nitrogen’s nucleus pull of the outer electrons harder (+5 attraction) than carbon’s nucleus (+4 attraction). Therefore nitrogen’s electrons are pulled closer to the nucleus making nitrogen smaller. 51 10. In a silicon atom, the force of attraction between the nucleus and outer energy level is +4 to –4. Using a Bohr diagram of a silicon atom as an illustration, explain in detail why this is true. By the time the +14 charge of the nucleus is felt by the 3rd energy level, it has to pass through a total of 10 electrons (2 in the 1st energy level and 8 more in the 2nd). This decreases the +14 charge by 10 to a +4 charge. Thus, the attraction from the nucleus is +4. Nucleus has a +14 charge 11. Draw Bohr diagrams for sulfur and chlorine. Nucleus has a +16 charge Nucleus has a +17 charge 12. a) Find the size of the charge attraction from the nucleus to the outer energy level for sulfur and for chlorine. Sulfur: +6 Chlorine: +7 b) Which atom do you predict to be larger: sulfur or chlorine? Sulfur c) Explain, in detail, your reasoning to part b. Sulfur has a weaker attraction than chlorine. Therefore, sulfur’s electrons won’t be held as tightly as chlorine’s. 13. Notice and compare the locations of boron, carbon and nitrogen on the periodic table. Now compare their sizes. Do the same with sulfur and chlorine. There is a general trend in size as you proceed from left to right across the periodic table. What is this trend? In other words, how do atoms in the same row of the periodic table compare to each other in size? As you go from left to right across a row in the periodic table, the size of atoms decreases. Information: Bohr Diagrams and the Size of Atoms Examine the following “Bohr Diagrams” of three atoms from the periodic table. FIGURE 3: Atom A Atom B Atom C Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 52 Critical Thinking Questions 14. Give the name and atomic number of each atom. Atom A Name of the element Sodium Atomic number 11 Atom B Atom C Lithium Hydrogen 3 1 15. a) Concerning atoms A, B and C, what is similar about their location in the periodic table? They are in the same column. b) In atoms A, B, and C compare the force of attraction from the nucleus to the outer level of electrons. The force of attraction in all of them is +1. 16. Draw Bohr diagrams for neon and argon. 17. a) What is similar about the location of neon and argon in the periodic table They are in the same column. b) Compare the force of attraction between the outer level electrons and the nucleus for neon and argon. Each of them has a +8 attraction from the nucleus. c) Using your answers to 15b and 17b, what can be said about elements in the same column and the force of attraction between their outer energy level and nucleus? Elements in the same column have the same attraction between the nucleus and outer level electrons. 18. Atom A is larger than atom B. The attraction between the nucleus and outer level electrons is equal in atoms A and B, so what other reason could there be for Atom A’s larger size? Propose an explanation based on that structure of atoms A and B. Atom A has an additional level of electrons. Each additional level increases the size of the atom. 19. Which is larger—neon or argon? Why is this atom the largest? Argon because argon has an additional energy level. 20. In general, there is a trend in the sizes of atoms as you move down a column of the periodic table. a) What is this trend? As you move down a column, atoms get larger. b) Why does this trend exist? (Explain the basis for the trend.) Going down a column also means that the number of energy levels are increasing. Increasing the energy levels causes the atoms to be larger. 21. Order the following lists of elements in order from smallest to largest. b) P, Sb, N c) S, Ca, Mg, Cl a) K, As, Br Br, As, K N, P, Sb Cl, S, Mg, Ca 53 ChemQuest 17 Name: ____________________________ Date: _______________ Hour: _____ Information: Separating Charges Examine Figure 1 below where there are three pairs of metal spheres that have different amounts of charge on them. The spheres in diagram C are closer than the others because they have the strongest attraction. FIGURE 1: Attraction between metal spheres. Diagram A +3 -3 +4 Diagram B Diagram C -4 +7 -7 Critical Thinking Questions 1. Which would be harder to separate: the two spheres in Diagram A or the two spheres in Diagram B? Why? The spheres in Diagram B would be harder to separate because the force of attraction is greater due to the higher charge difference (+4 and -4) and also the spheres are closer together which makes them harder to separate just like magnets that are very close together. 2. Draw Bohr diagrams of the following atoms: lithium, nitrogen and fluorine. 3. For each of the atoms in question two compare the attraction between the nucleus and the outer level of electrons. Which atom has the strongest attraction between the nucleus and outer electrons? Fluorine has the strongest attraction. A +7 force from the nucleus is attracting the outer level. (Nitrogen’s outer level feels a +5 attraction and lithium’s outer level feels a +1 attraction from the nucleus.) 4. Which would be more difficult: if you wanted to remove an outer electron from an atom of fluorine or from an atom of nitrogen? For similar reasons as question 1, it would take more energy to remove an outer electron from an atom of fluorine than from nitrogen. The outer electron from fluorine feels a stronger force of attraction from the nucleus. 5. Would it be easier to remove an outer electron from lithium or nitrogen? It would be easier to remove an outer electron from lithium because lithium’s outer electron experiences a weaker force of attraction from the nucleus. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 54 Information: Ionization Energy The amount of energy that it takes to completely remove an electron from an atom is called ionization energy. The first ionization energy is the energy required to remove one electron from an atom’s outer energy level. The second ionization energy is the energy needed to remove a second electron from the energy level. The second ionization energy is always higher than the first ionization energy. Because noble gases have eight electrons in their outer energy level, they are very stable and therefore it takes a very high amount of energy to remove an electron from a noble gas. Critical Thinking Questions 6. Which would have a higher first ionization energy: phosphorus or aluminum? (You may want to draw a Bohr diagram to help you determine the answer.) Phosphorus, because it has a higher attraction between (+5) the nucleus and outer electrons than aluminum (+3 attraction). 7. a) Phosphorus and aluminum are in the same row of the periodic table. Lithium, nitrogen and fluorine are also in the same row. Using your answers to questions 3, 4 and 6 what do you notice about the ionization energy of elements proceeding from left to right across a row of the periodic table? Does the ionization energy increase or decrease as you go across a period? As you move from left to right across a row (or “period”), the ionization energy increases. b) Explain why you believe this trend exists. This trend exists because as you move from left to right across a row, the attraction between the nucleus and the outer level electrons also increases. For atoms with a stronger attraction for the outer electrons, it requires more ionization energy to remove the electron. 8. Draw a Bohr diagram of sodium. Do you expect the first ionization energy to be high or low for sodium? Sodium has a low first ionization energy because of the low attraction from the nucleus (+1) for the electron in the third energy level. 9. The second ionization energy of all elements is higher than the first ionization energy, but in sodium the second ionization energy is extra large. Considering the structure of the sodium atom explain why this might be. (HINT: after one electron is removed, what is sodium’s electron arrangement like?). After the first electron is removed, sodium has the same electron configuration as neon with 10 electrons. It is like a noble gas and therefore to remove the next electron is very difficult. 10. A certain atom in the third period has a third ionization energy that is unusually high. Using the same reasoning you used for question 9, name this atom. Magnesium because it is in the 3rd row (period) of the periodic table and it has two more electrons than neon (a noble gas). After the first two electrons are removed, the 3rd one is extra hard to remove because it will need to be removed from a noble gas-like configuration. 55 Information: Trend in Ionization Energy in Groups Consider the following figure of diagrams of two magnets that you wish to separate. FIGURE 2: Separating Magnets Diagram D Diagram E Critical Thinking Questions 11. Would it be easier to separate the magnets in Diagram D or those in Diagram E in Figure 2? Assume that each magnet is attracted to the other and that the size of the attraction is the same. Take only the distance between magnets into consideration. Magnets are easier to separate when they are farther apart, so it will be easier to separate the magnets in Diagram E. 12. How does the distance between the outer level of electrons and the nucleus in phosphorus compare to the distance between the outer level of electrons and the nucleus in nitrogen? Since phosphorus is a larger atom, the distance between the nucleus and the outer level electrons is greater in it than in nitrogen. 13. Based on your answer to question 12, would it be easier to remove an electron from nitrogen or from phosphorus? It would be easier to remove an electron from phosphorus than from nitrogen since the outer electron is farther away in the phosphorus atom. 14. Nitrogen and phosphorus are in the same column on the periodic table. From your answers to questions 12 and 13 what happens to the ionization energy as you move down a column in the periodic table? As you move down a column of the periodic table, the ionization energy decreases because the size of the atoms increases. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 56 ChemQuest 18 Name: ____________________________ Date: _______________ Hour: _____ Information: Ions Figure 1: Below are four Bohr diagrams of atoms and ions. The two diagrams on the left are atoms; the two on the right are ions. 12 protons 12 protons Ion of Atom A Atom A 17 protons Atom B 17 protons Ion of Atom B Critical Thinking Questions 1. Prove that both Atom A and Atom B are neutral (have a charge of zero). Both atoms have an equal number of protons and electrons. 2. What is the identity of Atom A and of Atom B? The atom with 12 protons and 12 electrons is magnesium. 57 3. Given the above diagrams, how does an atom become an ion? An atom can become an ion by either gaining or losing electrons. 4. What is the charge on Ion A? What is the charge on Ion B? Atom A = 12 protons and 10 electrons = +2 charge Atom B = 17 protons and 18 electrons = -1 charge 5. Write the electron configuration (ex: 1s22s22p6….) for each ion and atom shown in the Bohr diagrams. Atom A: __1s22s22p63s2______________ Ion A: __1s22s22p6____________________ Atom B: __1s22s22p63s23p5____________ Ion B: ___1s22s22p63s23p6______________ 6. Consider the electron configuration that you wrote for Ion A. What atom has the same electron configuration as this ion? Neon 7. Consider the electron configuration that you wrote for Ion B. What atom has the same electron configuration as this ion? Argon 8. Bromine atoms always gain one electron when they become an ion. Which atom has the same number of electrons as a bromine ion? Krypton 9. Cesium atoms always lose one electron to become an ion. Which atom has the same number of electrons as a cesium ion? Xenon 10. Consider your answers to questions 6-9. What do all of the atoms you named have in common? They are all noble gases with eight outer-level electrons. 11. Knowing what you know about the atoms that you named in questions 6-9, why do you think atoms want to form ions the way they do? The noble gases have very stable electron configurations and so atoms want to gain or lose electrons so that their electron configuration is the same as a noble gas and more stable. Information: Ions As you know, all of the noble gases are very stable. Ions form in such a way so that the ion will have the same number of electrons as a noble gas. Take oxygen, for example. Oxygen has 8 electrons. To become like a noble gas it could either gain two to become like neon or it could lose six to become like helium. So what will oxygen do—gain two or lose six? As a general rule, atoms will gain or lose the fewest number of electrons possible. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 58 Critical Thinking Questions 12. What does an oxygen atom do when becoming an ion? (Does it gain or lose electrons and how many?) It will gain 2 electrons rather than losing 6. 13. An oxygen atom has an overall neutral charge because it has an even number of protons and electrons. What is the overall charge on an oxygen ion? After gaining 2 negative electrons the charge will be -2. 14. Consider an aluminum atom. a) To become like argon, would aluminum have to gain or lose electrons? How many? Aluminum would have to gain 5 electrons. b) To become like neon, would aluminum have to gain or lose electrons? How many? Aluminum would have to lose 3 electrons. c) Considering your answers to parts a and b, what does an aluminum atom do to become an ion? Since aluminum wants to gain or lose the fewest number of electrons possible, aluminum will lose 3 electrons. d) What is the charge on an aluminum ion? After losing 3 electrons, the charge will be a net +3. 15. When each of the following atoms becomes an ion, what will the charge be? a) Ca +2 b) Cl -1 c) N -3 d) K +1 e) S -2 f) B +3 g) P -3 59 ChemQuest 19 Name: ____________________________ Date: _______________ Hour: _____ Information: Naming Ions To write an ion, you write the symbol of the atom and put the charge in the upper right corner. Consider the following examples: Al3+, O2-, Mg2+. You should verify that each of the charges is correct. Positive and negative ions are named differently. Positive ions retain the same name as the parent atom. For example, Al3+ is called the “aluminum ion” and Mg2+ is called the “magnesium ion.” Negative ions are named a little differently. For negative ions, you change the ending of the name to “-ide”. Therefore, O2- is named oxide and P3- is named phosphide. Critical Thinking Questions 1. Write the symbol (including the charge) and name for each of the ions for each of the following: a) Ca b) Cl c) N d) K e) S f) B g) P Ca2+ ClN3K+ S2B3+ P3calcium ion chloride nitride potassium ion sulfide boron ion phosphide Information: Ionic Bonding and Formulas There are two ways in which atoms can “bond” to each other and form a compound. The means of bonding that we will consider now is called ionic bonding, which occurs between a metal and a nonmetal. As you know, opposite charges attract. Ionic bonding is when two ions of opposite charge attract and bond to each other forming an ionic compound. Consider the following examples of formulas for ionic compounds: • • • • One Na+ (sodium ion) and one Cl- (chloride ion) bond to make NaCl, “sodium chloride.” One Mg2+ (magnesium ion) and two F- (fluoride ion) bond to make MgF2, “magnesium fluoride.” Three Ca2+ (calcium ion) and two N3- (nitride ion) bond to make Ca3N2, “calcium nitride.” One Al3+ (aluminum ion) and one N3- (nitride ion) bond to make AlN, “aluminum nitride.” The small numbers at the bottom right of each symbol in a formula are called “subscripts”. Subscripts tell us how many of each type of atom are present. For example in the formula Mg3N2 there are three magnesium ions and two nitride ions. Critical Thinking Questions 2. Consider the formula NaCl in the above example. It tells us that one Na+ ion is bonded to one Clion. What is the overall charge for NaCl? Is it positive, negative, or neutral? Neutral: one +1 ion (Na+) and one -1 ion (Cl-) together are neutral. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 60 3. Consider MgF2. This formula tells us that one Mg2+ ion bonds with two F- ions. What is the overall charge on MgF2? Neutral: one +2 ion (Mg2+) and two -1 ions (F-) together are neutral. 4. What is the overall charge on any ionic compound? Neutral 5. Why is calcium nitride written like Ca3N2 and not something like CaN2 or Ca2N3? In other words why do exactly three calcium ions bond with exactly two nitride ions? Three +2 ions (Ca2+) are required to go along with two -3 ions (N3-) so that the overall charge is neutral. Mathematically: 3(+2) + 2(-3) = 0 6. The formula Ca3N2 can never be written as N2Ca3. To find out why, take note of each of the four example formulas given above. a) In terms of charge, what do the first ions named all have in common? They are positively charged. b) In terms of charge, what do the second ions named all have in common? They are negatively charged. c) Now, why can’t Ca3N2 ever be written like N2Ca3? The positive ion (Ca2+) must be written first. 7. There are two rules to follow when writing formulas for ionic compounds. One has to do with charges (see questions 4 and 5) and the other has to do with which atom to write first and which one to write second (see question 6). What are these two rules? 1. Ionic compounds need to be written with the ions combined so that the final compound is neutral. 2. The positive ion must be written first. 8. What is wrong with the following formulas? b) PNa3 a) Al2S This compound is not neutral. Two Al3+ ions will combine with three S2- ions to give the correctly written, neutral compound: Al2S3. c) Mg2S2 Although this compound is neutral, the positive ion (Na+) is not written first. The correct way to write it would be Na3P. It is generally best to write ionic compounds in their lowest multiple so it should be written MgS and not Mg2S2 or Mg3S3 or Mg4S4, etc. 9. Write the formula and name for the compound that forms when the following atoms form ionic compounds. The first is done for you. a) nitrogen and sodium b) barium and sulfur c) magnesium and iodine Na3N BaS MgI2 sodium nitride barium sulfide magnesium iodide d) oxygen and aluminum e) calcium and phosphorus f) sodium and sulfur Al2O3 Ca3P2 Na2S aluminum oxide calcium phosphide sodium sulfide 10. Given the following compounds, determine the charge on the unknown ion “X”. c) X3P2 a) X2S b) MgX X must be +1 since two +1 X ions would be needed to balance out the -2 sulfide ions. X must be -2 since a -2 X ion would be needed to balance out the +2 magnesium ion. X must be +2 since three +2 X ions would be needed to balance out the two -3 phosphide ions. 61 ChemQuest 20 Name: ____________________________ Date: _______________ Hour: _____ Information: Polyatomic Ions The word, “polyatomic” means “many atoms”. A polyatomic ion, therefore, is an ion that is made of more than one atom. An example of a polyatomic ion is the sulfate ion, SO42-. Sulfate is composed of one sulfur atom and four oxygen atoms and overall sulfate has a negative two charge. Some polyatomic ions: Sulfate: SO42Cyanide: CNAcetate: C2H3O2- Phosphate: PO43Ammonium: NH4+ Hydroxide: OH- Nitrate: NO3Chlorate: ClO3Carbonate: CO32- Critical Thinking Questions 1. What do all of the polyatomic ions that have the suffix “-ate” have in common? They all contain oxygen atoms. 2. Which two atoms do you think compose the polyatomic ion called “silicate”? silicon and oxygen 3. What is the difference between calcium nitride and calcium nitrate? The –ate ending indicates that calcium nitrate has oxygen in it, but calcium nitride does not. Information: Writing Formulas With Polyatomic Ions First of all, you must remember that you can never change the formula for a polyatomic ion. Sulfate is always SO42- and never S2O84- or something else. Following are some examples of chemical formulas that contain polyatomic ions. Ammonium chloride is formed from one ammonium ion (NH4+) and one chloride ion (Cl-) to give the formula: NH4Cl. Sodium sulfate requires two sodium ions (Na+) because sulfate (SO42-) has a negative two charge; the formula is: Na2SO4. Consider calcium hydroxide. Calcium has a positive two charge (Ca2+) and hydroxide has a negative one charge (OH-). We need two hydroxide ions to combine with one calcium ion so that the overall charge ends up being zero. We write calcium hydroxide like Ca(OH)2. Following are some more examples: potassium acetate: KC2H3O2 barium phosphate: Ba3(PO4)2 magnesium nitrate: Mg(NO3)2 calcium carbonate: CaCO3 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 62 Critical Thinking Questions 4. As mentioned above, calcium hydroxide is written like Ca(OH)2. Why can’t it be written like CaOH2? Ca(OH)2 means that two OH- have bonded with a Ca2+ ion. CaOH2 means that two H+ ions have bonded with one O2- ion and a Ca2+ ion. CaOH2 is not a neutral compound. 5. As mentioned above, barium phosphate is written as Ba3(PO4)2. Why can’t it be written like Ba3PO42? Ba3(PO4)2 means that two PO43- ions have bonded with three Ba2+ ions. Ba3PO42 means that three Ba3+ ions have bonded with one P3- ion and 42 O2- ions, which would not yield a neutral compound. 6. Name the following compounds. Each includes at least one polyatomic ion. a) Na3PO4 b) (NH4)2SO4 c) Mg(C2H3O2)2 sodium phosphate ammonium sulfate magnesium acetate d) (NH4)2S ammonium sulfide e) CaCO3 calcium carbonate f) Ba(NO3)2 barium nitrate 7. Write formulas for the following ionic compounds. Note that each includes a polyatomic ion. a) lithium phosphate b) ammonium oxide c) barium hydroxide Li3PO4 (NH4)2O Ba(OH)2 d) calcium cyanide Ca(CN)2 e) sodium chlorate NaClO3 f) potassium sulfate K2SO4 8. In question 3, you were asked the difference between calcium nitride and calcium nitrate. Now write the formula for each of them. calcium nitride: Ca3N2 calcium nitrate: Ca(NO3)2 Information: Formulas for Acids Acids are compounds that contain positive hydrogen ions (H+) bonded to a negative ion. For example, carbonic acid is formed when the carbonate ion (CO32-) bonds with two hydrogen ions (H+) to give H2CO3. Other common acids are listed below: Hydrochloric acid: HCl Sulfuric acid: H2SO4 Nitric Acid: HNO3 Acetic Acid: HC2H3O2 Critical Thinking Questions 9. Why do carbonic and sulfuric acid require two H+ ions to bond, but HCl and HNO3 only have one H+? Carbonate and sulfate both have a -2 charge and therefore require two H+ ions (each having a +1 charge) so that overall the formulas will be neutral. 10. Phosphoric acid is made from the phosphate ion and H+ ions. Write the formula for phosphoric acid. H3PO4 Three H+ ions are needed because phosphate has a -3 charge. 63 ChemQuest 21 Name: ____________________________ Date: _______________ Hour: _____ Information: Charges of Some Transition Elements So far you have learned that you can predict the charge that an ion will have based on its location on the periodic table. However, the transition elements are not easy to predict. A few common transition elements are listed below. You should memorize their charges. Silver: Ag+ Zinc: Zn2+ Cadmium: Cd2+ Critical Thinking Questions 1. Write the formulas for the following compounds: a) silver nitrate AgNO3 b) zinc phosphate Zn3(PO4)2 c) cadmium chloride CdCl2 Information: More Than One Possible Charge Many transition elements can have more than one charge when they become an ion. Copper ions, for example, can be Cu+ or Cu2+. As another example, iron ions are sometimes Fe2+ and sometimes Fe3+. Critical Thinking Questions 2. Copper and iron are in the “d block” and so you need to calculate their charge by comparing what bonds to them. Find the charge on copper and iron in each of the following compounds. a) CuCl2 +2 b) CuCl c) FeSO4 d) Fe2(SO4)3 +1 +2 +3 (This is similar to what you did in question 10 for ChemQuest 16) 3. Give your best attempt at naming the compounds from question 2. (They are rewritten below.) a) CuCl2 copper chloride b) CuCl copper chloride c) FeSO4 iron sulfate d) Fe2(SO4)3 iron sulfate Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 64 Information: Formulas Containing Roman Numerals You probably put the same name for the compounds in question 3a and 3b. You may also have put the same name for the compounds in 3c and 3d. BUT these are not the same compound! You cannot have the same name for two different compounds. Here are the correct names for the compounds in questions 2 and 3: a) CuCl2 copper(II) chloride b) CuCl copper(I) chloride c) FeSO4 iron(II) sulfate d) Fe2(SO4)3 iron(III) sulfate Critical Thinking Questions 4. Compare your answers for questions 2 & 3 with the names of the compounds given in the information section. What do the Roman numerals stand for? The Roman numerals stand for the charge on the transition metal ion. 5. Why is MnO2 called manganese(IV) oxide? Manganese has a charge of +4 indicated by the Roman numeral IV. 6. Name the following compounds. Note: assume that anytime you have a transition element (d block element) you must use a Roman numeral unless the element is silver, zinc, or cadmium. (The first one is done for you.) a) NiNO3 b) Cr2(CO3)3 c) FeNO3 nickel(I) nitrate chromium(III) carbonate e) Cu3(PO4)2 copper(II) phosphate f) MnS iron(I) nitrate g) ZnCl2 manganese(II) sulfide zinc chloride d) CoCl2 cobalt(II) chloride h) AgNO3 silver nitrate (No Roman numerals are needed for zinc or silver, but if you included them it is OK.) 7. Write the formulas for the following compounds. (The first one is done for you.) a) mercury(II) acetate b) chromium(III) sulfate Hg(C2H3O2)2 Cr2(SO4)3 c) iron(I) carbonate Fe2CO3 d) potassium carbonate e) strontium nitride f) manganese(IV) chlorate K2CO3 Sr3N2 Mn(ClO3)4 65 ChemQuest 22 Name: ____________________________ Date: _______________ Hour: _____ Information: Terminology Recall that an ionic bond results from the combination of a metal and a nonmetal. A covalent bond is the type of bond between two nonmetals. Covalent bonds are formed by neutral atoms that share electrons rather than by charged ions. When a compound is formed by sharing electrons, the compound is called a molecule or molecular compound. It is important to note that ionic compounds are not called molecules. The largest class of molecules are called organic molecules. Carbon is the distinguishing mark of organic compounds. Critical Thinking Questions 1. Circle any of the following compounds that would properly be called a “molecule”. a) H2O b) CO2 c) NaCl d) Mg3P2 e) N2O5 Any compound with no metal and no polyatomic ion is considered a “molecule” and covalent. Information: Naming Covalent Compounds There are several prefixes used to name molecules. The name “carbon oxide” is not sufficient because carbon and oxygen sometimes form CO2 and sometimes CO. Prefixes are necessary to distinguish between them. Formula Name N2 O4 dinitrogen tetraoxide SF6 sulfur hexafluoride XeCl5 xenon pentachloride SO3 sulfur trioxide CO carbon monoxide Critical Thinking Questions 2. Fill in the table to indicate which prefix is used to represent the numbers. The first one is done for you. Number Prefix 1 mono 2 di 3 tri 4 tetra 5 penta 6 Hexa Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 66 3. Name each of the following molecules. b) CF4 c) SCl3 d) SO a) N2O5 dinitrogen pentoxide carbon tetrafluoride sulfur trichloride sulfur monoxide (b, c, and d do not need to start with mono because whenever a name would start with mono, the mono is dropped and we assume there is only one of the atoms.) 4. Which of the above compounds would be classified as “organic”? Only carbon tetrafluoride (b) because it is the only one containing carbon. Information: Empirical Formulas Molecules can be represented by using either a molecular formula or an empirical formula. The molecular formula tells you exactly how many atoms of each element are in the compound. For example, in the table below, compound #2 has exactly 4 carbons and 8 hydrogens in each molecule. Observe the table below that shows four organic molecules along with a molecular and empirical formula for each one: Molecule Molecular Formula Empirical Formula #1 C 2 H4 CH2 #2 C 4 H8 CH2 #3 C 3 H8 C 3 H8 #4 C8H18 C 4 H9 Critical Thinking Questions 5. What is an empirical formula? An empirical formula is a way to show the lowest, simplified ratio of elements in a compound. It tells us the ratio of atoms in a compound and NOT how many atoms are in the compound. 6. How can molecules #1 and #2 have the same empirical formula even though they are different molecules? Both molecules #1 and #2 have a 1 to 2 ration of carbon to hydrogen and since an empirical formula gives the ratio, they both have the same empirical formula. 7. Given the empirical formula for a compound is it possible to determine the molecular formula? If so, explain how. No, because the empirical formula does not tell us how many of each atom is present in a compound. 8. Given the molecular formula for a compound is it possible to determine its empirical formula? If so, explain how. Yes, you must divide each subscript by the same number. For example if you are given the molecular formula C4H6, you could reduce it by dividing each subscript by 2 to obtain C2H3. 9. Give the empirical formula for each of the molecules below: b) C2H4O2 c) C4H14 a) N2O6 NO3 CH2O C 2 H7 d) C3H5 C 3 H5 (already empirical) 67 ChemQuest 23 Name: ____________________________ Date: _______________ Hour: _____ Information: Drawing Covalent Compounds For covalent bonding, we often want to draw how the atoms share electrons in the molecule. For example, consider CCl4 and NF3 as drawn below: Each line represents two electrons that are being shared Notice that the atoms share electrons so that they all have 8 electrons. If you count the electrons around carbon, you will get a total of eight (each line is two electrons). If you count the electrons around each chlorine atom, you will find that there are eight of them. Critical Thinking Questions 1. How many valence electrons does a carbon atom have (before it bonds)? Hint: find this based on carbon’s column on the periodic table. 4; since carbon is in column 14 (or “4A”) on the periodic table it has 4 valence electrons. 2. How many valence electrons does a chlorine atom have (before it bonds)? 7; since chlorine is in column 17 (or “7A”) it has 7 valence electrons. 3. Since CCl4 is made up of one carbon and four chlorine atoms, how many total valence electrons does CCl4 have? Hint: add your answer to question 1 and four times your answer to question 2. 4 + 4(7) = 4 + 28 = 32 4. Verify that there are 32 electrons pictured in the drawing of CCl4. Count each dot in the picture above: there are 24. There are also 8 more electrons represented by the 4 lines (each line represents two electrons). 5. Find the sum of all the valence electrons for NF3. (Add how many valence electrons one nitrogen atom has with the valence electrons for three fluorine atoms.) 26; nitrogen has 5 valence electrons; fluorine has 7. Since there are one nitrogen and 7 fluorine atoms, the total is obtained as follows: 5 + 7(3) = 5 + 21 = 26 6. How many electrons are pictured in the drawing of NF3 above? 26; count each dot in the picture above: there are 20. There are also 6 more electrons represented by the 3 lines (each line represents two electrons). Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 68 7. In CCl4 carbon is the “central atom”. In NF3 nitrogen is the “central atom”. What is meant by “central atom”? The central atom is the atom that is in the middle of other atoms in the Lewis structure. Other atoms branch off from the central atom. 8. In SF3 sulfur is the central atom. You can tell which atom is the central atom simply by looking at the formula. How does the formula give away which atom is the central atom? The central atom is usually the first atom written in the formula. Also, you can recognize the central atom in the formula because there is only one of the atom. For example, in the above formula for SF3 there is only one sulfur atom. 9. Identify the central atom in each of the following molecules: A) CO2 carbon B) PH3 phosphorus C) SiO2 silicon 10. For each of the compounds from question 9, add up how many valence electrons should be in the bonding picture. A is done for you. A) CO2 4 + 2(6) = 16 B) PH3 5 + 3(1) = 8 C) SiO2 4 + 2(6) = 16 11. The number of electrons that should appear in the bonding picture for CO3 is 22. The number of electrons that appear in the picture for CO32- is 24. Offer an explanation for why CO32- has 24 electrons instead of 22. (Where did the extra two electrons come from?) The -2 charge on CO32- means that it has two extra electrons. 12. The number of electrons that should be included in the picture of NH4 is 9. The number of electrons in the picture for NH4+ is 8. Offer an explanation for why NH4+ has 8 electrons instead of 9. The +1 charge on NH4+ means that it has one fewer electron. 13. Considering questions 11 and 12, we can formulate a rule: For each negative charge on a polyatomic ion, we must ____add______ an electron and for each positive charge we must add or subtract ___subtract____ an electron. add or subtract 14. For each of the polyatomic ions or molecules below, determine the total number of valence electrons. a) NO3b) SCl4 c) H3O+ d) PO435 + 3(6) + 1 = 24 6 + 4(7) = 34 3(1) + 6 -1 = 8 5 + 4(6) +3 = 32 69 Information: Steps for Drawing Lewis Structures for Covalent Compounds Study the two examples in the table of how to write structures for CO32- and NH3. Make sure you understand each of the five steps. Step #1: Add up the number of valence electrons that should be included in the Lewis Structure. CO32- NH3 4 + 3(6) + 2 = 24 (carbon has four and each oxygen has six; add two for the -2 charge) 5 + 3(1) = 8 (nitrogen has five; each hydrogen has one) Step #2: Draw the “skeleton structure” with the central atoms and the other atoms, each connected with a single bond. Step #3: Add six more electron dots to each atom except the central atom. Also, never add dots to hydrogen. (no change) Step #4: Any “leftover” electrons are placed on the central atom. Find the number of leftovers by taking the total from Step #1 and subtracting the number of electrons pictured in Step #3. 24 – 24 = 0 leftover electrons Step #5: If the central atom has 8, then you are done. If not, then move two electrons from a different atom to make a multiple bond. Keep making multiple bonds until the central atom has 8 electrons. a total of 4 electrons are shared here 2 electrons were moved to form a “double bond” 8 – 6 = 2 leftover electrons; placed around nitrogen (no change) (no change) Critical Thinking Questions 15. Write the Lewis Structure for nitrate, NO3-1. Hint: when you are done it should look very similar to CO32- in the table above. 16. Draw the Lewis Structure for SO2. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 70 ChemQuest 21 Name: ____________________________ Date: _______________ Hour: _____ Introduction Questions 1. Draw the Lewis structures for the following molecules. Part c is done for you. a) H2O (oxygen is central) b) SO2 c) N2 2. In question 1 above you should see three different kinds of bonds. Define each of these kinds of bonds as best you can. a) single bond: two electrons being shared between atoms; represented by one line b) double bond: four electrons being shared between atoms; represented by two lines together. c) triple bond: six electrons being shared between atoms; represented by three lines together. 3. In general, which kind of bond do you think would be harder to break—a single, double or triple bond? Why? Triple bonds are harder to break because six electrons being shared means that there is more “glue” than between atoms that share fewer electrons. Information: Bond Order, Bond Length and Bond Energy Study the following table relating bond order, bond length, and bond energy. Note that bond length is the distance between two bonding atoms in picometers and bond energy is the amount of energy (in kilojoules) required to break one mole of bonds. Carbon—Carbon Bonds (note: these are drawings of bonds, not entire molecules) Bond Order for C—C bonds C—C Bond Length (pm) C—C Bond Energy (kJ/mole) 1 150 350 2 130 600 3 120 830 71 Critical Thinking Questions 4. Is your answer to question 3 consistent with the data in the table? Hopefully you see that triple bonds require more energy to break—they are harder to break than other bonds. 5. What is the relationship between bond order and single, double, and triple bonds? A single bond has a bond order of 1, a double bond has bond order of 2, and a triple bond has a bond order of 3. 6. What relationship exists between bond length and bond energy? Shorter bonds are also harder to break. 7. Which bonds are shortest—single, double or triple? Triple bonds are the shortest. 8. A structure for benzene is given below. H H All of these single bonds are 1st order and have lengths of approximately 150 pm (as estimated from the table above) C C C H H C C C All of these double bonds are 2nd order and have lengths of approximately 130 pm (as estimated from the table above) H H a) Based on the Lewis structure, what is the bond order for each C—C bond? Label your predictions on the structure above. See labels above. b) What would be the approximate lengths of the carbon-carbon bonds? Label these predictions on the structure also. See the labels where the double bonds have lengths of approximately 130 pm and single bonds have lengths of about 150 pm. Information: Resonance Structures Experimentally, all of the carbon-carbon bonds in benzene are exactly the same length. All of the bonds also require the same energy to break. This would indicate that all of the carbon-carbon bonds are identical. The bond order of each of the C—C bonds is approximately 1.5. The bonds have characteristics in between those of first order and second order bonds. Therefore, the structure of benzene is best represented by what is called a resonance structure. The structure is given at the top of the next page. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 72 The resonance hybrid representation of benzene: H H H H C H C H H C C C C C C C C C H H H C H H Critical Thinking Questions 9. a) The nitrate ion, NO3-, is best represented by three resonance structures. Draw the three structures below. b) Why were three structures required instead of two? The double bond can be in three different, but equivalent places and thus, three different resonance structures are needed. c) Describe in general terms what you think nitrate’s nitrogen-oxygen bonds are like in terms of bond order, bond length, and bond energies. Each bond has a bond order of 1.333 (or 1 1/3). Each bond will be equal in length, somewhere between 130 pm and 150 pm . Each bond will have the same bond energy, between 350 kJ and 600 kJ. (These values are given on the table above.) As an explanation for the bond order you could mentally picture it like this: The bond order is 1 and 1/3 because the double bond is at each of the positions 1/3 of the time. This means that each bond is a single bond and 1/3 of another bond. 73 ChemQuest 25 Name: ____________________________ Date: _______________ Hour: _____ Information: More than one possible Lewis Structure. Sometimes when you draw a Lewis structure you discover that there is more than one possible way to draw it. For example, consider the following Lewis Structures for sand, which is silicon dioxide: Diagram #1: Structure A Structure B Critical Thinking Questions 1. What is the total number of electrons allowed in the Lewis structure for SiO2? Do both of the above Lewis structures have the correct number of electrons pictured? 16; you find this by adding up the total valence electrons of each atom involved. Oxygen has 6 valence electrons since it is in column 16. Each silicon has 4 valence electrons. Adding it all up looks like this: 6(2) + 4 = 16. 2. Is each of the proposed structures above a legitimate Lewis structure for SiO2? (In other words, in each of the proposed structures are all of the “rules” that you know followed?) Explain. Both Lewis Structures look good according to the rules we know of so far. There are the correct number of electrons in the picture (16) and every atom is sharing enough electrons so that each has 8. 3. According to a large number of experiments, both of the silicon-oxygen bonds in SiO2 are identical. Given this information, which Lewis structure, A or B, is a better description of the bonding in SiO2. Explain. Given this experimental evidence, Structure A is better because both of the bonds in Structure A are identical. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 74 Information: Formal Charges When there are two structures that are possible for a compound, then scientists in a lab can determine which one is the correct one. Other than laboratory work, there is another way to determine which Lewis structure is correct—using “formal charges”. Let’s examine Structure B for SiO2. In the structure that was drawn, each atom has the eight electrons that they need. Let’s look at how many formal electrons each atom has. To understand “formal electrons” study the diagram of Structure B below: Diagram #2: Of the two electrons being shared, one is a formal electron for oxygen and one is a formal electron for silicon. Electrons that aren’t shared belong to the atom that they are around, so these six electrons are six of oxygen’s formal electrons. Of the six electrons being shared, three are formal electrons for oxygen and three are formal electrons for silicon. Oxygen Atom #2 Oxygen Atom #1 Electrons that aren’t shared belong to the atom that they are around, so these two electrons are two of oxygen’s formal electrons. You should see from the above diagram that the silicon atom has a total of four formal electrons, oxygen atom #1 has 7 formal electrons, and oxygen atom #2 has 5 formal electrons. Now we can calculate the formal charge of each atom. Found from the column of the periodic table that the atom is in Formal charge = (# of valence electrons) – (# of formal electrons) Here are the formal charges for each of the atoms in structure B for SiO2. Oxygen atom #1: 6 – 7 = -1 Oxygen atom #2: 6 – 5 = 1 Si = 4 – 4 = 0 Critical Thinking Questions 4. Verify that the formal charge for each of the atoms is zero in Structure A from Diagram #1. Each oxygen has six valence electrons and six formal electrons formal charge is zero. Silicon has four valence electrons and four formal elcectrons formal charge is zero. 5. Consider questions 3 and 4. If two different structures are possible, does the best structure have the fewest or the most formal charges? The best structure will have the fewest formal charges. 6. Draw a Lewis structure for the carbonate ion, CO32-. On your drawing label the formal charge of each atom. This carbon’s formal charge is zero because C has 4 These oxygens have a formal charge of -1, having 6 valence electrons and 7 formal electrons in the picture: 6 – 7 = – 1 valence electrons and there are 4 formal electrons pictured here: 4 – 4 = 0 This oxygen’s formal charge is zero because oxygen has 6 valence electrons and here there are 6 formal electrons; 6 – 6 = 0 75 7. Add up all of the formal charges for all the atoms from question 6. (-1) + (-1) + 0 + 0 = – 2 8. Draw the Lewis structure for the ammonium ion, NH4+ and label the formal charge of each atom. Each hydrogen has a formal charge of zero since hydrogen has one valence electron and each has one formal electron in the picture. Nitrogen’s formal charge here is +1 since nitrogen has 5 valence electrons and in the picture it has 4 formal electrons. 5 – 4 = +1 9. Add up all of the formal charges for the atoms from question 8. +1 + 0 + 0 + 0+ 0 = +1 10. Draw the Lewis structure for SO3 and label the formal charge of each atom. This sulfur has a formal charge of +2, having 6 valence electrons and only 4 formal electrons in the picture: 6 – 4 = + 2 These oxygens have a formal charge of -1, having 6 valence electrons and 7 formal electrons in the picture: 6 – 7 = – 1 This oxygen’s formal charge is zero because oxygen has 6 valence electrons and here there are 6 formal electrons; 6 – 6 = 0 11. Add up all of the formal charges for the atoms from question 10. +2 + (-1) + (-1) + 0 = 0 12. Consider questions 6-11 and then fill in the blanks: The SUM of the formal charges for all the atoms in a structure always equals the _charge_ of the molecule or ion. For example, the charge on CO32- is ___-2___ and the sum of the formal charges (question 6) is ___-2___. 13. Which of the following is the best structure for CH2O2? Explain your reasoning. Structure #1 Structure #2 Structure #1 is better because all of the atoms have a formal charge of zero. In Structure #2, one of the oxygen atoms has a formal charge of -1 and the other oxygen has a formal charge of +1. The best structure always has the least number of formal charges. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 76 ChemQuest 26 Name: ____________________________ Date: _______________ Hour: _____ Information: Definition of Electronegativity Electronegativity is a measure of how much an atom attracts an electron. The higher the electronegativity, the greater the atom’s attraction for electrons. Atoms that become negative ions have a much greater electronegativity than atoms that become positive ions. Below is a table of the electronegativities of many elements from the periodic table. 1 H 2.20 3 4 Li Be 0.97 1.47 11 12 Na Mg 1.01 1.23 19 20 K Ca 0.91 1.04 5 6 7 8 9 B C N O F 2.01 2.50 3.07 3.50 4.10 13 14 15 16 17 Al Si P S Cl 1.47 1.74 2.06 2.44 2.83 31 32 33 34 35 Ga Ge As Se Br 1.82 2.02 2.20 2.48 2.74 2 He — 10 Ne — 18 Ar — 36 Kr — Critical Thinking Questions 1. Why do you think there are no values for the noble gases? Noble gases are so stable that they do not attract electrons. 2. In terms of electrons, what is the difference between a covalent bond and an ionic bond? Electrons are shared in a covalent bond, but atoms lose or gain them in ionic bonds. 3. What type of bond (covalent or ionic) would you expect to form between an atom with a high electronegativity and an atom of low electronegativity. Explain and give an example. Ionic bond because the atom with high electronegativity will attract an electron away from the atom with low electronegativity. Example: NaF (between a metal and a nonmetal) 4. What type of bond (covalent or ionic) would you expect to form between two atoms of somewhat high electronegativity? Explain and give an example. Covalent bond because both atoms will be trying to attract an electron and so they will end up sharing it. Example: CO2 (both atoms have high electronegativity compared to the metals) 5. Consider the ionic compound, sodium chloride (NaCl). Which atom has a greater attraction for electrons—sodium or chlorine? Which atom forms the negative ion? Chlorine has the highest electronegativity and therefore the strongest attraction. It also forms the negative ion and sodium forms a positive ion. 77 6. In an ionic bond, the atom with the highest electronegativity will always form a _______negative_________ ion. (positive or negative) 7. Consider the covalent compound, carbon monoxide (CO). a) Draw the Lewis dot structure for carbon monoxide. b) In the Lewis structure you drew, you should see that there is a triple bond between carbon and oxygen. The carbon and oxygen share 6 electrons. All 6 electrons are not shared equally, however, because carbon and oxygen don’t have equal attraction for electrons. The 6 electrons spend a little more time near one of the atoms—predict which one and explain. The electrons will spend more time near the oxygen because oxygen has a greater electronegativity than carbon and therefore attracts the electrons more. 8. Why are the electrons in a nitrogen-phosphorus covalent bond NOT shared equally? Which atom do the electrons spend more time around? Explain. They are not shared equally because nitrogen and phosphorus do not have the same electronegativity. The atoms spend more time around the most electronegative atom— nitrogen. 9. True or false: In an ionic bond, the difference in electronegativities between the two bonding atoms is greater than the difference in a covalent bond. True; there is a greater difference between a metal and nonmetal than between 2 nonmetals. 10. In terms of electronegativity, explain why this statement is true: “Carbon monoxide is more ‘ionic’ than carbon monosulfide”. The electronegativity difference is greater between carbon and oxygen (like in carbon monoxide) than between carbon and sulfur (like in carbon monosulfide) 11. Which bond is more like an ionic bond—a nitrogen-oxygen bond or a carbon-oxygen bond? Explain. A carbon oxygen bond is more like an ionic bond because there is a greater difference in electronegativity between carbon and oxygen than between nitrogen and oxygen. 12. Which compound is more like an ionic compound—NH3 or PH3? Explain. NH3, because of the greater electronegativity difference between N and H than between P and H. Information: “Polar” Bonds In critical thinking questions 10, 11, and 12 we used the term “ionic” when describing covalent bonds. This can get confusing and so instead of the term “ionic” we will use the term “polar”. A polar covalent bond then is a covalent bond in which the electrons are not shared equally by the two atoms involved in the bond. For example, in carbon monoxide the electrons spend more time near oxygen than carbon because oxygen has a greater electronegativity. Because of oxygen’s greater electronegativity, it attracts the electrons more than carbon. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 78 Critical Thinking Questions 13. Which bond is more polar—a phosphorus-chlorine bond or a phosphorus-fluorine bond? Explain why. P—F bond is more polar than the P—Cl bond because there is a greater electronegativity difference between P and F than between P and Cl. 14. Consider a carbon-fluorine bond. One atom in the bond is “partially negative” and the other atom is “partially positive”. Which atom is which? Explain how you know. The fluorine is “partially negative” because it has a higher electronegativity than carbon and therefore the electrons that are shared between carbon and fluorine spend more time near fluorine. 15. In your own words explain what you think the term “partially negative” means and explain why an atom might be partially negative when it bonds covalently. (I.e. What makes it partially negative?) If an atom is “partially negative” it means that the electrons are more attracted to that atom than to the other atom covalently bonded to it. 16. In your own words explain what you think the term “partially positive” means and explain why an atom might be partially positive when it bonds covalently. (What makes it partially positive?) If an atom is partially positive, then the electrons it shares with another atom will spend more time around that other atom. Information: Polar vs. Nonpolar Not all covalent compounds are categorized as polar. For example, a carbon-hydrogen bond is made up of two atoms that have very similar electronegativities. Because of this, carbon and hydrogen share the electrons just about equally and we say that a carbon-hydrogen bond is “nonpolar”. As a general measure, if the difference in electronegativity between two bonding atoms is less than about 0.5, then the bond is nonpolar. Critical Thinking Questions 17. For the following bonds, indicate whether they are ionic (I), polar covalent (P), or nonpolar covalent (NP). __NP_ a) C—P __P__ b) S—O __I__ c) Fe—O 18. What are the three most electronegative atoms on the periodic table? Nitrogen, oxygen, and fluorine __P__ d) N—H 79 ChemQuest 27 Name: ____________________________ Date: _______________ Hour: _____ Information: Shapes of Molecules Name Lewis Structure Methane, CH4 H Ammonia, NH3 H H C H H Tetrahedral shape Water, H2O H N O H H Trigonal pyramidal shape H Bent shape 3-D Shape Bond angle =109.5o Bond angle =106.5o Bond angle =104.5o Total # of electron regions # of Bonding electron regions # of lone pair electron regions 4 4 0 4 3 1 4 2 2 Name Carbonate, CO32- Ozone, O3 Carbon dioxide, CO2 Lewis Structure O Trigonal planar shape 3-D Shape Bond angle =120o Total # of electron regions # of bonding electron regions # of lone pair electron regions 3 3 0 Bent shape Bond angle =118.6o C O Linear shape Bond angle =180o 3 2 1 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 2 2 0 80 Critical Thinking Questions 1. What is an electron region? A place around the central atom where electrons (either bonding or lone pair) can be found. 2. What is a "lone pair electron region"? A place around the central atom where electrons not bonding with another atom can be found. 3. What is a "bonding electron region"? A place around the central atom where electrons are shared with another atom. 4. The number of electron regions determines the bond angle. With this in mind, complete the following sentence: "Any molecule that has bond angles of approximately 105-109o will have ____4_______ total electron regions; any molecule that has bond angles of approximately 120o how many? will have ____3______ total electron regions; and any molecule with bond angles of how many? approximately 180o will have ____2______ total electron regions." how many? 5. The molecules in the above table are representative of many other molecules. Therefore, it can be said that any molecule with 3 bonding electron regions and 1 lone pair electron region has a geometrical shape called "trigonal pyramidal". Draw Lewis dot structures for the following structures and name the geometrical shape. A) NO3B) NF3 C) CF4 trigonal planar trigonal pyramidal tetrahedral 6. A certain molecule has a bent shape with bond angles of about 119o. Is the molecule SO2 or SH2? Explain. (Hint: draw the Lewis structures for SO2 and SH2.) The molecule is SO2 because SO2 has 3 electron domains which corresponds to bond angles near 120o. SH2 has 4 electron domains which would correspond to bond angles near 109o. Information: VSEPR The geometry of molecules is based on a theory called "Valence Shell Electron Pair Repulsion" (VSEPR) theory. The word "repulsion" is the key word because this theory states that all the electron pairs repel each other and so they want to get as far away from each other as possible. The atoms in a tetrahedral molecule are as far apart as geometrically possible at bond angles of 109.5o. There is no way that the atoms can get farther apart. Critical Thinking Questions 7. In the tables above, there are 3 molecules that have a total of 4 electron regions. The bond angles are slightly different because of lone pair electrons. What takes up more room--a lone pair of electrons or a bonding pair of electrons? Offer proof from the table above. A lone pair takes up more room, which “squeezes” atoms a little closer together and causes the smaller bond angles. 81 8. If you know how many bonding regions and lone pair regions surround an atom you can predict the bond angles around the atom, even in complex situations. Examine the following "big" molecules. By each arrow that points to an atom, write the bond angle for that atom; you should write 109o, 120o, or 180o to represent the approximate bond angle. One of them is done for you. 109o H C H H H H H C C C C H C C H H C H N H 109o 120o because of 3 bonding regions and no lone pair regions H H H H O 120o 109o 120o H H C H H C C H H H H H H C H N C H H O H O C C C N O 180o 109o Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 82 ChemQuest 28 Name: ____________________________ Date: _______________ Hour: _____ Information: Introduction to Reactions During a chemical reaction, new substances are formed. Reactants are transformed into different products. Atoms are never created or destroyed, but they are rearranged. A chemical equation represents what happens during a reaction. The following is an example of a chemical equation: Example Equation: Ca + HNO3 Ca(NO3)2 + H2 This equation describes the reaction of calcium (Ca) with nitric acid (HNO3) to produce calcium nitrate (Ca(NO3)2) and hydrogen gas (H2). You may notice that there are more total atoms on the right side than there are on the left side of the equation. If this seems strange to you, don’t worry about it now; we’ll fix this later. Note in the above equation that hydrogen gas is written as H2 and not simply as H. There are a few elements that exist as diatomic molecules. If a substance is diatomic then the substance must always be bonded to something. A hydrogen atom is diatomic and so it must be bonded to something else like in HCl or HNO3. If nothing is available for it to bond to, it will bond to itself by forming H2. All of the diatomic substances are listed below: Br I N Cl H O F When by themselves these elements exist as Br2, I2, N2, Cl2, H2, O2, and F2. By the way, you can remember these by recalling a made-up name: Mr. Brinclhof. Critical Thinking Questions 1. Consider the bromine atoms in this reaction: LiBr + P Li3P + Br2. a) Why is bromine written as Br2 on the right side? Bromine is a diatomic molecule and always needs to be bonded to something; even bonding to itself works. b) Why is it not necessary for LiBr to be written as LiBr2? The Br is already bonded to something else (the Li). If it were written LiBr2 it would not be a neutral compound. 2. What are the reactants in the example equation in the above information section? Reactants are written on the left side of the equation and so the reactants are Ca and HNO3. 83 3. Answer the questions that follow based on this chemical equation: Na + MgCl2 NaCl + Mg. a) Why can’t NaMg be produced? Na+ cannot bond with Mg2+ because they are both positive. b) Why can’t NaCl2 be produced? Na+ only requires one Cl-. NaCl2 is not a neutral compound. c) Are NaCl and Mg the only products that can be produced? Yes. 4. Given the following equation: Li + Ca3(PO4)2 Li3PO4 + Ca. a) Why can’t CaLi2 be produced? Ca2+ and Li+ won’t bond because they are both positive. b) Why can’t Li3P be produced? Although Li3P is neutral, there is no P3- in the equation. There is only PO43- and we will almost never be breaking up polyatomic ions like PO43-. Don’t mess with polyatomic ions! c) Are Li3PO4 and Ca the only substances that can be produced? Yes. 5. Write chemical equations for the following reactions. a) Aluminum sulfate reacts with barium to produce barium sulfate and aluminum. Al2(SO4)3 + Ba BaSO4 + Al b) Magnesium reacts with copper(I) nitrate to produce magnesium nitrate and copper. Mg + CuNO3 Mg(NO3)2 + Cu c) Sodium reacts with calcium phosphide to produce sodium phosphide and calcium. Na + Ca3P2 Na3P + Ca d) Phosphorus reacts with sodium chloride to produce sodium phosphide and chlorine. (Note that chlorine is diatomic!) P + NaCl Na3P + Cl2 6. Each of the reactions you wrote in question 5 follows a similar pattern. The same pattern is followed by the equations in questions 3 and 4. Describe this pattern. A single atom reacts with a compound and replaces one of the atoms in that compound. 7. a) How are reactions 5c and 5d different? Na forms a positive ion (Na+) and replaces another positive ion, but P forms a negative ion (P3-) and replaces another negative ion. b) How are reactions 5c and 5d similar? In both reactions a single atom replaces another atom from a compound that it is reacting with. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 84 8. Complete the following reactions: a) NaCl + Ag AgCl + Na (note: order is not important, so you could also write Na + AgCl) b) Li + Ca3(PO4)2 Li3PO4 + Ca (again, order is not important) Information: Single and Double Replacement Reactions Each of the equations that you looked at in the above section is called a single replacement reaction. Notice that in each of them, a single atom replaces an ion from another reactant. Study what happens in the following reactions. They are called double replacement reactions. MgCl2 + NaF NaCl + MgF2 Al2S3 + Na2O Al2O3 + Na2S Ca(NO3)2 + Na3P NaNO3 + Ca3P2 Critical Thinking Questions 9. What is the difference between single replacement reactions and double replacement reactions? In single replacement reactions, one of the reactants is made of only one kind of atom. In double replacement reactions, both reactants contain more than one kind of atom. 10. Complete the following reactions by providing the formulas for the missing compound(s). a) Cu(NO3)2 + _____NaCl________ NaNO3 + CuCl2 b) ZnI2 + ______Al2(SO4)3________ ZnSO4 + AlI3 c) K2O + MgBr2 ____MgO_______ + ______KBr_______ 11. Name the two products in the reaction between calcium phosphate and sodium iodide. Calcium iodide and sodium phosphate (The reaction is Ca3(PO4)2 + NaI CaI2 + Na3PO4) 12. Explain why when you mix the following reactants, no reaction occurs: Na2SO4 + NaCl Both reactants contain sodium. Therefore, if the sodium atoms replaced each other, the same products would be formed—no change would occur. Information: Combustion, Synthesis, and Decomposition Reactions Another type of reaction is a combustion reaction. During combustion, a hydrocarbon reacts with oxygen. The products for complete combustion are always the same—water and carbon dioxide and energy. The following equation is an example of the combustion of a hydrocarbon. C3H8 + O2 CO2 + H2O 85 Two other types of reactions are synthesis and decomposition. During a synthesis reaction, several reactants combine to make a single product. During a decomposition, one reactant decomposes into two or more products. The following table shows some examples of these types of reactions. Synthesis H2 + O2 H2 O Na + Cl2 NaCl Decomposition H2 O H 2 + O 2 NaCl Na + Cl2 Critical Thinking Questions 13. Categorize each of the following reactions as single replacement (SR), double replacement (DR), synthesis (S), decomposition (D) or combustion (C). __S__ a) Ca + O2 CaO __SR_ b) Mg(NO3)2 + Cu CuNO3 + Mg __C__ c) C4H10 + O2 CO2 + H2O __D__ d) Al2O3 Al + O2 __SR_ e) SrCl2 + F2 SrF2 + Cl2 __DR_ f) BaF2 + Na2O BaO + NaF 14. Write an equation for the combustion of C3H6. C3H6 + O2 CO2 + H2O (Combustion reactions always involve combining with O2 to form CO2 and H2O.) 15. Write an equation for the decomposition of calcium oxide. CaO Ca + O2 (Oxygen is diatomic, so it is written as O2 when by itself.) Practice Problems 1. Complete the following reactions. a) Na2CO3 + AlN Na3N + Al2(CO3)3 (Remember that the order is never important.) b) BaCl2 + F2 BaF2 + Cl2 c) CuNO3 + Ag AgNO3 + Cu 2. Fill in the blanks for the missing reactant or product and then in the blank to the left of each equation indicate whether the reaction is a single replacement (SR), double replacement (DR), synthesis (S), decomposition (D) or combustion (C). __DR_ a) LiCl + ___Zn(NO3)2____ ZnCl2 + LiNO3 __SR_ b) ____Na______ + CaBr2 NaBr + Ca __S__ c) K + Cl2 ____KCl_______ Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 86 ChemQuest 29 Name: ____________________________ Date: _______________ Hour: _____ Introduction Questions 1. Write the equation for the reaction of sodium and chlorine to form sodium chloride. Na + Cl2 NaCl 2. Write the equation for the reaction of calcium nitride and sodium chloride to produce calcium chloride and sodium nitride. Ca3N2 + NaCl CaCl2 + Na3N Information: Subscripts and Coefficients A subscript is a small number that tells you how many atoms are in a compound. For example, in CaCl2 the two is the subscript and it tells us that there are two chloride ions bonded to one calcium. A coefficient tells also tells us how many atoms or compounds there are, but in a different way. For example in the expression “3 H2O” the three is the coefficient. The three tells us that there are three molecules of water present. In the expression “3 H2O” there are a total of 6 hydrogen atoms and 3 oxygen atoms. Critical Thinking Questions 3. Verify that in 4Ca3(PO4)2 there are 32 oxygen atoms present. Multiply the subscripts by each other and then by the coefficient: 4 x 2 x 4 = 32 4. How many oxygen atoms are in each of the following: __3__ a) Al2O3 __3__ b) 3 Na2O __16_ c) 4 Na2SO4 __30_ d) 5 Mg(NO3)2 Information: How To Balance Equations Consider the reaction of sodium and chlorine to produce sodium chloride from question one: Na + Cl2 NaCl Remember that when chlorine is by itself it is always written as Cl2. On the reactant side of the reaction (left side) there are a total of two atoms of chlorine, but on the product side there is only one atom of chlorine. Atoms cannot simply disappear. In order for the equation to make sense, we need to balance the equation. This can be done, first, by adding a “2” to the product side: Na + Cl2 2 NaCl Now the equation reads that one atom of Na reacts with one molecule of Cl2 to produce two units of NaCl. However, now the Na atoms are not balanced because there is one atom of the reactant side, but two atoms of Na on the product side. This can be fixed by adding another two: 2 Na + Cl2 2 NaCl 87 Let us consider another example, the equation you wrote in question two above: Ca3N2 + NaCl CaCl2 + Na3N Notice that none of the atoms are “balanced”. There are three calcium atoms on one side and only one on the other. There are two nitrogen atoms on one side and one on the other. How can we fix this? Begin by “balancing” one atom at a time: 1. First, let’s balance the calcium atoms by placing a three in front of CaCl2: Ca3N2 + NaCl 3 CaCl2 + Na3N 2. Next, let’s balance the nitrogen atoms, by placing a 2 in front of Na3N: Ca3N2 + NaCl 3 CaCl2 + 2 Na3N 3. Now we will balance the sodium atoms by placing a 6 in front of NaCl. Ca3N2 + 6 NaCl 3 CaCl2 + 2 Na3N 4. Finally, examine the chlorine atoms and notice that they are already balanced. 5. Double check each atom to make sure there are equal numbers of each on both sides of the equation. When balancing equations you NEVER change subscripts. Only change the coefficients. Critical Thinking Questions 5. Which of the following equations are properly and completely balanced? (Circle as many as apply.) A) 2 NaCl + Cu CuCl2 + Na B) C3H8 + 3 O2 3 CO2 + H2O C) MgCl2 + F2 MgF2 + Cl2 D) FeCl2 + O2 2 FeO + Cl2 6. Balance each of the following equations by inserting the correct coefficient in each blank. Remember to balance one atom at a time. If the number “one” belongs in the blank you may either leave it blank or insert the number one. A) _____ CaCl2 + _____ Li2O _____ CaO + __2__ LiCl B) _____ Al2S3 + __3__ Cu __3__ CuS + __2__ Al C) __2__ Na3P + __3__ MgBr2 _____ Mg3P2 + __6__ NaBr Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 88 7. Here are the answers to question 6. Double check your answers to question 6: A) CaCl2 + Li2O CaO + 2 LiCl B) Al2S3 + 3 Cu 3 CuS + 2 Al C) 2 Na3P + 3 MgBr2 Mg3P2 + 6 NaBr Information: Balancing Equations Containing Polyatomic Ions When an equation contains polyatomic ions, it may look a little more difficult to balance. But balancing an equation containing polyatomic ions is really not much different from the ones you just did. In the equations above, you kept in mind that you must balance only one atom at a time. With polyatomic ions, keep in mind that you balance one polyatomic ion at a time. For example, consider the following unbalanced equation. Na2SO4 + Ca3(PO4)2 Na3PO4 + CaSO4 The first thing you should do is take note of which atoms/ions are not balanced. Keep the polyatomic ions together. In other words, do not balance the phosphorus and oxygen atoms separately; instead, balance the phosphate ions on each side of the reaction. Follow these steps: 1. Balance the sodium so that there are 6 sodium atoms on each side of the reaction: 3 Na2SO4 + Ca3(PO4)2 2 Na3PO4 + CaSO4 2. Now there are three sulfate ions on the left and one on the right. Add a three to the right. 3 Na2SO4 + Ca3(PO4)2 2 Na3PO4 + 3 CaSO4 3. Take inventory of all the atoms and ions to make sure they are balanced. The equation is now balanced. Critical Thinking Questions 8. Which of the following equations are properly balanced? A) Ba(NO3)2 + Fe2SO4 2 FeNO3 + BaSO4 B) 3 SrCl2 + Al(NO3)3 3 Sr(NO3)2 + AlCl3 C) Ca3(PO4)2 + 3 Na2CO3 2 Na3PO4 + 3 CaCO3 D) Fe(NO3)2 + Mg Mg(NO3)2 + Fe 9. Balance each of the following equations by inserting the correct coefficient in each blank. Remember to balance one atom/ion at a time. If the number “one” belongs in the blank you may either leave it blank or insert the number one. A) _____ Ca(NO3)2 + __2__ Na __2__ NaNO3 + _____ Ca B) _____ Al2(CO3)3 + __3__ MgCl2 __2__ AlCl3 + __3__ MgCO3 C) _____ Ba3(PO4)2 + __3__ Na2CO3 __2__ Na3 PO4 + __3__ BaCO3 89 10. Make sure you got the following answers for question 9: A) Ca(NO3)2 + 2 Na 2 NaNO3 + Ca B) Al2(CO3)3 + 3 MgCl2 2 AlCl3 + 3 MgCO3 C) Ba3(PO4)2 + 3 Na2CO3 2 Na3 PO4 + 3 BaCO3 11. Complete and balance the following equations. A) 2 HCl + Mg MgCl2 + H2 B) Na2S + Be(OH)2 2 NaOH + BeS C) BaF2 + D) Ca3(PO4)2 + E) Zn(NO3)2 + F) 3 Ca(NO3)2 + 2 NaNO3 Ba(NO3)2 + 2 NaF 3 K2SO4 2 K3PO4 + 3 CaSO4 Mg Mg(NO3)2 + Zn Al2(CO3)3 3 CaCO3 + 2 Al(NO3)3 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 90 ChemQuest 30 Name: ____________________________ Date: _______________ Hour: _____ Information: Atomic Mass Using Atomic Mass Units (amu) and Grams (g) One atomic mass unit (amu) is equal to 1.6611x10-24 grams. Critical Thinking Questions 1. According to the periodic table, a single carbon atom has a mass of 12.011 amu. What is the mass of a single carbon atom in grams? (12.011 amu) x (1.6611x10-24 g/amu) = 1.9951x10-23 2. How many carbon atoms does it take to equal 12.011 grams? (12.011g) / (1.9951x10-23 g/atom) = 6.0201x1023 atoms 3. According to the periodic table, a single phosphorus atom has a mass of 30.973 amu. What is the mass of a single phosphorus atom in grams? (30.973 amu) x (1.6611x10-24 g/amu) = 5.1449x10-23 4. How many phosphorus atoms does it take to equal 30.973 grams? (30.973g) / (5.1449x10-23 g/atom) = 6.0201x1023 atoms 5. Compare your answers to questions 2 and 4. They are both approximately the same. Therefore, it takes about 6.02x1023 atoms of carbon to equal the atomic mass in grams and it takes 6.02x1023 atoms of phosphorus to equal its atomic mass in grams. Information: What is a Mole? Hopefully, you found that your answers to questions 2 and 4 were about the same. Both answers should be about 6.02x1023. The quantity, 6.02x1023 is Avogadro’s constant and we call it the “mole”. Just like the quantity “12” is called a “dozen”, so the quantity 6.02x1023 is called a “mole”. By definition, a mole is the quantity of atoms necessary to equal the element’s atomic mass in grams. So, according to the periodic table, one atom of sodium has an atomic mass of about 22.99 amu. If you weighed out 22.99 grams on a balance, you would have 6.02x1023 atoms of sodium present. Look at your periodic table and find gold (atomic number = 79). What is the mass of one gold atom? You should note that one gold atom has a mass of 196.97 amu. How many gold atoms would you need to get 196.97 grams? You would need to put 6.02 x 1023 atoms of gold on the balance before you would have 196.97 grams of gold. One mole of an atom will always equal the atom’s atomic mass in grams. 91 Critical Thinking Questions 6. What is the mass of one atom of aluminum? (include units) 26.98 amu (from the periodic table) 7. If you had 6.02 x 1023 atoms of aluminum, what mass of aluminum would you have? (include units) Since 6.02x1023 is a “mole” there will be 26.98 grams of aluminum. 8. What is atomic mass? It is the mass of a single atom, usually measured in units of amu (atomic mass units). 9. If you had one mole of pennies, how many pennies would you have? You would have 6.02x1023 pennies. 10. If you had 3 moles of sand, how many grains of sand would you have? (6.02x1023)(3) = 1.81x1024 grains of sand. Information: Molecular Mass (also known as Formula Mass) and Molar Mass Just as atomic mass is the mass of an atom, molecular mass is that mass of a molecule. It is found by adding up all of the masses of the atoms in the molecule. Because ionic compounds are not properly called molecules, the term formula mass is used in place of molecular mass for ionic compounds. Consider the following examples: 1. The atomic mass of hydrogen 1.0 amu and the atomic mass of oxygen is 16.0 amu. One molecule of water (H2O) has a molecular mass of 18.0 amu. This number is obtained by adding the masses of two hydrogens (each at 1.0 amu) and the mass of one oxygen (16.0 amu). 2. Aluminum chloride (AlCl3) has a formula mass of about 133.5 amu. This is found by adding the mass of one aluminum atom (27.0 amu) to the mass of three chlorine atoms (3 x 35.5 amu). Verify this on your calculator. Just as one mole of atoms equals the atomic mass of an atom in grams, so also one mole of molecules equals the molar mass of the molecule in grams. Molar mass is the mass (in grams per mole) of one mole of a substance. Therefore we expect that 6.02 x 1023 molecules of water will have a mass of 18.0 g and 6.02 x 1023 formula units of AlCl3 will have a mass of 133.5 g. We say, then, that the molar mass of water is 18.0 g/mol and the molar mass of AlCl3 is 133.5 g/mol. (g/mol is read grams per mole where mol is the abbreviation for mole.) Critical Thinking Questions 11. Verify using a periodic table and calculator that the molecular mass of N2O5 is approximately 108 amu. 2(14.0) + 5(16.0) = 108 amu; note: 14.0 and 16.0 are found on the periodic table for nitrogen and oxygen, respectively. 12. How many molecules of N2O5 are required to equal 108 grams? 6.02x1023 Since 108 amu is the molecular mass, it will take 6.02x1023 molecules to equal 108 grams. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 92 13. Why is the term “molecular mass” applied to water, but the term “formula mass” applied to aluminum chloride? Although molecular and formula masses are calculated the exact same way, the term “molecular” cannot apply to any substance that has a metal in it (such as aluminum). 14. Find the molecular or formula mass for each of the following (include units): a) magnesium phosphide b) sodium sulfate Mg3P2 Na2SO4 3(24.31) + 2(30.97) = 134.87 amu 2(23.0) + (32.06) + 4(16.0) = 142.06 amu (NOTE: all numbers in parentheses are found on the periodic table.) c) Ca(NO3)2 (40.1) + 2(14.0) + 6(16.0) = 164.1 amu d) C4H8 4(12.0) + 8(1.01) = 56.1 amu 15. Find the molar mass of each of the following (include units): a) CaCl2 b) barium nitrate 40.1 + 2(35.5) = 111.1 g/mol Ba(NO3)2 137.3 + 2(14.0) + 6(16.0) = 261.3 g/mol 16. What is the difference between the terms molecular mass and molar mass. Molecular refers to the mass of a single molecule, but molar refers to the mass of a mole of something (or the mass of 6.02x1023 atoms or molecules) . Information: Beginning Mole Conversions The mole (often abbreviated as mol) is the link between the microscopic (atoms and molecules) and the macroscopic (things measured in grams). If you know how many grams of a substance you have and you know the molar mass, you can find out how many molecules you have. Two examples of how to do this using a method similar to converting units is shown below: 1. How many atoms of carbon does it take to equal 23.5 g? 23.5 g • 1mol 6.02x1023 atoms • = 1.18x1024 atoms 12 .0 g mol molar mass of carbon, from the periodic table 2. How many molecules of carbon monoxide gas does it take to equal 50.0 g? 50.0g • mol 6.02 x10 23 molecules • = 1.08x10 24 molecules 28.0g mol molar mass of carbon monoxide found by adding molar mass of C and of O 93 Critical Thinking Questions 17. Using your calculator, verify that if you have 125 g of gold, you have about 3.82x1023 atoms of gold. Show the calculations below. mol 6.02x10 23 atoms 125g • • = 197.0 g mol 3.82x10 23 atoms 18. Consider 210 g of N2O5. How many molecules are present? mol 6.02x10 23 molecules 210g • • = 1.17 x10 24 molecules 108g mol 19. If you exhale 7.25 x 1024 molecules of CO2… a) How many moles of CO2 do you exhale? (Hint: use the conversion factor that one mole = 6.02x1023 molecules) 7.25 x10 24 molecules • mol = 12.04 mol 6.02 x10 23 molecules b) How many grams of CO2 do you exhale? (Hint: find how many grams are in a mole by finding the molar mass of CO2. Use this as a conversion factor.) 12.04mol • 44.0 g = 529.9 g mol 20. In a bag full of pennies, you may have 2.15 moles of copper. How many grams do you have? 2.15mol • 63.55 g = 136.6 g mol Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 94 ChemQuest 31 Name: ____________________________ Date: _______________ Hour: _____ Information: Percent Composition Sometimes it is needful to know the composition of a compound. For example, 39.3% of the mass of sodium chloride is due to sodium. The other 60.7% of the mass is from chlorine. So, in a 100 g sample of sodium chloride, there are 39.3 g of sodium and 60.7 g of chlorine. This type of data is known as percent composition. The percent composition tells you the percentage by mass of an element in a compound. There is a convenient formula for finding the percent composition of an element in a compound: (obtained from periodic table) percent composition of element " X" = mass of x in one mole of the compound • 100 mass of one mole of the compound (obtained from periodic table) Let us look at how the percent composition of calcium (Ca) in calcium chloride (CaCl2) was determined. percent composition of Ca = mass of Ca in one mole of CaCl 2 • 100 mass of one mole of CaCl 2 from periodic table for calcium 40.1 g percent composition of Ca = • 100 = 36.1% 111.1 g from periodic table for calcium + 2 chlorines; 40.1 + 2(35.5) = 111.1 As another example, consider calculating the percent composition of nitrogen in Ca3N2: From periodic table for 2 nitrogen atoms: 2(14.0)=28.0 percent composition of N = 28.0 g • 100 = 18.9% 148.3 g from periodic table for 3 calcium + 2 nitrogen atoms 3(40.1) + 2(14.0) = 148.3 Critical Thinking Questions 1. Verify that in C4H10 the percent composition of carbon is approximately 82.6%. 4(12.0) •100 = 82.6% [4(12.0) + 10(1.01)] Note: 12.0 and 1.01 are the molar masses for carbon and hydrogen on the periodic table. 95 2. Calculate the percent composition of sodium in Na2S. 2(23.0) •100 = [2(23.0) + (32.1)] 58.9% Information: Formulas and Percent Composition Table 1: Percent composition and formulas of some compounds. Name Structural Formula Hexene H2C Propene CH CH2 H2C CH2 CH CH2 CH3 CH3 Molecular Formula % Comp. of H % Comp. of C C6H12 14.4 85.6 C 3 H6 14.4 85.6 C 6 H6 7.8 92.2 C 4 H4 7.8 92.2 C 6 H6 7.8 92.2 CH Benzene HC CH HC CH CH HC CH HC CH CH2 CH2 Cyclobutadiene 1,5-hexadi-yne HC C C CH Critical Thinking Questions 3. Verify that the percent composition of C and H given for hexene in Table 1 are correct. For carbon: For hydrogen: 100-85.6 = 14.4% 6(12.0) • 100 = 85.6% [6(12.0) + 12(1.01)] 4. Fill in the blanks in Table 1 by determining the percent composition and the molecular formulas of each compound. See table above. 5. Can you determine a compound’s structural formula if you are given the molecular formula? Explain. No, for example, hexene and cyclohexane each have the same molecular formula, but different structural formulas. 6. What is true about the percent composition of two different compounds that each have the same molecular formula? If two compounds have the same molecular formula they will have the same percent composition as well. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 96 7. Can you determine a molecule’s molecular formula solely from the percent composition? Explain. No. As can be seen in the Table 1, compounds with different molecular formulas may still have the same percent composition. 8. Using only the information in Table 1 (no calculators or periodic tables), predict the percent composition of each of the following compounds: Molecular Formula % Composition of H % Composition of C C 8 H8 7.8 92.2 C10H20 14.4 85.6 Note: any compound with a 1:1 ratio of carbon to hydrogen will have 92.2% carbon and 7.8% hydrogen. Any compound with a 1:2 ratio will have 85.6% carbon and 14.4% hydrogen. Information: Empirical Formulas An empirical formula is a formula that describes the lowest whole-number ratio of elements in a compound. An example of an empirical formula is CH, which is the empirical formula for benzene whose molecular formula was given in Table 1. Critical Thinking Questions 9. What is the empirical formula of a compound whose percent composition is 92.2% carbon and 7.8% hydrogen? (See question 8 and Table 1.) The empirical formula must be CH as demonstrated in question 8 and Table 1. 10. If you know the percent composition of each atom in a molecule, can you determine the empirical formula for the compound? Yes you can, although right now you may not be sure exactly how to do this. Information: Calculating the Empirical Formula When you know the percent composition of each element in a compound, you can calculate the empirical formula of that compound. The following example will illustrate how to do this. Example 1: A certain compound is 30.4% nitrogen and 69.6% oxygen by mass. What is the empirical formula of the compound? Step #1: Divide each percentage by the molar mass from the periodic table: For Nitrogen: 30.4 = 2.17 For Oxygen: 69.6 = 4.35 14.0 16.0 Step #2: Find the ratio of nitrogen to oxygen. To do this, find the smallest number obtained in step #1. In this example, the smallest answer is 2.17. Now divide each of your answers to step #1 by this smallest number. In this example, you should divide each answer by 2.17: 2.17 4.35 = 1.00 = 2.00 For Nitrogen: For Oxygen: 2 . 17 2 . 17 Step #3: Write the formula. The answers from step #2 are the subscripts in the formula! The formula therefore is NO2. 97 If in step #2 you get something like Nitrogen = 1.00 and Oxygen = 2.50 then the formula you write in step #3 would be NO2. 5. This doesn’t make sense because all subscripts must be whole numbers. You would need to double each subscript. The formula would be N1x2O2.5x2 = N2O5. Critical Thinking Questions 11. Find the empirical formula for a compound that contains 82.4% nitrogen and 17.6% hydrogen. 5.89 is smaller than 17.4 82 . 4 For hydrogen: 17.6 For nitrogen: = 5.89 = 17.4 14.0 1.01 Divide by the smallest: 5.89/5.89 = 1 17.4/5.89 = 2.95 3.0 The formula therefore is N1H3, or just NH3. Information: Calculating the Molecular Formula from the Empirical Formula Remember that the empirical formula is just a simplification of the molecular formula. For example, consider the empirical formula NO. There are several possible molecular formulas including: N2O2, N3O3, N4O4, etc. Which one is it? Notice that the possible formula N2O2 is made up of two of the empirical formulas, NO. Similarly, N3O3 is made up of three of the empirical formulas, NO. How do we know which empirical formula is correct? We need is the molar mass of the molecular formula. Critical Thinking Questions 12. The empirical formula for a certain compound is NO. The molar mass of the compound is 60.0 g/mol. a) What is the molar mass of the empirical formula? (Use the periodic table.) 14.0 + 16.0 = 30.0 b) Divide the molar mass of the compound (given in the question) by the molar mass of the empirical formula found in part a. 60.0/30.0 = 2.0 c) Your answer to part b tells you how many empirical formulas are in the molecular formula. You now should be able to write the correct molecular formula, which is N2O2. Verify that the correct molecular formula is N2O2. We found in part b that there are 2 empirical formulas in the molecular formula, therefore the molecular formula must be N1x2O1x2 = N2O2. 13. a) What is the empirical formula of a compound whose percent composition by mass is 85.7% carbon and 14.3% hydrogen? 14.3 85.7 = 7.14 = 14.2 For carbon: For hydrogen: 12.0 1.01 Divide by smallest: 7.14/7.14 = 1 14.2/7.14 = 1.99 2 The empirical formula is therefore: CH2 b) If the compound has a molar mass of 56 g/mol, what is the molecular formula? Molar mass of empirical formula from part a = 12.0 + 2(1.01) = 14.02 56/14.02 = 3.99 4; Molecular formula = C1x4H2x4 = C4H8 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 98 ChemQuest 32 Name: ____________________________ Date: _______________ Hour: _____ Information: Mole Ratios in Equations Propane is burned in many rural homes for heat in the winter. Below is the balanced equation for the combustion of propane (C3H8). C3H8 + 5 O2 3 CO2 + 4 H2O For each molecule of propane that is burned, there needs to be five molecules of oxygen present. Likewise, if there were a dozen molecules of propane, five dozen molecules of oxygen would be required. Similarly, for each mole of propane, five moles of oxygen are needed. Also, for each mole of propane burned three moles of carbon dioxide and 4 moles of water are produced. The numbers of moles of each substance in a chemical equation are related by the ratio of the coefficients of each substance. Critical Thinking Questions Note: For questions 1-6, refer to the balanced equation for the combustion of propane. 1. a) How many moles of water are produced when 1.45 moles of propane are combusted? It is a 4:1 ration so the moles of water = (1.45)(4) = 5.8 moles b) How many molecules of water is this? (Remember each mole has 6.02x1023 molecules.) (5.8)(6.02x1023) = 3.49x1024 2. If 2.35 moles of CO2 are produced in a reaction, how many moles of H2O would be produced? It is a 4:3 ration so the moles of water = 2.35(4/3) = 3.13 moles 3. Why is this statement false: “If 10 grams of propane burn, you need 50 grams of oxygen.” The coefficients in the balanced equation relate mole ratios and not gram ratios. The above statement would be true if it read: “If 10 moles of propane burn, you need 50 moles of oxygen.” 4. a) If 27.3 moles of carbon dioxide are produced during the combustion of a certain amount of propane, how many moles of propane were combusted? Since it is a 1:3 ratio, the moles of propane = 27.3(1/3) = 9.1 moles b) How many grams of propane was this? We need the molar mass of propane using the periodic table: 3(12.0) + 8(1.01) = 44.08 g/mol Now, using our answer from part a: (9.1 moles)(44.08 g/mol) = 401.1 g 99 5. If you have 410 grams of propane and want to know how many grams of oxygen are required to burn it, you can follow these steps… a) Find the number of moles of propane that you have. Convert grams to moles! 410g/(44.08g/mol) = 9.30 mol; (note: 44.08 is the molar mass found using the periodic table) b) The moles of propane are related to the moles of oxygen by the ratio of coefficients in the balanced chemical equation. Find the number of moles of oxygen you need given the moles of propane from part a. Since it is a 5:1 ratio, the moles of oxygen are: 9.30(5) = 46.5 mol c) Find the grams of oxygen from the moles of oxygen. Convert the moles of oxygen (answer to part b) to grams of oxygen (O2)! (Note: use the molar mass for O2, not just O. You should get approximately 1490 g of oxygen.) (46.5 mol)(32.0 g/mol) = 1488 g 6. Verify that this statement is correct: If 315 grams of propane combusts, then approximately 515 grams of water are produced. (315 g) / (44.08g/mol) = 7.15 mol propane 4:1 ratio so the mol of water = (7.15)(4) = 28.6 mol (28.6 mol)(18 g/mol) = 514.8 g 7. Consider the decomposition of ammonia: 2 NH3 3 H2 + N2. If you start with 425 g of NH3, how many grams of H2 and N2 can be produced? (425 g) / (17.0 g/mol) = 25 mol NH3 (25 mol NH3)(3/2) = 37.5 mol H2 (37.5 mol H2)(2.0 g/mol) = 75 g H2 (25 mol NH3)(½) = 12.5 mol N2 (12.5 mol N2)(28.0 g/mol) = 350 g N2 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 100 ChemQuest 33 Name: ____________________________ Date: _______________ Hour: _____ Information: Limiting Reactant Again consider the combustion of propane: C3H8 + 5 O2 3 CO2 + 4 H2O. If you had 10 moles of propane to burn, you would need 50 moles of oxygen according to the ratio in the balanced equation. If you only had 20 moles of oxygen you could not combust all 10 moles of propane. The reaction has been limited by the amount of oxygen you have—you don’t have enough oxygen to burn all of the propane. In this case, oxygen is called the “limiting reactant” because it limits how much propane can react. Notice that the limiting reactant isn’t always the substance that is present in the fewest number of moles. In this example, propane (C3H8) is the “excess reactant” because after the reaction there will be some of it left over. It is important to remember that everything in a chemical equation is related by mole ratios. If you only know the mass (grams) of the substances, you need to convert to moles. Critical Thinking Questions 8. a) In the above discussion, it was evident that 20 moles of oxygen was not sufficient to combust 10 moles of propane. How many moles of the propane can be combusted with 20 moles of oxygen? 4 moles. For every 5 moles of oxygen, 1 mole of propane can be combusted, so the number of moles of propane that can be combusted with 20 moles of oxygen is 20/5 = 4 moles b) How many moles of carbon dioxide will be produced? (Base the answer to this question on the number of moles of propane that actually get combusted—which is your answer to part a.) 12 moles. For every mole of propane that combusts 3 moles of CO2 are produced, so the number of moles of CO2 that can be produced when 4 moles of propane combusts = 4(3) = 12. c) Verify that if 12.5 moles of propane and 63.2 moles of oxygen were present, then propane is the “limiting reactant” and oxygen is the excess reactant. For each mole of C3H8 five moles of O2 are required, so for 12.5 moles of C3H8, the number of moles of O2 needed are (12.5)(5) = 62.5 moles. Since we have more than 62.5 moles (according to the question we have 63.2 moles), then we have excess O2 and therefore C3H8 is the limiting reactant. 101 9. Consider the following chemical reaction: 3 MgCl2 + 2 Na3PO4 6 NaCl + Mg3(PO4)2. Assume that 0.75 mol of MgCl2 and 0.65 mol of Na3PO4 are placed in a reaction vessel. a) Verify that Na3PO4 is the excess reactant and MgCl2 is the limiting reactant. Using the 3:2 ratio, we find that for 0.75 mol of MgCl2, we need (0.75)(2/3) = 0.5 moles of Na3PO4. We have more than that and therefore Na3PO4 is the excess reactant and MgCl2 the limiting. b) How many moles of the excess reactant are left over after the reaction stops? We have 0.65 mol and we use up 0.5 mol (calculated in part a), so the number of moles left over is 0.65 – 0.5 = 0.15 mol c) How many moles of NaCl will be produced in this reaction? (Remember—you must base this answer on how many moles of the limiting reactant that reacted.) 1.5 mol. 0.75 mol of MgCl2 is the limiting reactant, so we use the 6:3 ratio of NaCl to MgCl2 and multiply (0.75)(6/3) = 1.5 mol. 10. Consider the double replacement reaction between calcium sulfate (CaSO4) and sodium iodide (NaI). If 34.7 g of calcium sulfate and 58.3 g of sodium iodide are placed in a reaction vessel, how many grams of each product are produced? (Hint: Do this problem in the steps outlined below.) a) Write the balanced chemical equation for the reaction. CaSO4 + 2 NaI CaI2 + Na2SO4 b) Find the limiting reactant. First, convert 34.7g and 58.3g from grams to moles using the molar masses from the periodic table. Next, compare the number of moles of each reactant. Ask yourself: Do I have enough NaI to use up all of the CaSO4? Do I have enough CaSO4 to use up all of the NaI? Whichever one will get used up is the limiting reactant. (34.7g) ÷ (136.2g/mol) = 0.255 mol CaSO4; (58.3g) ÷ (149.9g/mol) = 0.389 mol NaI To use up all of the NaI, I need (0.389)(½) = 0.1945 mol CaSO4 I have more than this much, so NaI is limiting reactant. c) Use the number of moles of the limiting reactant to calculate the number of moles of each product produced using the coefficients from the balanced chemical equation in part a. mol of CaI2 = mol of Na2SO4 because the coefficients are both “1” in the balanced equation. Using the mol of limiting reactant and the mole ratio of 1:2, you can calculate the mol of CaI2 and Na2SO4. (0.389)(½) = 0.1945 mol. d) In part c you found the moles of each product produced. Now convert moles to grams using the molar mass from the periodic table. You have now answered the question. g of CaI2 = (0.1945 mol)(293.9g/mol) = 57.2g g of Na2SO4 = (0.1945 mol)(142.1g/mol) = 27.6g Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 102 11. If 181.1g of Al(NO3)3 react with 102.1g of CaO in a double replacement reaction, how many grams of each product will be produced? (Note: this is just like the last question, but parts a-d are not spelled out for you.) First, write the balanced equation: 2 Al(NO3)3 + 3 CaO 3 Ca(NO3)2 + Al2O3 Next, find the limiting reactant: (181.1g) ÷ (213 g/mol) = 0.850 mol Al(NO3)3 (102.1g) ÷ (56.1g/mol) = 1.82 mol CaO To use up all 0.850 mol of Al(NO3)3, I need (0.850)(3/2) = 1.275 mol CaO. Since you have more than this amount, CaO is present in excess and Al(NO3)3 is the limiting reactant. Use the moles of limiting reactant to calculate the moles of each product produced: mol Ca(NO3)2 = (0.850)(3/2) = 1.275 mol mol Al2O3 = (0.850)(½) = 0.425 mol Convert the mol of each product to grams of each product: Grams Ca(NO3)2 = (1.275 mol)(164.1g/mol) = 209.2 g Grams Al2O3 = (0.425mol)(102g/mol) = 43.4 g 103 ChemQuest 34 Name: ____________________________ Date: _______________ Hour: _____ Information: Sources of Error in the Real World When we perform experiments in the laboratory we never get all of the product we theoretically could get. Reactions in the laboratory are not ideal. For example, consider the reaction of sodium metal and chlorine gas to produce salt: 2 Na + Cl2 2 NaCl If we react 23.0g of sodium metal with plenty of chlorine gas, calculations tell us that we should get about 58.5g of sodium chloride. However, if you tried this in the lab and then took the mass of your final product on a balance, you would usually get somewhat less than 58.5g. The more careful you are, the closer you can get to the mark of 58.5g, but you will never be able to make 58.5g of sodium chloride. Critical Thinking Questions 1. What are some practical things—some “sources of error”—that can happen in the lab and in a reaction that would cause us to get less than 100% of the product that we are trying to produce? Spilling some of the product before weighing; evaporation of some product that are liquids 2. The information section above made it sound like we always would get less than 58.5 g of salt. However, it is possible that after the reaction we could take the mass of the product and find that it is greater than 58.5g. According to our calculations this should be impossible, but what “sources of error” may cause this? Contamination of product with a substance that would add weight. 3. When 40.5 g of sodium metal reacts with plenty of chlorine gas, how many grams of sodium chloride should be produced? (Note: when questions are worded this way, you automatically know that sodium is the limiting reactant.) 40.5g ÷ 23g/mol = 1.76 mol Na = mol NaCl because of a 1:1 mol ratio (1.76 mol NaCl)(58.5 g/mol) = 103g NaCl Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 104 Information: Calculating Percent Yield Percent yield is a measure of how much of a product you actually obtained in a reaction compared to the amount that you could have obtained according to calculations. There is a handy formula for calculating percent yield: percent yield = actual yield •100 theoretical yield “Theoretical yield” is the amount of a product that we should be able to make. You calculated the theoretical yield of sodium chloride in question 3; hopefully your answer was about 103g. Now let’s say that you actually attempted this experiment in a lab with 40.5g of sodium and when you were finished you collected 84.0g of sodium chloride—this amount is your “actual yield”, the amount of product you actually collected in the lab. Using the formula given above, we can calculate our percent yield: 84.0g percent yield = •100 = 81.6% 103g Critical Thinking Questions 4. Consider the combustion of 215.0g of butane, C4H10 with plenty of oxygen. If you are able to collect 295.3g of water in the laboratory, what was your percent yield. Hint: first calculate your theoretical yield using the balanced equation and mole ratios! 215 g 333.6g theoretical yield divide 2 C4H10 + 13 O2 8 CO2 + 10 H2O 58 g/mol 18 g/mol multiply 3.71 mol 18.53 mol x 10/2 Percent yield = (295.3g) ÷ (333.6) · 100 = 88.5% 5. Consider the double replacement reaction of excess sodium chloride with 203.9g of silver nitrate. If you are able to produce 150.4g of silver chloride what was your percent yield? 203.9 g 171.98g theoretical yield Divide NaCl + AgNO3 NaNO3 + AgCl 169.87g/mol 143.32 g/mol Multiply 1.20 mol 1.20 mol Percent yield = (150.4) ÷ (171.98) · 100 = 87.45% 6. If you make 46.8g of calcium carbonate by reacting 87.5g of calcium nitrate with plenty of sodium carbonate, what is your percent yield? 87.5 g 53.35g theoretical yield Divide Ca(NO3)2 + Na2CO3 CaCO3 + 2 NaNO3 164.1g/mol 100.1g/mol multiply 0.533 mol 0.533 mol Percent yield = (46.8) ÷ (53.35) · 100 = 87.7% 105 ChemQuest 35 Name: ____________________________ Date: _______________ Hour: _____ Information: Gas Pressure Figure 1: Two containers of gas molecules Container 1 Container 2 Gas molecules move randomly in their containers, colliding with the walls of their containers causing “gas pressure.” Pressure can be defined as the force pushing on an area. It can be described with the equation: F 2 P= A where P is pressure (in kPa), F is force (in N) and A is area (in m ). The more molecules collide with the wall and the faster the molecules are going when they strike the wall, the greater the force on the wall and therefore, the higher the pressure. Critical Thinking Questions 1. Use the pressure equation to explain why it would be more likely for an ice skater to fall through the ice on a lake than it would be for someone walking across the lake with regular shoes on. The blade of an ice skate has a much smaller surface area than the sole of a shoe. With a smaller area (A), the pressure (P) on the ice is larger. 2. Which container in Figure 1 has the highest pressure? Explain. Container 2, because there are more gas molecules just like a tire filled with more air. 3. If I heated container 1 and did not heat container 2, could I get the pressure in container 1 to equal container 2? Explain. Yes, increasing the temperature increases pressure by speeding up gas molecules. 4. If container 2 was made of an elastic material and if I expanded container 2, could I make the two containers have equal gas pressures? Explain. Yes, increasing volume decreases pressure giving both containers an equal number of molecules per volume. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 106 Information: Gas Laws Observe the following experimental data concerning a container of gas. The pressure, volume and temperature of a gas are all related. The table of data was obtained by making measurements of the pressure, volume and temperature of a sample of a gas. Several different kinds of gases were used and all had identical results. The variable that was not changed was the amount of gas present. The number of moles of gas always remained the same during these trials. Table 1: Experimental Data for Gases Trial A B C D E F G H Pressure (P) Units: kPa 120 135 195 150 135 100 225 262 Volume (V) Units: L 3.2 2.5 2.3 2.0 4.2 3.0 3.2 2.8 Temperature (T) Units: K 324.3 285.0 378.7 254.4 480.8 254.4 608.0 620.4 Critical Thinking Questions P1 P2 = 5. Verify that this equation is true when the volume is unchanged:T1 T2 (Hint: You must use two sets of data where the volume does not change like in trials A and G. Note: the subscript 1 refers to the pressure and temperature for the first trial you select and the subscript 2 refers to the pressure and temperature for the second trial you select.) PA PG 120 225 = = = 0.370 TA TG 324.3 608.0 6. Scientists often look for relationships between variables. If you wanted to see how the volume and pressure are related you would need to compare data from different trials when the temperature does not change. Why? Temperature would affect volume and pressure. 7. Find two sets of data in the table that have constant temperature. Which of the following mathematical relationships is true (there may be more than one) when the temperature remains unchanged? This relationship is known as “Boyle’s Law”, named after the person who first discovered it. a) P1 P2 b) (P1)(V1) = (P2)(V2) c) P1 + V1 = P2 + V2 d) P1 V1 = = V1 V2 P2 V2 B is the correct choice. Using data from trials D and F, we see: (PD)(VD) = (PF)(VF) (150)(2.0) = (100)(3.0) = 300 107 8. At constant pressure, which of the following equations is/are true? This relationship is known as V1 T1 “Charles’ Law”, named after the person who first discovered it. = a) V1 V2 b) (T1)(V1) = (T2)(V2) c) P1 + V1 = P2 - V2 d) V2 T2 = T1 T2 Substituting data from trials B and E you will see that a is correct. 9. Complete the following. You may want to consider the equations from questions 5, 7 and 8. a) At constant volume, as the temperature increases, the pressure always ___increases______. b) At constant temperature, as the volume increases, the pressure always ___decreases______. c) At constant pressure, as the temperature increases, the volume always ___increases______. 10. If the volume is not constant, is the statement you completed in 9a always true? Justify your answer by citing experimental data from the data table. No, in trial A, the temperature is greater, but the pressure is lower than in trial B. 11. If the temperature or pressure is not constant are your statements in 9b and 9c correct? Justify your answer. No. In trial C, the volume is greater, but the pressure is higher than in trial D. In trial C, the temperature is greater, but the volume is lower than in trial B. 12. Would the equations you discovered still be true if the temperature was measured in degrees Celsius (oC) instead of Kelvin (K)? Recall that K = oC + 273 or oC = K – 273. No, if you changed all the temperatures to oC, none of the equations would work. 13. Which of the following quantities is a constant? a) PV b) PV + T c) PT T V For each trial, if you multiply the pressure and volume and then divide by the temperature you get approximately 1.18. P1 V1 P2 V2 = 14. Based on your answer to question 13, verify that this equation is true: This T T 1 2 equation is called the “Combined Gas Law”. Notice that it contains all of the equations combined into one! Again, the pressure times the volume divided by the Kelvin temperature equals 1.18 for every trial in the table. 15. What needs to remain constant in order for equation 14 to be true? (You may need to refer to the information section.) The number of moles of gas in the container must be the same. If the moles changes we probably would not get (P)(V)/T to equal 1.18. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 108 16. Prove that when the temperature remains constant, the combined gas law becomes Boyle’s Law. If the temperature is constant, then T2 = T1 and we can write: P1V1 P2V2 multiplying both sides by T1 P1V1 = P2V2 = T1 T1 17. Prove that when the pressure remains constant, the combined gas law becomes Charles’s Law. If pressure is constant, then P2 = P1 and we can write: P1V1 P1V2 = dividing both sides by P1 T1 T2 V1 V2 = T1 T2 18. You have discovered several new mathematical relationships among gases. Now is your chance to practice using these equations! a) At constant temperature, the volume of a gas expands from 4.0 L to 8.0 L. If the initial pressure was 120 kPa, what is the final pressure? P1V1 = P2V2 (120)(4.0) = P2 (8.0) P2 = 60 kPa b) At constant pressure, a gas is heated from 250 K to 500 K. After heating, the volume of the gas was 12.0 L. What was the initial volume of the gas? Notice: as the temperature doubled, what happened to the volume? V1 V2 = T1 T2 V1 12.0 = V1 = 6.0 L 250 500 c) The volume of a gas was originally 2.5 L; its pressure was 104 kPa and its temperature was 270K. The volume of the gas expanded to 5.3 L and its pressure decreased to 95 kPa. What is the temperature of the gas? P1V1 P2V2 (104)(2.5) = (95)(5.3) = T1 T2 270 T2 T2 = 522.9 kPa 19. At constant temperature, if you increase the volume by a factor of two (doubling the volume), the pressure ____decreases_____ by a factor of ______2_______. (Refer to 18a for a hint.) increases or decreases what number 109 ChemQuest 36 Name: ____________________________ Date: _______________ Hour: _____ Information: The Gas Constant We previously examined the fact that PV is a constant as long as the number of moles of a gas T remains unchanged. This means that any time you measure a gas, the pressure times the volume divided by the temperature (in Kelvin) will always equal the same number unless the amount of gas in the container changes. We are now going to explore how the pressure, volume and temperature depend on the number of moles of gas that are present. Table 1: Experimental Data for Three Different Samples of Gas That Contain the Same Amount of Gas (# of moles of gas is constant) Gas A B C # of moles of Gas in container (mol) 0.3008 0.3008 0.3008 Volume of Container (L) 14.0 16.0 10.0 Temperature of Gas (K) 672.0 1280 340.0 Pressure of Gas (kPa) 120.0 200.0 85.0 Table 2: Experimental Data for Three Different Samples of Gases That Contain Different Amounts of Gas (# of moles of gas is not constant) Gas D E F # of moles of Gas in container 3.3485 0.7306 0.85950 Volume of Container (L) 40.0 22.5 16.0 Temperature of Gas (K) 230.0 315 280 Pressure of Gas (kPa) 160.0 85 125 Critical Thinking Questions 1. Prove that PV is a constant for the data in Table 1, but not a constant for the data in Table 2. T PAVA/TA = PBVB/TB = 2.5; but in Table 2, PDVD/TD does not equal PEVE/TE PV 2. Why is T a constant in Table 1, but not in Table 2? The number of moles of gas is not constant in Table 2. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 110 3. Which of the following is a constant for Table 1 and Table 2? (Note that “n” means number of moles of gas in the container.) A) PT B) PTn C) (n2)PV+T D) PV nV V nT 4. Consider your answer to question 3. Compute the numerical value of the constant by putting values into the equation indicated by your answer to question 3. This constant is given a special name, “The Ideal Gas Constant” and it has the symbol “R”. PV = 8.31 = R nT 5. Which of the following equations is true? The equation is known as the “Ideal Gas Law”. A) PT = nRV B) PV = nRT C) TV = nRP PV = R PV = nRT nT 6. At 215 kPa of pressure and a temperature of 318 K, 2.35 mol of a gas has what volume? (Use the ideal gas law and the ideal gas constant, R, to calculate V. Your answer should be 28.9 L.) PV = nRT (215)V = (2.35)(8.31)(318) V = 28.88 L 7. What is the temperature of a gas if 4.11 mol of a gas has a pressure of 315 kPa and a volume of 12.5 L? PV = nRT (315)(12.5) = (4.11)(8.31)T T = 115.3 K 8. 23.5 g of CO2 gas is at a temperature of 45oC and a pressure of 125 kPa. What is the volume of the container? (Hint: change grams to moles and 45oC to Kelvin.) 23.5g ÷ 44.0g/mol = 0.534 mol; T = 45oC + 273 = 318K PV = nRT (125)V = (0.534)(8.31)(318) V = 11.3 L Information: Molar Volume Molar volume: the volume of one mole of a gas. Depending on the temperature and pressure a mole of gas may have different volumes. Whenever we are speaking of molar volume, we can assume that we are talking about one mole of the gas. Thus, whenever we need to calculate the molar volume, n=1. 111 Critical Thinking Questions 9. What is the molar volume of oxygen gas if the gas has a pressure of 120 kPa and a temperature of 38oC? Use the ideal gas law (from question 5) with n=1 for one mole of gas and solve for V; remember to use Kelvin temperature and that R always equals 8.31. PV = nRT (120)V = (1)(8.31)(311) V = 21.5 L 10. Prove that if two different gases are at the same pressure and temperature, each gas has the same molar volume. In question 9 we looked at oxygen gas at 120 kPa and 38oC (311 K). Now, I’ll pick another gas—nitrogen—at 120 kPa and 38oC. PV = nRT (120)V = (1)(8.31)(311) V = 21.5 L 11. What is the molar volume of any gas at STP? Recall that STP is standard temperature (273 K) and standard pressure (101.325 kPa). PV = nRT (101.325)V = (1)(8.31)(273) V = 22.4 L 12. Why do you think all gases have the same molar volume at STP? As we found in question 10, the molar volume of a gas depends on the temperature and pressure and not the identity of the gas. Information: Using the Ideal Gas Law to Calculate Molar Mass You should know that to find the number of moles (n) of a substance, you take the mass (m) of the substance and divide it by its molar mass (M) according to the following equation: # of moles = mass Molar mass ⇒ n= m M Now consider the Ideal Gas Law, PV=nRT. Since n=m/M we can replace n with m/M in the ideal gas law to get: m PV = RT M By rearranging it a bit, we can get a more useful form of the equation: MPV = mRT. This equation can then be used when you are dealing with grams and molar mass instead of moles. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 112 Critical Thinking Questions 13. If 128g of a certain gas in a container with a volume of 21.5 L has a pressure of 132 kPa and a temperature of 45oC, what is the molar mass of the gas? MPV = mRT M(132)(21.5) = (128)(8.31)(318) M = 119.2 g/mol 14. How many grams of CO2 are in a 5.0 L container if the pressure in the container is 101 kPa and the temperature is 280 K? MPV = mRT (44.0)(101)(5.0) = m(8.31)(280) 9.55 g 15. If 4.0 L of a gas were produced at STP and the mass of the gas was found to be 12.8g, then what is the molar mass of the gas? MPV = mRT M(101.325)(4.0) = (12.8)(8.31)(273) 71.6 g/mol 113 ChemQuest 37 Name: ____________________________ Date: _______________ Hour: _____ Critical Thinking Questions 1. How many moles and how many grams of hydrogen gas can be produced by reacting 32.8 g of magnesium according to the following balanced equation? Mg (s) + 2 HCl (aq) MgCl2 (aq) + H2 (g) 2.72 g divide 32.8g 2.72 g Mg (s) + 2 HCl (aq) MgCl2 (aq) + H2 (g) 24.3 g/mol 2.02 g/mol 1.35 mol 1.35 mol multiply 2. Considering your answer to question 1, what volume of hydrogen gas was produced if this reaction was carried out at STP? n = 1.35, so using PV=nRT (101.325)V = (1.35)(8.31)(273) V = 30.2 L 3. Nitrogen gas can be produced by reacting Na3N with chlorine gas. If the reaction was carried out at STP with 34.8 g of Na3N, what volume of nitrogen gas can be produced? (Hint: first use stoichiometry to calculate the number of moles of nitrogen produced and then use the ideal gas law.) 34.8g 2 Na3N + 3 Cl2 N2 + 6 NaCl 83 g/mol 0.419 mol x ½ = 0.2095 mol N2 = n PV=nRT (101.325)V = (0.2095)(8.31)(273) V = 4.7 L Information: Mole Ratios in Chemical Equations and Volume As you know, the coefficients in balanced equations relate the number of moles of reactants to each other. For gaseous reactions, the coefficients relate not only the moles but also the number of liters of reactants and products to each other. For example, consider the following reaction, noting that “(g)” means that the substances are gaseous: N2 (g) + 2 O2 (g) 2 NO2 (g) The coefficients tell you that for every mole of N2, two moles of O2 react and two moles of NO2 are produced. It also tells you that for each liter of N2, two liters of O2 react and two liters of NO2 are produced. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 114 Critical Thinking Questions 4. Verify that for the following reaction to be completed, 4 liters of hydrogen would require at least 2 liters of oxygen gas at the same pressure and temperature. 2 H2 (g) + O2 (g) 2 H2O (g) It is a 2:1 ratio of hydrogen to oxygen, so for every two liters of hydrogen one liter of oxygen is required. 5. In a certain combustion reaction involving propane, 34.4 L of CO2 was produced. How many liters of propane (C3H8) were combusted? Assume the reaction took place at STP. C3H8 + 5 O2 3 CO2 + 4 H2O There is a 3:1 ratio of CO2 to C3H8, so there needs to be three times as much CO2 as there is C3H8. Therefore, the volume of C3H8 required is 34.4÷3 = 11.5 L 6. How many grams of sodium are required to produce 31.9 L of H2 at 97 kPa and 290 K according to the following balanced equation? 2 HNO3 + 2 Na 2 NaNO3 + H2 PV=nRT (97)(31.9) = n(8.31)(290) n = 1.28 mol H2 (1.28 mol H2)(2) = 2.56 mol Na (2:1 mol ratio of Na to H2) (2.56 mol Na)(23.0g/mol) = 58.9 g 7. At room temperature (25oC) and pressure (100 kPa), 381g of octane (C8H18) was burned in an atmosphere of plenty of oxygen gas. How many liters of each product were produced? 2 C8H18 + 25 O2 16 CO2 + 18 H2O Liters of C8H18: MPV = mRT (114.18)(100)V = 381(8.31)(298) V = 82.6 L Liters are related by the ratios of coefficients in the balanced equation: Liters CO2 = 82.6(16/2) = 660.8 L Liters H2O = 82.6(18/2) = 743.4 L 115 ChemQuest 38 Name: ____________________________ Date: _______________ Hour: _____ Information: Collecting Gas Over Water When gas is collected in a container, it is often collected using a technique called, “water displacement.” In water displacement, a container is filled with water and then gas is bubbled into the container. In this way, the container can be filled with relatively pure gas without air in it. Figure 1A: A beaker is filled with water and then turned upside down in a bucket of water. Rubber tubing is attached to a source of gas so that the gas is bubbling up through the water in the beaker. These gas bubbles force some of the water out of the beaker. Beaker upside down in water Rubber tubing carrying gas Gas bubbles Gas Generator Water Bucket Figure 1B: The same setup after 5 minutes. Rubber tubing carrying gas Beaker upside down in water Collected Gas Gas Generator Water Bucket Critical Thinking Questions 1. To collect the gas, why is the beaker first filled completely with water? What purpose does the water serve? By filling the beaker with the water you are making sure that there is no air in the beaker. 2. Examine the beaker in Figure 1A and 1B. What causes the water level to go down after 5 minutes? The gas has replaced the water and the water has gone into the bucket. 3. In Figure 1B, is the gas in the beaker pure? Explain. It is quite pure; however, there is some water vapor left behind. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 116 Information: Dry Gas The gas that is collected is not “dry” because there is some water vapor left behind in the beaker. If you attempt to collect pure oxygen, you will actually get mostly pure oxygen with a little water vapor. You can get an idea of how much water is left behind by examining the water vapor pressure at various temperatures. Table 1: Vapor pressure of water at various temperatures. Temperature (oC) 0 5 10 15 20 25 30 35 40 45 Vapor Pressure (kPa) 0.6 0.9 1.2 1.6 2.3 3.2 4.2 5.6 7.4 9.8 Critical Thinking Questions 4. Why is it logical to expect that the vapor pressure of water would increase as the temperature increases? As the temperature increases, water is more likely to evaporate and the evaporated molecules move faster, colliding with more force (or pressure). 5. Examine the two containers below. Both contain gases that were collected over water. Which one was collected at the higher temperature—gas A or gas B? Explain your answer. Gas A = Gas B = Water vapor = Water vapor = Gas B was collected at higher temperatures because there is more water vapor mixed with the gas and the water vapor pressure increases as the temperature increases. 6. Consider Gas A from question 5. The total pressure in the container is 104 kPa. If the temperature of the container is 20oC, calculate the pressure of Gas A when it is “dry”. Hint: find the vapor pressure of water at 20oC from Table 1 and then subtract it from the total pressure in the container. 104 – 2.3 = 101.7 kPa 7. Consider Gas B from question 5. The total pressure in the container is 110 kPa. If the temperature of the container is 35oC, calculate the pressure of Gas B when it is “dry”. 110 – 5.6 kPa = 104.4 kPa 117 Information: Dalton’s Law of Partial Pressures John Dalton was one of the first scientists to quantitatively state a mathematical relationship involving the total gas pressure in a container and the individual gases in the container. Dalton’s law states that the total pressure of any mixture of gases in a container is the sum of all the individual gas’s partial pressures. In equation form, Dalton’s law can be written as: PTotal = PGas A + PGas B + PGas C +… Critical Thinking Questions 8. A container of gas with a pressure of 450 kPa contained three different gases—hydrogen, oxygen and nitrogen. If the partial pressure of hydrogen was 210 kPa and the partial pressure of oxygen was 125 kPa, what was the partial pressure of nitrogen? 450 – (210 + 125) = 115 kPa 9. A tank held neon gas at a pressure of 350 kPa, helium at a pressure of 275 kPa and argon at a pressure of 410 kPa. What was the pressure in the tank? 350 + 275 + 410 = 1035 kPa 10. A certain gas was collected over water. The total pressure of the container was 100.0 kPa. The pressure of the dry gas was 94.4 kPa. At what temperature was the gas collected? 100.0 – 94.4 = 5.6 kPa (the water vapor pressure)This corresponds to a temperature of 35oC 11. Hydrogen gas was collected over water at a pressure of 102.5 kPa and a temperature of 25oC. After sealing the container, it was heated to 210oC. What is the final pressure of the dry hydrogen gas? (Hints: You must first find P1 for the gas by subtracting out the water vapor from the given pressure; solve for P2 using the combined gas law with constant volume.) P1 = 102.5 kPa – 3.2 kPa = 99.3 kPa; T1 = 25oC + 273 = 298K; T2 = 210oC+273= 483 K P1 P2 99 .3 P2 = → = → P2 = 160 .9 kPa T1 T2 298 483 12. Oxygen gas was collected over water at 30oC. The pressure in the 2.5 L container was 110 kPa. If the container was allowed to expand to 7.0 L and if the temperature was decreased to -30oC, what is the final pressure of the dry oxygen gas? P1=110-4.2 = 105.8 kPa; T1 = 30oC+273 = 303 K; T2 = -30oC + 273 = 243 K P1 V1 P2 V2 105.8( 2.5) P2 (7.0) = → = → 30.3 kPa T1 T2 303 243 13. A quantity of oxygen gas was collected over water at 30oC in a 475 mL container. The pressure in the container was measured to be 105 kPa. How many grams of oxygen gas were collected? P = 105 – 4.2 = 100.8 kPa; molar mass of O2 = 32.0g/mol; V = 0.475 L (not mL); T = 303 K MPV = mRT (32.0)(100.8 kPa)(0.475) = m(8.31)(303) m = 0.608 g Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 118 ChemQuest 39 Name: ____________________________ Date: _______________ Hour: _____ Information: Molarity Concentration is a term that describes the amount of solute that is dissolved in a solution. Concentrated solutions contain a lot of dissolved solute, but dilute solutions contain only a little. Critical Thinking Questions 1. Consider the terms "concentrated" and "dilute". Are these qualitative or quantitative terms? These are qualitative terms: very general and do not include the magnitude or quantity. 2. One way of quantitatively measuring solution concentration is with units of molarity, symbolized by M. You see 1.7 liters (L) of a sodium chloride and water solution. The label on the bottle reads "1.5 M NaCl". You don't know what molarity is, but you decide to find out. After evaporating the water out of the solution you discover that there are about 149 grams of salt. Using this information, which of the following formulas is/are correct for finding molarity? grams of solute moles of solute A) Molarity = B) Molarity = moles of solute liters of solution 149g ÷ 58.5g/mol = 2.547 mol 1.5 = 2.547 mol ÷ 1.7 L 3. Using the equation you discovered in question two, calculate the molarity of each of the following solutions. A) A solution is prepared by dissolving 24.9 g of CaCl2 in 210 mL (which 0.210 L) of solution. 1.07 M Change g to mol: 24.9g ÷ 111.1g/mol = 0.224 mol Molarity = mol ÷ L = 0.224 ÷ 0.210 = 1.07 M B) A solution contains 12.9 g of Na2SO4 in 325 mL of solution. 0.279 M Change g to mol: 12.9 ÷ 142.1g/mol = 0.09078 mol Molarity = mol ÷ L = 0.09078mol ÷ 0.325L = 0.279 M 4. Verify that I need 2.15 moles of Ca(NO3)2 to make 358 mL of a 6.00 molar solution. mol = (Molarity)(Liters) = (6.00M)(0.358L) = 2.15 mol 5. Verify that it takes 80.8 g of sodium chloride to make 425 mL of a 3.25 M solution. mol = (Molarity)(Liters) = (3.25M)(0.425L) = 1.38 mol (1.38 mol)(58.5g/mol) = 80.7 g 119 6. Consider 670 mL of a 4.10 M solution of Mg(NO3)2 setting in a beaker. If you evaporate all 670 mL of the solution, how many grams of solute would be left in the beaker? 407.8g mol = (Molarity)(Liters) = (4.10M)(0.670L) = 2.747 mol convert mol to g: (2.747mol)(148.3g/mol) = 407.8g Information: Molality Molality is another way of expressing solution concentration. The symbol for molality is m. Whereas molarity (M) represents the ratio of moles solute to liters of solution, the molality (m) is the ratio of moles solute to kilograms of solvent. It can be expressed using the following formula: moles of solute molality = kg solvent Critical Thinking Questions 7. Consider a solution that is prepared by adding 1.34 moles of sodium nitrate to 2.5 kg of water. What is the molality of the solution? 0.536 m m = 1.34mol ÷ 2.5kg = 0.536 m 8. Considering the data given in question 7, is this enough data to find the molarity? If so, calculate the molarity. If not, explain why not. No, because we don’t know the total volume of the solution. 9. What is the molality of a solution that is made by dissolving 32.6 g of Na2SO4 in 475 g of water? 0.483 m Convert g to mol: 32.6g ÷ 142.1g/mol = 0.229 mol molality = mol/kg = 0.229mol ÷ 0.475kg = 0.483 m 10. Consider 2.35 moles of sodium chloride are dissolved in 1.21 kg of solution to make 1.29 liters. Calculate and compare the molarity and molality. Molarity: 1.82 M M = mol ÷ L = 2.35mol ÷ 1.29 L = 1.82 M molality: 1.94 m m = mol ÷ kg = 2.35mol ÷ 1.21 kg = 1.94 m 11. If 26.45g of Na2SO4 are dissolved in 1.10 kg of solution to make 1.24 L, calculate both the molarity and the molality of the resulting solution. Convert g to mol: 26.45g ÷ 142.1g/mol = 0.186mol Molarity: 0.150 M M = mol ÷ L = 0.186mol ÷ 1.24 L = 0.150 M molality: 0.169 m m = mol ÷ kg = 0.186mol ÷ 1.10kg = 0.169 m Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 120 Information: Mole Fraction Another way of expressing solution concentration is called “mole fraction”. The mole fraction (symbolized by X) of the solute or of the solvent can be calculated using the following equations: mol solute mol solvent X solute = X solvent = (mol solute + mol solvent ) (mol solute + mol solvent ) Note: both the solute and the solvent must be converted to moles when finding the mole fraction! Critical Thinking Questions 12. Prove that the mole fraction of salt (XNaCl) equals 0.049 when 14.25 g of NaCl is dissolved in 85.0 g of H2O. 85.0g ÷ 18.0g/mol = 4.722 mol H2O 14.25g ÷ 58.5g/mol = 0.2436 mol NaCl mol NaCl 0.2436 X NaCl = = = 0.049 (mol NaCl + mol H 2O ) (0.2436 + 4.722) 13. Find the mole fraction of water (Xwater) for the solution described in question 12. mol H 2O 4.722 X H 2O = = = 0.951 (mol H 2O + mol NaCl ) (0.2436 + 4.722) 14. Prove that Xsolute + Xsolvent = 1. The answer from question 12 + answer to question 13 = 1 15. In a certain salt water solution, the mole fraction of salt is 0.18. Find the mole fraction of water. 1.00 – 0.18 = 0.82 Information: Mass Percent Composition Mass percent composition is similar to the mole fraction except the amounts of solute and solvent are in grams instead of moles. Here is the formula for finding the mass percent of a solute: mass solute mass% solute = • 100 (mass solute + mass solvent ) Critical Thinking Questions 16. Prove that the mass percent of salt is 14.36% in the solution described in question 12. mass NaCl 14.25 mass% NaCl = • 100 = • 100 = 14.36% (mass NaCl + mass H 2 O ) 14.25 + 85.0 17. Calculate the mass percent of sodium phosphate if 12.5g of it are dissolved in 250 mL of water. (Note: 1 mL of water has a mass of 1 g.) Because 1 mL = 1g, the mass of H2O = 250 g mass Na 3 PO 4 12.5 mass% Na 3 PO 4 = • 100 = • 100 = 4.76% (mass Na 3PO 4 + mass H 2 O ) 12.55 + 250 121 ChemQuest 40 Name: ____________________________ Date: _______________ Hour: _____ Introduction Question: Melting Ice 1. In colder climates during the winter, people put salt on the roads and walkways to melt ice. Why do people do this? Why does salt melt the ice? Salt actually lowers the freezing point of water so that water will not freeze until much lower temperatures. Salt water remains a liquid at 0oC Information: Dissociation and Total Molality of Particles When you dissolve a solute in a solvent, the resulting solution has slightly different properties than the original solvent. For example, salt water has a different freezing point and boiling point than pure water. The salt interferes with water’s ability to freeze and boil. When ionic compounds dissolve, they dissociate. When an ionic compound dissociates that means that it breaks up into ions. For example, salt (sodium chloride) breaks up into sodium ions and chloride ions. This process is represented in the following balanced equation: NaCl Na+ + ClNote for the above equation that Cl- does not need to be written as Cl2 because Cl- is a chloride ion and not a lone chlorine atom. Since calcium nitrate is an ionic compound it also dissociates as shown below: Ca(NO3)2 Ca2+ + 2 NO3Covalent molecules do not dissociate. Although they may dissolve, they do not break up into ions. Critical Thinking Questions 2. Write the balanced equation for the dissociation of magnesium chloride. MgCl2 Mg2+ + 2 Cl3. Write the balanced equation for the dissociation of ammonium sulfate. (NH4)2SO4 2 NH4+ + SO42- Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 122 4. Consider calcium nitrate. Each calcium nitrate breaks up into one calcium ion and two nitrate ions according to the balanced equation given in the information section. If you take one mole of calcium nitrate and put it in water, it will dissociate. a) How many moles of calcium ions and how many moles of nitrate ions will there be in the solution? One mole of calcium ions and two moles of nitrate ions. b) What is the total number of moles of all ions in the solution? 3; one mole of calcium ions plus two moles of nitrate ions equal a total of three moles. 5. A solution is made so that it is 2.5 M Ca(NO3)2. Therefore the concentration of Ca2+ is 2.5 M and the concentration of NO3- is 5.0 M. The total concentration of all particles is 7.5 M. Explain. Since each mole of Ca(NO3)2 breaks up into 1 mole of Ca2+, the concentration of Ca2+ would be the same as the concentration of Ca(NO3)2. Since each mole of Ca(NO3)2 breaks up into 2 moles of NO3-, the concentration of NO3- is twice the concentration of Ca(NO3)2. 6. A solution is made so that it the concentration is 3.0 m MgCl2. What is the molality of Mg2+ and Cl- ions? What is the total molality of all particles in the solution? Mg2+ ___3.0m___ Cl- ___6.0m___ Total molality of all particles: ___9.0m_____ 7. A solution is prepared by dissolving 45.7 g of sodium carbonate in 200 g of water. a) What is the molality of the sodium carbonate? 45.7 g 0.431mol = 0.431mol = 2.16m 106g / mol 0.200kg b) What is the total molality of all particles in the solution? 6.48m; Na2CO3 2 Na+ + CO32- ; Since Na2CO3 breaks into 3 total ions, the total molality is 3(2.16) = 6.48m 8. Consider sugar (C6H12O6) , a covalent molecule. If a solution is made so that the concentration is 3.5 m in sugar, then what is the total molality of particles? 3.5m; Covalent compounds don’t break up into ions so the total molality is still 3.5m. Information: Total Molality of Particles and Changes in Boiling/Freezing Points You may be wondering how all of this ties together. We have seen that adding a solute changes the boiling and freezing points of solvents. The amount of the change depends on how much solute is added. Equations relating the change in boiling or freezing point and the molality is shown below: ∆Tbp = (mT)(Kbp) for boiling point ∆Tfp = (mT)(Kfp) for freezing point Note: mT is the total molality of particles. Kbp and Kfp are constants called the molal boiling point elevation constant and the molal freezing point depression constant respectively. Kbp for water is 0.515 oC/m and Kfp for water is 1.853 oC/m. 123 Critical Thinking Questions 9. What is the freezing point of a 2.5 m solution of salt water. Hints: first find ∆Tfp and then subtract the change from the original freezing point (0oC for water). Also, remember mT is not 2.5 m in this problem. mT = 2.5(2) = 5.0 (recall that NaCl breaks into Na+ and Cl-) ∆Tfp = (mT)(Kfp) = (5.0)(1.853) = 9.3oC Tfp = 0oC – 9.3oC = – 9.3oC 10. Find the boiling point of a 3.7 m solution of calcium chloride. mT = (3.7)(3) = 11.1 (recall that CaCl2 breaks into Ca2+ and two Cl-) ∆Tbp = (mT)(Kbp) = (11.1)(0.515) = 5.72oC Tbp = 100oC + 5.72oC = 105.72oC 11. What is the freezing point of a sugar solution in which the concentration of sugar is 2.25m? Note: sugar is covalent so it dissolves but it does not dissociate. ∆Tfp = (mT)(Kfp) = (2.25)(1.853) = 4.17oC Tfp = 0oC – 4.17oC = – 4.17oC Information: Raoult’s Law A solution will almost always have a lower vapor pressure than the pure solvent. For example, salt water will have a lower vapor pressure than pure water. The vapor pressure of a solution (Psolution) is related to the vapor pressure of the pure solvent (Psolvent) by the mole fraction of the solvent (Xsolvent) in an equation known as Raoult’s Law: Psolution = (Xsolvent)(Psolvent) Critical Thinking Questions 12. What is the vapor pressure of water at 20oC is 2.3 kPa. What is the vapor pressure of a solution formed by dissolving 21.5g of LiCl in 84.3g of H2O? 21.5 g = 0.507mol 42.44 g / mol x solvent = 84.3g = 4.68mol 18.02 g / mol 4.68 = 0.902 Psolution = (Xsolvent)(Psolvent) = (0.902)(2.3) = 2.08 kPa (4.68 + 0.507) Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 124 ChemQuest 41 Name: ____________________________ Date: _______________ Hour: _____ Information: Molar Mass As you know, the molar mass of any substance is how much mass (measured in grams) one mole of a substance has. The units for molar mass are g/mol. If you know that 2.0 moles of water have a mass of 36.0 g, you can find the molar mass of water by dividing 36.0 g by 2.0 moles to get 18.0 g/mol. Critical Thinking Questions 1. If 2.75 moles of a certain compound has a mass of 125.9 g, what is the molar mass of the compound? 125.9 g = 45.8 g/mol 2.75mol 2. If you put 2.53x1024 molecules of an unknown compound on a balance you will discover that the mass is 757.8 g. What is the identity of the unknown compound? (Hint: find the molar mass and then calculate the molar masses of the following compounds.) A) C10H12O 2.53 x10 24 = 4.20 mol 6.02 x10 23 B) C6H12O6 C) C8H18 D) C10H8NO 757.8 g = 180.3 g/mol B has a molar mass of 180 g/mol also 4.20mol Information: Relating Molar Mass and Colligative Properties One of the ways to determine the molar mass of a compound is by experiments involving colligative properties. By measuring the temperature changes of solutions, it is possible to calculate the molality of the solution and from the molality you can determine the molar mass. The following calculations walk you through the process. Please note that the process used here is valid only for covalent compounds. 125 Critical Thinking Questions 3. When 135 g of an unknown covalent compound is dissolved in 450 g of water, the freezing point of the solution is –3.21oC. Find the molar mass of the covalent compound. Follow these steps… a) What is the molality of the solution? (Use ∆Tfp = mT Kfp and solve for mT. Note that since this is a covalent compound mT equals the molality of the solute because covalent compounds don’t dissociate.) ∆Tfp = mT Kfp mT = ∆Tfp ÷ Kfp = (3.21) ÷ 1.853 = 1.73 m b) How many moles of solute were dissolved? (Multiply your answer to part a by the kilograms of solvent.) (1.73m)(0.450kg) = 0.779 mol c) Calculate the molar mass of the compound. (Take the mass of the solute given in the problem and divide by your answer to part b.) 135g ÷ 0.779mol = 173 g/mol 4. Find the molar mass of a covalent compound if 210 g of the substance is dissolved in 810 g of water changes the boiling point of the solution to 101.3 oC. ∆Tbp = mT Kbp mT = ∆Tbp ÷ Kbp = (1.3) ÷ 0.515 = 2.52 m (2.52m)(0.810kg) = 2.04 mol 210 g ÷ 2.04 mol = 103 g/mol 5. Find the molar mass of a covalent compound if 38.5 g dissolves in 520 g of water to give a freezing point of –2.15oC. ∆Tfp = mT Kfp mT = ∆Tfp ÷ Kfp = 2.15 ÷ 1.853 = 1.16 m (1.16m)(0.520 kg) = 0.603 mol 38.5g ÷ 0.603 mol = 63.8 g/mol Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 126 6. When 282.5 g of a covalent compound is dissolved in 630 g of water, the vapor pressure lowers from 3.2 kPa to 2.1 kPa. What is the molar mass of the compound? Use the following steps… a) Use Raoult’s Law to find the mole fraction of the water. (Solve for xsolvent.) Psolution = (Xsolvent)(Psolvent) Xsolvent = Psolution ÷ Psolvent = 2.1 ÷ 3.2 = 0.656 b) Convert grams of water to moles of water. 630g ÷ 18.02 g/mol = 34.96 mol c) Use the mole fraction equation (below) and your answer to part b to solve for molsolute. mol solvent x solvent = (mol solvent + mol solute ) mol solute = mol solvent 34.96 − mol solvent = − 34.96 = 18.33 mol 0.656 x solvent d) Divide the mass of the compound given in the question by your answer to part c to find the molar mass. 282.5g ÷ 18.33 mol = 15.4 g/mol 7. Find the molar mass of a covalent solute if when 371 g of the solute are added to 650 g of water, the vapor pressure changes from 4.2 kPa to 2.8 kPa. Psolution = (Xsolvent)(Psolvent) Xsolvent = Psolution ÷ Psolvent = 2.8 ÷ 4.2 = 0.667 650 g ÷ 18.02g/mol = 36.07 mol of water mol solvent 36.07 mol solute = − mol solvent = − 36.07 = 18.0 mol 0.667 x solvent 371g ÷ 18.0 mol = 20.6 g/mol 8. 230 g of a covalent solute are dissolved in 520 g of water. The vapor pressure changes from 3.5 kPa to 2.9 kPa. What is the freezing point of the solution? Hint: first find the moles of solute using Raoult’s Law and then find the molality and use ∆Tfp = mT Kfp. Psolution = (Xsolvent)(Psolvent) Xsolvent = Psolution ÷ Psolvent = 2.9 ÷ 3.5 = 0.829 molsolvent = 520g ÷ 18.02g/mol = 28.86 mol mol solvent 28.86 − mol solvent = − 28.86 = x solvent 0.829 molality = mT = 5.95mol ÷ 0.520kg = 11.45 m ∆Tfp = mT Kfp = (11.45)(1.853) = 21.2 Tf = 0oC – 21.2oC = –21.2oC mol solute = 5.95 mol 127 ChemQuest 42 Name: ____________________________ Date: _______________ Hour: _____ Information: Average Rate of Reaction Recall that during a chemical reaction reactants are transformed into products: A + B C + D. A very important question is: how fast do such processes happen? For example, we need to know how long it will take for a medicine to work in our bodies and how long will it take to produce chemicals in industry. How fast do reactants A and B disappear? How fast do products C and D form? We can express such questions in the form of an equation as shown below. rate of disappearance change in molarity of reactant A ∆[reactant A] =− of reactant A = − change in time ∆time Keep in mind that all rates are written as positive numbers; thus, the function for the negative sign in the above equation is to yield a positive result for the rate. Critical Thinking Questions 1. What is molarity? Molarity is a measure of the moles of solute per liter of solution. 2. What do the symbols ∆ and [ ] mean in the above equation? ∆ means “change” and [ ] means concentration in molarity. 3. What units would you expect for the rate of disappearance of a reactant? (Assume time is measured in seconds.) Molarity per second 4. How is the change in molarity of a reactant calculated? Would this be a positive or a negative number? Final molarity minus initial molarity. It would be a negative number because the molarity of a reactant decreases as the reaction proceeds. 5. How is the change in molarity of a product calculated? Would this be a positive or a negative number? Final molarity minus initial molarity. It would be a positive number because the molarity of a product increases as the reaction proceeds. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 128 6. When writing the equation for the rate of formation of a product, the negative sign in the above equation is not needed. Explain why. The only purpose of the negative sign is to give a positive number and the negative sign is only needed for calculating the change in concentration for a reactant because the change will be a negative number, as mentioned in our answer to question 4. Information: Stoichiometry and Average Rate Consider the decomposition of N2O5 and the experimental data for the reaction taking place inside a container that has a volume of 3.0 L. 2 N2O5 (g) 4 NO2 (g) + O2 (g) Time (s) 0 600 1200 1800 Moles N2O5 0.4320 0.4194 0.4104 0.4032 Moles NO2 0 0.0252 0.0432 0.0576 Moles O2 0 0.0063 0.0108 0.0144 Critical Thinking Questions 7. Calculate the change in moles of each reactant and product between 600 and 1200 seconds. change in moles of N2O5: 0.4194 – 0.4104 = 0.0090 change in moles of NO2: 0.0432 – 0.0252 = 0.0180 change in moles of O2: 0.0108 – 0.0063 = 0.0045 8. Compare your answers in question 7. What is the relationship between the coefficients in the balanced chemical equation and the number of moles used up or produced in a reaction? The change in N2O5 is twice the change in O2 which corresponds to the 2:1 ratio of the balanced equation. Also, the change in NO2 is twice the change of N2O5, which also corresponds to the 4:2 ratio of the balanced equation. 9. Fill in the missing blanks in the table above. ∆[O2] = 0.0144-0.0108 = 0.0036; ∆[NO2] = 4(0.0036) = 0.0144; ∆[N2O5]=2(0.0036) = 0.0072 10. Calculate the following values. Be sure to use molarity in your calculations. a) Rate of disappearance of N2O5 between time 0 and 600 s. 0.4194 mol 0.4320 mol − 0.1398 − 0.144 3.0 L 3.0 L = = −7.0 x10 −6 M/s 600 s - 0 s 600 b) Rate of appearance of NO2 between time 0 and 600 s. 0.0252 mol 0 mol − 3.0 L 3.0 L = 0.0084 = 1.4 x10 − 5 M/s 600 s - 0 s 600 c) Rate of appearance of O2 between time 0 and 600 s. 0.0063 mol 0 mol − 3.0 L 3.0 L = 0.0021 = 3.5 x10 − 6 M/s 600 s - 0 s 600 129 11. Compare each of the rates. What relationship exists between the rates and the coefficients in the balanced chemical equation? They are related by the coefficients in the balanced equation so that Rate O2 = 2(Rate N2O5) = 4(Rate NO2) 12. Calculate the following values. Be sure to use molarity in your calculations. a) Rate of disappearance of N2O5 between time 600 and 1200 s. 0.4104 mol 0.4194 mol − 0.1368 − 0.1398 3.0 L 3.0 L = = −5.0 x10 − 6 M/s 600 s - 0 s 600 b) Rate of appearance of NO2 between time 600 and 1200 s. Rate NO2 = 2(RateN2O5) = 2(5.0x10-6) = 1.0x10-5 M/s c) Rate of appearance of O2 between time 600 and 1200 s. Rate O2 = 1/2(Rate N2O5) = 2.5x10-6 M/s 13. As the reaction proceeds are the rates constant? What affects the rate? No, they are not constant. The rates are gradually slowing. For example, between 0 and 600 seconds N2O5 was disappearing at a rate of 7.0x10-6 M/s, but between 600 and 1200 seconds N2O5 was disappearing at a rate of 5.0x10-6 M/s. The other reactants also slowed. It seems that rate is dependent on concentration. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 130 ChemQuest 43 Name: ____________________________ Date: _______________ Hour: _____ Information: Reaction Order Below is a table of data corresponding to the following balanced equation: 2 ClO2 (aq) + 2 OH- (aq) ClO3- (aq) + ClO2- (aq) + H2O (l) . Six experiments were carried out with differing concentrations of ClO2 and OH- in each experiment. The measurement of how quickly ClO2 disappears is given in the table for each of the six experiments. How quickly a reactant disappears (or how quickly a product forms) is a good measurement of how fast a reaction takes place. Table 1: Experimental data for the reaction of ClO2 Experiment Initial [ClO2] Initial [OH-] Initial Rate of disappearance of ClO2 (M/s) 1 0.020 0.030 0.00276 2 0.040 0.030 0.01104 3 0.020 0.060 0.00552 4 0.040 0.060 0.02208 5 0.040 0.090 0.03312 6 0.120 0.030 0.09936 Critical Thinking Questions 1. What happens to the rate of a reaction as the concentrations of the reactants increases? Justify your answer with data from the table above. The higher the concentration of reactants, the faster the rate. For example, the rate corresponding to Experiment 4 is greater than the rate in Experiment 1 and Experiment 4 also has a greater concentration of reactants. 2. Consider the molecular level of what is happening when ClO2 reacts with OH- to form products. Offer an explanation for why changing the concentration of reactants changes the rate of a reaction. Changing the concentration changes how many molecules collide and react with each other. 3. Does the reaction depend on the concentration of ClO2 and the concentration of OH- equally? In other words, is the rate more dependent on ClO2, on OH-, or is it equally dependent on the concentration of both. Justify your answer. The rate depends more on the concentration of ClO2 than on OH-. For example, comparing experiments 1 and 2 we see that when [ClO2] is doubled and [OH-] held constant the rate increases by a factor of 4. When we compare experiments 2 and 4 when [OH-] is doubled, we see that the rate only increases by a factor of 2. 131 4. If you wanted to know how the rate of reaction depends on the concentration of ClO2 you could compare experiments 1 and 2. But if you compared experiments 1 and 4, you would not be able to accurately see how the reaction depends on the concentration of ClO2. Why? You always need a control--one of the concentrations needs to remain constant. In experiments 1 and 4 both the [ClO2] and [OH-] change. 5. Which two experiments would you want to compare to determine how much the rate of reaction depends upon the concentration of OH-? A) 1 and 4 B) 5 and 6 C) 1 and 6 D) 1 and 3 6. Considering [ClO2] in experiments 1 and 2, complete the following sentence. When [ClO2] increases by a factor of 2, the rate of reaction increases by a factor of 2 to the _2nd_ power. 7. Considering [OH-] in the two experiments you identified in question 5, complete the following sentence. When [OH-] increases by a factor of 2, the rate of reaction increases by a factor of 2 to the _1st_ power. 8. Considering [ClO2] in experiments 2 and 6, complete the following sentence. When [ClO2] increases by a factor of 3, the rate of reaction increases by a factor of 3 to the _2nd_ power. 9. Considering [OH-] in experiments 2 and 5, complete the following sentence. When [OH-] increases by a factor of 3, the rate of reaction increases by a factor of 3 to the _1st_ power. 10. The rate dependence with respect to [ClO2] is said to be second order. Given your answers to questions 6 and 8, explain what “second order” means. Second order means that if the concentration increases by a factor of X, then the rate increases by a factor of X to the 2nd power. 11. The rate dependence with respect to [OH-] is said to be first order. Given your answers to questions 7 and 9, explain what “first order” means. First order means that if the concentration increases by a factor of X, then the rate increases by a factor of X to the 1st power. 12. Can you find the order for a reactant just by looking at the balanced equation? No, you must have experimental data. The orders of reactants are not the same as the coefficients in the balanced equation as can be seen in the balanced equation given at the beginning of the information section. 13. The overall “order” of the reaction for this reaction is third order. Explain how the overall order of a reaction is found. Overall order is found by adding the orders for each of the reactants. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 132 Information: Rate Law Once you know the order with respect to each reactant, you can determine the “rate law” for the reaction. Each reaction has a different rate law. The rate law is a convenient way of expressing how the rate of a reaction depends upon concentration. The rate law for the reaction we have been considering so far is Rate = k[ClO2]2[OH-]1 Each reaction has a rate constant, given the symbol k. As you will soon see, the rate constant can be determined from experiments in a similar fashion to how you determined the orders for reactants. Critical Thinking Questions 14. What is the relationship between the order of the reactant and the exponent for the reactant in the rate law? The order for the reactant equals the exponent in the rate law. 15. Using the data from any of the six experiments in Table 1, verify that the rate constant is 230 1/(M2s). For example, let’s pick experiment 3 to use. The rate is given and so are the concentrations of ClO2 and OH-. Plug these given data into the rate law and solve for k. Use units in your calculation to verify that the units for k are 1/(M2s). Rate = k[ClO2]2[OH-]1 0.00552M/s = k (0.020M)2(0.060M) k = 230 1/M2s 16. Now that you know the rate constant, you can calculate the rate for any concentration of reactants. For example, calculate the rate of reaction when the concentration of ClO2 is 0.32 and the concentration of OH- is 0.42. Rate = k[ClO2]2[OH-]1 Rate = (230)(0.32)2(0.42) = 9.89 M/s Skill Practice 17. In a certain reaction, it is discovered that if the concentration of a reactant is tripled, then the rate of the reaction increases from 0.0670 M/s to 1.809 M/s. What is the order with respect to this reactant? 1.809 ÷ 0.0670 = 27; 27 = 33; since the exponent is a 3, the order is 3rd order. 18. Given the following data, write the rate law for the reaction. Then find the rate constant (include units). H2O2 + 2 HI 2 H2O2 + I2 Experiment [H2O2] [HI] Rate (M/s) 1 0.1 0.1 0.0076 2 0.1 0.2 0.0608 3 0.2 0.2 0.2432 Comparing experiments 1 and 2 we see that the rate increases by a factor of 23 when [HI] doubles. Comparing experiments 2 and 3 we see that the rate increases by a factor of 22 when [H2O2] doubles. Therefore we can write: Rate = k[H2O2]2[HI]3. Rate = k[H2O2]2[HI]3 (plug in data from expt. #1 0.0076 = k(0.1)2(0.1)3 k = 760 1/M4s 133 ChemQuest 44 Name: ____________________________ Date: _______________ Hour: _____ Information: First Order Reactions with One Reactant As we consider what affects the rate of reactions it is desirable to examine how the concentration of a reactant changes with time. Let us consider reactions that have only one reactant and we will further restrict our considerations to first order reactants. As an example, consider the following reaction: SO2Cl2 SO2 + Cl2 Table 1: Experimental data for the decomposition of SO2Cl2 Time (s) [SO2Cl2] 0 0.0250 60 0.0228 120 0.0208 180 0.0190 It can be shown that the natural log of the concentration of a first order reactant varies directly with the time. So in this case ln[SO2Cl2] varies in direct proportion to the time. 1. Using the above data, prove on the graph below that ln[SO2Cl2] = - kt + ln[SO2Cl2]0 is a straight line when graphed. Note the expression [SO2Cl2]0 is the concentration of SO2Cl2 at a time of zero seconds. Label the axes. -3.680 Calculate ln[SO2Cl2] values and plot: Time ln[SO2Cl2] 0 -3.689 60 -3.781 120 -3.873 180 -3.963 -3.730 -3.780 ln[SO2Cl2] -3.830 -3.880 -3.930 -3.980 0 30 60 90 120 150 180 210 240 Time (s) 2. Given this relationship between concentration and time (ln[SO2Cl2] = - kt + ln[SO2Cl2]0), find the rate constant k. Choose values to plug into equation; I'll use at time 120: ln(0.0208) = -k (120) + ln(0.0250) k = 0.00153 1/s Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 134 3. Find the half-life for this reaction. What this means is that you need to find the time it takes for half of the reactant to get used up. Find the time (t) when [SO2Cl2] = 1/2(0.0250) = 0.0125 ln(0.0125) = -(0.00153)(t) + ln(0.0250) t = 453 seconds Information: Second Order Reactions with One Reactant Consider the following reaction: 2 NO2 2 NO + O2. The following experimental data was gathered for this reaction: Table 2: Experimental data for the decomposition of NO2 Time (s) [NO2] 0 0.0370 45 0.0338 90 0.0311 135 0.0288 If you attempted a plot of ln vs. t as you did in question 2 above you would not get a straight line. Instead, for second order reactants, the inverse of the concentration varies directly with time. Critical Thinking Questions 4. Using the following graph and the above data, prove that the following equation yields a straight line. 1 1 = kt + [ NO 2 ] [ NO 2 ]0 37 35 Calculate 1/[NO2] and plot this data: Time (s) 1/[NO2] 0 27.03 45 29.59 90 32.15 135 34.72 33 1/[NO2] 31 29 27 25 0 20 40 60 80 100 120 140 160 Time (s) 5. What is the value of the rate constant, k, for this reaction? 1/0.0311 = k (90) + 1/0.0370 k = 0.0570 1/Ms 135 6. Find the half-life for this reaction. Need to find the time, t, when [NO2] = 1/2(0.0370) = 0.0185 M 1/0.0185 = (0.0570) (t) + 1/0.0370 t = 474 s Skill Practice Question 7. Given the following reaction and table of experimental data, answer the following questions. 2 N2O5 4NO2 + O2 Time (s) [N2O5] 0 0.0200 100 0.0169 200 0.0142 300 0.0120 400 0.0101 500 0.0086 600 0.0072 700 0.0061 a) Is the reaction 1st order or second order with respect to N2O5? How do you know? It is 1st order because the data fits the equation ln[N2O5] = -kt + ln[N2O5]0 much more closely than it fits the 2nd order equation. b) What is the value for the rate constant? Using ln[N2O5] = -kt + ln[N2O5]0 and plugging in data… ln(0.0086) = -k(500) + ln(0.0200) k = 0.0017 1/s c) What is the half life for this reaction? ln(0.0100) = -(0.0017)(t1/2) + ln(0.0200) t = 408 s d) How many seconds are required for the concentration of N2O5 to reach a level of 0.0025 M? ln(0.0025) = -(0.0017)(t) + ln(0.0200) 1223 s Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 136 ChemQuest 45 Name: ____________________________ Date: _______________ Hour: _____ Information: Forward and Reverse Processes When most people think of chemical reactions, they think of reactants being transformed into products. For example, take the reaction of carbon monoxide with hydrogen gas to form methane gas and water vapor: CO (g) + 3 H2 (g) CH4 (g) + H2O (g) If you were watching the molecules of this reaction, you would see that at the very beginning, there is no methane and no water in the container. Soon methane and water would begin to form and then the reaction would appear to stop. However, at this point there would still be some carbon monoxide and hydrogen present. What happens at the molecular level is this: as the products (methane and water) begin to form, they react with each other and begin forming the reactants (carbon monoxide and hydrogen) again. There is a forward reaction and a reverse reaction. The forward reaction is written above. The reverse reaction is below: CH4 (g) + H2O (g) CO (g) + 3 H2 (g) The best way to represent the reaction, then, is as follows: CO (g) + 3 H2 (g) CH4 (g) + H2O (g) The reaction appears to stop when the rate of the forward reaction equals the rate of the reverse reaction. At this point we say that the reaction has reached equilibrium. The reaction is still occurring, but the products and reactants are being formed at the same time so there is no net change in their amounts. Critical Thinking Questions 1. Consider the reaction above involving carbon monoxide and hydrogen. At the beginning of the reaction, there are 2.50 moles of carbon monoxide and 5.10 moles of hydrogen gas placed in a 5.0 L container. Of course, at the very beginning there is no methane or water in the container. When equilibrium is reached, there are 1.02 moles of water in the container. Calculate the number of moles of methane, hydrogen and carbon monoxide in the container. Hint: Calculate the change in the number of moles of water; by how many moles did the water increase? The number of moles of methane increased by this same amount. The number of moles of carbon monoxide decreased by this same amount. The number of moles of hydrogen decreased by three times this amount. We know this because of the coefficients in the balanced chemical equation. Change in moles of H2O, CO and of CH4 = 1.02 because coefficients in balanced equation are equal. Change in moles of H2 = 3(1.02) = 3.06. Therefore the final moles of each are as follows… CH4 = 1.02 mol; H2 = 5.10-3.06 = 2.04 mol; CO = 2.50 – 1.02 = 1.48 mol 137 2. Consider the following reaction: N2 (g) + 3 H2 (g) 2 NH3 (g). Initially, 4.25 moles of nitrogen gas and 6.33 moles of hydrogen gas are placed in a 3.35 L container. At equilibrium, 2.15 moles of NH3 (ammonia) was present. Calculate the number of moles of nitrogen and hydrogen at equilibrium. There were initially 0 moles of NH3, but at equilibrium there were 2.15 moles. From this we can calculate the change in moles of N2 and H2 and then the amount at equilibrium N2 (g) + 3 H2 (g) 2 NH3 (g) Initial moles: 4.25 6.33 0 Change in moles: -1.075 -3.225 +2.15 Final moles: 3.175 3.105 2.15 3. As mentioned already, equilibrium occurs when the rate of the forward reaction equals the rate of the reverse reaction. Assume that all reactions discussed so far are elementary reactions. This means that the exponents in the rate law are the same as the coefficients in the balanced equation. a) Write the rate law for the forward reaction of carbon monoxide and hydrogen. Use kf to symbolize the rate constant for the forward reaction. Rate = kf [CO][H2]3 b) Write the rate law for the reverse reaction of carbon monoxide and hydrogen. Use kr to symbolize the rate constant for the reverse reaction. Rate = kr [H2O][CH4] 4. Now divide the reverse rate law by the forward rate law and complete the following equation. rate reverse = rate forward kr [H2O][CH4] kf [CO][H2]3 5. At equilibrium, the reverse rate equals the forward rate. What does the left side of the equation in question 4 equal? Since ratereverse = rateforward, then ratereverse ÷ rateforward = 1 6. Taking into account your answer to question 5, rearrange the equation that you wrote in number four and get kf and kr on the left side of the reaction and everything else on the right side. kf kr = [H2O][CH4] [CO][H2]3 Information The Equilibrium Constant The equilibrium constant is a constant that allows us to compare the concentrations of products and reactants in a chemical reaction. The equilibrium constant (K) is defined as kf/kr. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 138 Critical Thinking Questions 7. Given your answer to question 6 and also the information above, write the expression for the equilibrium constant for the reaction of carbon monoxide with hydrogen. [H2O][CH4] K= [CO][H2]3 8. Calculate the equilibrium constant for the reaction using your answers to question number 1. You will first need to find the molarity of each reactant and product. You should get a value of 2.07. We need to divide each of the final moles from Q 1 by 5.0L to obtain molarity. [CH4] = [H2O] = 1.02 ÷5= 0.204 M; [H2]=2.04÷5= 0.408 M; [CO]=1.48÷0.296 M [H2O][CH4] (0.204)(0.204) = [CO][H2]3 = 2.07 (0.296)(0.408)3 9. Verify that the equilibrium constant expression for the reaction described in question two can be written as K= [NH 3 ] 2 [ N 2 ][H 2 ] 3 Yes, in general, equilibrium constants are written as the concentration of the products divided by the concentration of the reactants, with each substance raised to the power of its coefficient. 10. Calculate the numeric value of the equilibrium constant from question 9 using your answers and the data in question 2. [NH3] = 2.15mol÷3.35L = 0.642M; [N2] = 3.175mol÷3.35L = 0.948M; [H2]=3.105÷3.35L= 0.927M [NH3]2 (0.642)2 [N2][H2]3 = (0.948)(0.927)3 = 0.546 11. Considering questions 7 and 9, what relationship exists between the coefficients in the balanced chemical equation and the expression for the equilibrium constant? In general, equilibrium constants are written as the concentration of the products divided by the concentration of the reactants, with each substance raised to the power of its coefficient. 12. Write the equilibrium constant expression for each of the following reactions. a) 2 HI H2 + I2 b) 2 CO2 2 CO + O2 [H2][I2] [CO]2[O2] [HI]2 [CO2]2 139 Information: Calculating Equilibrium Constants Consider a 100 L container that holds 80 moles of hydrogen iodide. Over time, the hydrogen iodide decomposes into hydrogen and iodine. At equilibrium, there are 8.84 moles of iodine. The balanced equation for this process is: 2 HI H2 + I2 Critical Thinking Questions 13. Find the initial concentration of HI and the equilibrium concentration of I2. [HI] = 80 ÷ 100 = 0.80 M [I2] = 8.84 ÷ 100 = 0.0884 M 14. Consider the balanced equation for a moment. How will the change in iodine concentration compare to the change in hydrogen iodide? (Hint: it depends on the coefficients in the balanced equation.) The change in HI will be twice the change in I2. 15. What was the initial concentration of I2? Note: “initial” means before any reaction takes place. Zero 16. What was the initial concentration of H2? Zero 17. What was the change in concentration for I2? (Remember that change in concentration is simply the final minus the initial concentration.) 0.0884 M 18. Calculate the change in H2 and the change in HI concentration. ∆ in [H2] = ___0.0884 M__ ∆ in [HI] = (0.0884)(2) = 0.177 M 19. What is the equilibrium concentration (i.e. concentration at equilibrium) of HI? Note the equilibrium concentration of HI is equal to the initial concentration of HI minus the change in concentration. 0.80 – 0.177 = 0.623 M 20. What is the equilibrium concentration of H2? Note the equilibrium concentration of H2 is equal to the initial concentration of H2 plus the change in concentration of H2. 0 + 0.0884 = 0.0884 M 21. In question 19, you subtracted the change in concentration, but in question 20, you added it. Why? Question 19 referred to a reactant and the concentration of a reactant is decreasing with time. Question 20 referred to a product and the concentration of product increases with time. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 140 22. Write the equilibrium constant expression for this reaction. [H2][I2] [HI]2 23. Calculate the equilibrium constant for this reaction. (0.0884)(0.0884) = 0.0201 (0.623)2 24. This problem combines all of the previous eleven questions and asks you to find the equilibrium constant for a reaction. Consider the following reaction: 2 NO + O2 2 NO2. 5.25 moles of NO and 3.15 moles of O2 are combined in a 12.0 L container. At equilibrium 3.20 moles of NO2 are in the container. Verify that the equilibrium constant for this reaction is 18.88. Don’t forget to use molarity instead of moles! Initial concentrations: [NO] = 5.25 ÷ 12.0 = 0.4375 M [O2] = 3.15 ÷ 12.0 = 0.2625 M Equilibrium (final) concentrations: [NO2] = 3.20 ÷ 12.0 = 0.2667 M Changes in concentration: ∆[NO2] = 0.2667 M (because there was zero to start with and at equilibrium there was 0.2667 M) ∆[NO] = ∆[NO2] = 0.2667 M (because coefficients are same in balanced equation) ∆[O2] = ½ (∆[NO2]) = 0.1333 M (because there is a 1:2 ratio in balanced equation.) Equilibrium (final) concentrations: [NO2] = 0.2667 M [NO] = 0.4375 – 0.2667 = 0.1708 M [O2] = 0.2625 – 0.1333 = 0.1292 M [NO2]2 K= [NO]2[O2] (0.2667)2 = (0.1708)2(0.1292) = 18.87 141 ChemQuest 46 Name: ____________________________ Date: _______________ Hour: _____ Information: Equilibrium Constant for Gas Pressure So far we have looked at equations involving gases in terms of the molarity of the gas. For example, if 3 moles of a gas was in a 1.5 L container we used the value 2.0 M in calculating the equilibrium constant. The equilibrium constant, K, when dealing strictly with molarity actually has the symbol Kc. It has been unnecessary to write Kc until now. Molarity is not the only way to express the concentration of a gas in a container. Pressure, for example, may also be used. We know that for a 1.5 L container, the higher the pressure the greater the concentration. When only pressure information about a gaseous reaction we can still calculate an equilibrium constant, Kp. It is calculated in a very similar way as Kc is calculated. Consider the following reaction. N2 (g) + 3 H2 (g) 2 NH3 (g) The equilibrium constant expression, Kp for this reaction is: 2 p NH 3 Kp = 3 pN 2 pH 2 Note: p stands for the partial pressure of each gaseous reactant or product. Critical Thinking Questions 1. Consider the following reaction: 2 NO (g) + Br2 (g) 2 NOBr (g). Write the expression for Kp . [NOBr]2 Kp = [NO]2[Br2] Information: Relating Kc and Kp The values Kc and Kp are related by the following equation: Kp = Kc(RT)∆n R = 0.0821 (L-atm)/(mol-K) or 8.31 (L-kPa)/(mol-K). It is customary to use atmospheres (atm) to measure pressure, so we will use the value 0.0821 for R. T = Kelvin temperature ∆n = the change in the number of moles of gas as the reaction proceeds. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 142 Critical Thinking Questions 2. Verify that ∆n = -1 for the reaction in question 1. (Show how you can obtain -1.) There are three moles of gaseous reactants and only two moles of gaseous products. Therefore, the number of moles of gas has decreased by one; thus, ∆n = -1. 3. Verify that ∆n = -2 for the reaction N2 (g) + 3 H2 (g) 2 NH3 (g). The four total moles of gaseous reactants decreases by two moles to produce two moles of gaseous products. 4. For the reaction referred to in questions 1 and 2 Kc equals 0.45 at 650oC. What is Kp at this temperature? You should get a value of about 0.00594. Kp = Kc(RT)∆n = (0.45)[(0.0821)(923)]-1 = 0.00594 5. For the reaction in question 3, it was found that Kp equals 1.25x10-4 at 425oC. What is Kc at this temperature? Kp = Kc(RT)∆n Kc = Kp ÷ (RT)∆n = 1.25x10-4 ÷ [(0.0821)(698)]-2 = 0.410 Information: Equilibrium Constants from the Sum of Chemical Equations Consider the following two reactions for which the equilibrium constants are known: Reaction 1: CO + 3 H2 CH4 + H2O; K1 = 3.92 Reaction 2: CH4 + 2 H2S CS2 + 4 H2; K2 = 3.3x104 Now consider the following reaction for which the equilibrium constant is unknown: Reaction 3: CO + 2 H2S CS2 + H2O + H2; K3 = ? It is possible to obtain the equilibrium constant K3 from K1 and K2. The following questions will show you how. Critical Thinking Questions 6. Which of the following is the relationship between reaction 3 and reactions 1 and 2? A) Reaction 3 = Reaction 1 + Reaction 2 B) Reaction 3 = Reaction 2 – Reaction 1 7. Write the equilibrium constant expressions for K1 and K2. [CS2][H2]4 [CH4][H2O] K1 = [CO][H2]3 8. Now write the equilibrium constant expression for K3. [CS2][H2O] K3 = [CO][H2O]2 K2 = [CH4][H2S]2 143 9. Consider your answers to questions 6 through 8. Which of the following equations is true: A) K3 = K2/K1 B) K3 = K1 + K2 C) K3 = K1K2 D) K3 = K1/K2 10. Calculate the equilibrium constant K3. K3 = K1K2 = (3.92)(3.3x104) = 1.29x105 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 144 ChemQuest 47 Name: ____________________________ Date: _______________ Hour: _____ Critical Thinking Questions 1. If Kc for a given reaction is very large would there be a large amount of products or reactants in the mixture? Large amounts of products. 2. If Kc for a given reaction is very small would there be a large amount of products or reactants in the mixture? Large amounts of reactants. 3. Offer a mathematical explanation for your answers to questions 1 and 2. Since Kc equals the concentration of products divided by reactants, a large Kc means a large numerator (products) whereas a small Kc indicates a small numerator. Information: The Reaction Quotient The reaction quotient, Qc, is calculated in the same way as you would calculate the equilibrium constant. For the reaction aA + bB cC + dD, the reaction quotient is: [C] c [ D ] d Q= [A] a [ B] b It is important to keep in mind that the reaction quotient does not involve equilibrium concentrations. The concentrations used to calculate Qc are at any time, not just at equilibrium. Critical Thinking Questions 4. Consider the following reaction: CO + 3H2 CH4 + H2O. While carrying out a reaction between carbon monoxide and hydrogen, a scientist analyzed the mixture and found that in the 3.5 L container there were 0.35 moles of CO, 0.42 moles of H2, 0.29 moles of CH4, and 0.38 moles of H2O. What is the reaction quotient for this mixture? [CO] = 0.35÷3.5 = 0.10 M; [H2] = 0.42÷3.5 = 0.12 M; [CH4] = 0.29÷3.5 = 0.0829 M; [H2O] = 0.38÷3.5 = 0.109 M [CH4][H2O] (0.0829)(0.109) Qc = = = 52.3 3 3 [CO][H2] (0.10)(0.12) Information: What Qc Tells Us As a reaction proceeds it will always tend to go toward equilibrium. For example, the equilibrium constant for the reaction described in question 4 is 3.92. The concentration of products and reactants will adjust themselves so that as the reaction progresses until the products divided by reactants (raised to the appropriate power) will equal 3.92. 145 Critical Thinking Questions 5. Given your answer to question 4 and the fact that Kc equals 3.92 for the reaction, what must happen for the reaction to reach equilibrium? A) more products must form B) more reactants must form Qc is 0.75 and it must increase to 3.92 as the reaction proceeds. To increase the value of Q, the concentration of the products must increase. 6. At a certain time during a reaction whose equilibrium constant was 12.5, it was found that the reaction quotient was 4.2. Predict what will happen to the concentration of reactants and products as the reaction progresses. 4.2 must increase to 12.5 by increasing the concentration of products and decreasing the concentration of reactants. 7. At a certain time during a reaction whose equilibrium constant was 0.45, it was found that the reaction quotient was 2.1. Predict what will happen to the concentration of products and reactants as the reaction progresses. 2.1 must decrease to 0.45 by decreasing the concentration of products and increasing the concentration of reactants. 8. Given your answers to questions 6 and 7, complete the following sentences. If Qc is greater than Kc, then the concentration of products needs to __decrease______. If Qc is less than Kc, then the concentration of products needs to ____increase________. 9. Consider the equilibrium reaction of hydrogen gas reacting with nitrogen gas to produce ammonia, NH3. Kc for the reaction is 0.500. A 50.0 L reaction vessel contains 1.00 mol N2, 3.00 mol H2, and 0.500 mol of NH3. Will more NH3 be formed or will more N2 and H2 form as the reaction proceeds? 3 H2 + N2 2 NH3 [H2] = 3.00÷50.0 = 0.0600 M; [N2] = 1.00 mol÷50.0 = 0.0200 M; [NH3] = 0.500÷50.0 = 0.0100 M [NH3]2 Q= 3 [H2] [N2] (0.0100)2 = = 23.1 (0.0600) (0.0200) 3 Since Q is greater than Kc, the amount of product (NH3) needs to decrease and more reactants (H2 and N2) will form. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 146 ChemQuest 48 Name: ____________________________ Date: _______________ Hour: _____ Information: Definitions of Acids and Bases Arrhenius definitions 1) acid: substance that when dissolved in water increases [H+]; (note: H+ exists bonded to water as the hydronium ion, H3O+, so [H+] and [H3O+] are equivalent expressions) 2) base: substance that when dissolved in water increases [OH-] Bronsted-Lowry definitions 1) acid: substance that donates a proton, H+, in a reaction 2) base: substance that accepts a proton, H+, in a reaction Table 1: Equilibrium constants (at 25oC) for some acid-base equilibrium reactions. Reaction Kc 1.07 x 10-11 1. C2H3O2- + H2O HC2H3O2 + OH4.9 x 10-11 2. HCN + SO42- + H2O HSO4- + CN- + H2O 3. HC2H3O2 + H2O H3O+ + C2H3O23.09 x 10-7 + 4. H2CO3 + H2O H3O + HCO3 7.82 x 10-9 5. HCl + H2O H3O+ + Cl2.0 x 104 Critical Thinking Questions 1. List all of the reactants in Table 1 that are Arrhenius acids. HC2H3O2, H2CO3, and HCl 2. List all of the reactants in Table 1 that are Arrhenius bases. C2H3O23. List all of the reactants in Table 1 that are Bronsted-Lowry acids. H2O, HCN, HC2H3O2, H2CO3, and HCl 4. List all of the reactants in Table 1 that are Bronsted-Lowry bases. C2H3O2-, SO42-, H2O 5. Is it possible for an ion to act as a base? Explain. Yes, C2H3O2- and SO42- act like bases in the above reactions. 6. Can a substance act as both an acid and a base under different conditions? Explain. Yes, for example in the above table H2O acts like a base and an acid in different reactions. 7. Is this statement true: “All substances that are Arrhenius acids are also Bronsted-Lowry acids”? Explain. Yes, all of the substances in our answer to question 1 also are found in our answer to question 3. 147 8. If a substance is a Bronsted-Lowry acid, can we conclude that the substance is also an Arrhenius acid? Explain. No because not all of the substances from question 3 are listed in question 1. 9. a) What is the strongest acid among the reactants in reactions 3-5 inTable 1? Explain. HCl because the large Kc indicates that it forms H3O+ in large concentrations. b) What is the weakest acid among the reactants in reactions 3-5 inTable 1? Explain. HCN, because of its very small Kc. 10. Consider Reaction 3. a) What substance is formed (by the acid) after the acid loses a proton? C2H3O2b) Is this substance an acid or a base? (Hint: look at reaction 1.) It is a base since it produces OH- in reaction 1. 11. Drawing a conclusion from question 10, what can be said about a substance after it loses a proton? Is the substance formed acidic or basic? After an acid loses an H+, the substance formed is a base. Information: Conjugate Acid-Base Pairs After an acid loses a proton in a reaction, the substance formed behaves like a base. Verify this by examining Reactions 3 and 1 in Table 1. Notice from reaction 3 that HC2H3O2 is an acid. After it loses a proton it becomes the acetate ion, C2H3O2-. The acetate ion is a base, as seen in reaction 1; there is a special name for this base: it is a conjugate base. So, C2H3O2- is the conjugate base of HC2H3O2. Similarly, HSO4- is the conjugate acid of SO42-. Verify this by examining Reaction 2. Critical Thinking Questions 12. Describe how a conjugate base is formed. It is formed by an acid losing a H+. 13. How is a conjugate acid formed? It is formed by a base gaining a H+. 14. For each of the acids below, write the reaction of the acid with water and circle the formula of the conjugate base in your reaction. a) H2SO4 H2SO4 + H2O H3O+ + HSO4b) HCO3- HCO3- + H2O H3O+ + CO32- c) HF HF + H2O H3O+ + F- 15. For each of the bases below, write the reaction of the base with water and circle the formula of the conjugate acid in your reaction. a) NH3 NH3 + H2O NH4+ + OHb) OH- OH- + H2O H2O + OH- c) NO3- NO3- + H2O HNO3 + OHCopyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 148 ChemQuest 49 Name: ____________________________ Date: _______________ Hour: _____ Information: Measuring Acidity Many people have heard of “pH”, and many people know that it probably refers to acidity, but not very many understand what exactly it measures. The pH is a measure of the concentration of hydrogen ions (H+) in a solution. Recall that in water H+ ions bond to water to form H3O+ (hydronium ions). Keep this in mind because hydrogen (H+) and hydronium (H3O+) are often used interchangeably. The pH of a solution can range from 0-14. The equation for calculating pH is: pH = -log[H+] Critical Thinking Questions 1. A certain solution has a concentration of hydrogen ions of 2.5 x 10-3 M. What is the pH? pH = -log[H+] = -log(2.5x10-3) = 2.60 2. The same solution as question 1 has a concentration of hydroxide ions of 4.0 x 10-12. What is the pOH? pOH = -log[OH-] = -log(4.0x10-12) = 11.40 3. A certain solution has a pH of 5.2. What is the concentration of hydrogen ions? [H+] = 10(-pH) = 10(-5.2) = 6.3x10-6 M 4. The same solution as question 3 has a pOH of 8.8. What is the concentration of hydroxide ions? [OH-] = 10(-pOH) = 10(-8.8) = 1.6x10-9 M 5. Use the information given and your answers to questions 1 and 2 to find the following: a) Divide the pH by the pOH of the solution. 2.60 ÷ 11.40 = 0.228 b) Multiply the pH by the pOH of the solution. 2.60 x 11.40 = 29.64 c) Add the pH and the pOH together. 2.60 + 11.40 = 14.00 d) Multiply [H+] and [OH-] together. 2.5 x 10-3 x 4.0 x 10-12 = 1.0x10-14 e) Divide [H+] by [OH-]. 2.5x10-3 ÷ 4.0x10-12 = 6.25x108 149 6. Use the information given and your answers to questions 3 and 4 to find the following: a) Divide the pH by the pOH of the solution. 5.2 ÷ 8.8 = 0.591 b) Multiply the pH by the pOH of the solution. 5.2 x 8.8 = 45.76 c) Add the pH and the pOH together. 5.2 + 8.8 = 14.0 d) Multiply [H+] and [OH-] together. 6.3x10-6 x 1.6x10-9 = 1.0x10-14 e) Divide [H+] by [OH-]. 6.3x10-6 ÷ 1.6x10-9 = 3937.5 7. Consider your answers to questions 5 and 6. Were there any that gave the same answer (or just about the same answer)? If so, which ones? 5c and 6c: pH + pOH = 14 5d and 6d: [H+]x[OH-] = 1.0x10-14 8. Given your answer to number 7, you should be able to answer the following questions. a) Find the pH of a solution that has a pOH of 4.6. 9.4; pH + pOH = 14 pH = 14 – pOH = 9.4 b) The [H3O+] of a solution is 2.55x10-3 M. What is the [OH-]? 3.92x10-12 M; [H+] x [OH-] = 1.00x10-14 [H+] = 1.00x10-14 ÷ 2.55x10-3 = 3.92x10-12 M 9. What is the pOH of a solution that has a [H+] = 4.22 x 10-3 M. 11.625; pH = -log(4.22x10-3) = 2.375 pOH = 14 – pH = 14 – 2.375 = 11.625 10. A certain solution has a pH of 3.25. a) Find the pOH of the solution. pOH = 14 – 3.25 = 10.75 b) Find the [H+] of the solution. [H+] = 10(-pH) = 10(-3.25) = 5.623x10-4 M c) Find the [OH-] of the solution. [OH-] = 1x10-14 ÷ [H+] = 1x10-14 ÷ 5.623 x 10-4 = 1.778x10-11 M Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 150 11. Find the pH of a 0.035 M solution of HNO3. (Hint: First find the [H+] by realizing that nitric acid dissociates according to this balanced equation: HNO3 H+ + NO3-. If the concentration of HNO3 is 0.035, then [H+] is also 0.035.) pH = -log(0.035) = 1.46 12. Find the pH of a 0.16 M solution of HCl. pH = -log(0.16) = 0.80 13. Find the pH of a 0.045 M solution of NaOH. (NaOH Na+ + OH-) 12.65; pOH = -log[OH-] = -log(0.045) = 1.35; pH = 14 – pOH = 14-1.35 = 12.65 14. Find the pH of a 0.0045 M solution of LiOH. 11.65; pOH = -log[OH-] = -log(0.0045) = 2.35; pH = 14 – pOH = 14-2.35 = 11.65 15. If 14.5 g of NaOH is dissolved in 720 mL of water, what is the pH? 13.702 14.5g ÷ 40 g/mol = 0.363 mol; 0.363 mol ÷ 0.720 L = 0.503 M = [NaOH] = [OH-] pOH = -log[OH-] = -log(0.503) = 0.298 pH = 14 – pH = 14 – 0.298 = 13.702 16. A certain solution contains 0.35 mol of HCl in 1200 mL of water. a) Find the pH and pOH of the solution. pH = 0.535 pOH = 13.465 [HCl] = [H+] = 0.35mol ÷ 1.200L = 0.292 M pH = -log(0.292) = 0.535; pOH = 14 – pH = 14 – 0.535 = 13.465 b) Find the [H+] and the [OH-] in the solution. [H+] = 10(-pH) = 10(-0.535) = 0.292 M [OH-] = 10(-pOH) = 10(-13.465) = 3.428x10-14 M 151 ChemQuest 50 Name: ____________________________ Date: _______________ Hour: _____ Information: Constants and Equations You have discovered two fundamental constants represented by the following equations: 1. pH + pOH = 14 2. [OH-][H+] = 1x10-14 A third equation is related to equation 2 above: 3. (Ka)(Kb) = 1x10-14 This equation holds true for any conjugate acid-base pair. The Ka for the acid multiplied by the Kb for the conjugate base will always equal 1x10-14. The following questions will give you some practice using the above three relationships combined with the equations for pH and pOH that you should already be familiar with. Critical Thinking Questions 1. The Ka for a certain weak acid is 1.8x10-9. What is the Kb for the conjugate base of this acid? Kb = 1x10-14 ÷ Ka = 1x10-14 ÷ 1.8x10-9 = 5.6x10-6 2. The Kb for NH3 is 1.8x10-5. Write the formula for and calculate the Ka for the conjugate acid of NH3. The formula is NH4+ since conjugate acids are formed when a base gains a H+. Ka = 1x10-14 ÷ 1.8x10-5 = 5.6x10-10 3. The pH of a certain solution was 1.45. What was the pOH? pOH = 14 – 1.45 = 12.55 4. If the hydroxide ion concentration of a certain solution was 2.6x10-4 M, what is the pH? pOH = -log(2.6x10-4) = 3.59 pH = 14 – 3.59 = 10.41 Information: Strong Acids and Bases Acids and bases are called “strong” if they dissociate completely (or almost completely) in water. For example, hydrochloric acid (HCl) exists almost completely as H+ ions and Cl- ions in water. Therefore HCl is a strong acid. If you put 2.0 moles of HCl in water, the HCl will dissociate and you will end up having about 2.0 moles of H+ and 2.0 moles of Cl- in the water. Each HCl molecule breaks up into two ions when dissolved: HCl H+ + Cl-. For every mole of HCl added to water, you will get one mole of H+. Critical Thinking Questions 5. Consider a strong acid like HCl. If the concentration of the acid is 0.025 M, what is the concentration of hydrogen ions? Explain your reasoning. 0.025 M because all of the HCl breaks up into H+ and Cl- ions. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 152 6. Consider a strong base such as NaOH. If the concentration of the base is 0.75 M, what is the concentration of the hydroxide ions? Explain. For a strong base, [OH-] = [base]. Therefore, here the [OH-] = 0.75 M 7. What is the pH of a solution that has a concentration of HCl equal to 0.0049 M? [H+] = [HCl] = [0.0049]; pH = -log(0.0049) = 2.31 8. If 2.5 moles of HCl were dissolved in 42 L of water, what would the pH be? What would the pOH be? [H+] = [HCl] = 2.5mol ÷ 42L = 0.595 M pH = -log(0.595) = 0.23 pOH = 14 – 0.23 = 13.77 9. The strong base, NaOH, was dissolved in water. If 4.2 moles of NaOH was dissolved in 245 L of water, what is the pH of the solution? [OH-] = [NaOH] = 4.2mol ÷ 245L = 0.017 M; pOH = -log(0.017) = 1.77 pH = 14 – 1.77 = 12.23 Information: Weak Acids and Bases Weak acids and bases do not dissociate completely in water. For example, consider acetic acid (HC2H3O2): HC2H3O2 H+ + C2H3O2- ; Ka = 1.7x10-5 For HCl, the concentration of HCl equaled the concentration of H+ so finding the pH was easy. However, for acetic acid, [HC2H3O2] does not equal [H+] because not all of the HC2H3O2 dissociates into H+ ions. To find the pH of a solution of acetic acid, you first must calculate the [H+] using the Ka. This will be an equilibrium problem! Once you know [H+], you calculate pH using pH = log[H+]. Critical Thinking Questions 10. What is the pH of a solution that was 0.75 M acetic acid? (Note: 0.75 M is the initial concentration of acetic acid.) a) Find the equilibrium concentration of H+ using the equilibrium constant, Ka, as shown below. Recall that [C2H3O2-] and [H+] will equal “x” and [HC2H3O2] will equal 0.75x. [C H O - ][H + ] Ka = 2 3 2 [HC 2 H 3O 2 ] 1.7 x10 −5 [x][ x] x2 −5 = → 1.7 x10 = → x = 0.036 = [ H + ] [0.75 - x ] 0.75 b) Now that you know the equilibrium concentration of H+, calculate the pH. pH = -log(0.036) = 2.45 153 11. Find the pH of a 0.85 M solution of hydrocyanic acid, HCN, whose Ka = 4.9x10-10. pH = 4.69 [CN-][H+] Ka = (x)(x) = [HCN] 4.9x10 -10 (0.85-x) x2 = x = 2.04x10-5 = [H+] 0.85 pH = -log(2.04x10-5) = 4.69 12. Find the pH of a 0.5 M solution of ammonia, NH3, whose Kb is 1.8x10-5. pH = 11.48 [NH4+][OH-] Kb = 1.8x10-5 = [NH3] (x)(x) x = 0.003 M = [OH-] (0.5 – x) pOH = -log(0.003) = 2.52 pH = 14 – 2.52 = 11.48 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 154 ChemQuest 51 Name: ____________________________ Date: _______________ Hour: _____ Information: Dilutions When water is added to a solution, the concentration decreases. It is often desirable to be able to calculate the concentration of solutions that have been diluted. For doing this, keep in mind that molarity is equal to the moles of solute divided by the total liters of solution. Therefore, the following equations are valid where M is molarity, Lsolution is liters of solution and molsolute is the moles of solute: Equation #1: Equation #2: molsolute M= molsolute = (M)(Lsolution) Lsolution Critical Thinking Questions For the following questions, assume that liquid volumes are additive. 1. A certain solution is prepared by dissolving 4.0 moles of salt (NaCl) in enough water to make 400 mL of solution. Later, the solution was diluted with enough water so that the volume of the solution was 650 mL. Calculate the molarity of the solution before and after dilution. Mbefore = 4.0mol ÷ 0.400L = 10.0 M Mafter = 4.0mol ÷ 0.650L = 6.15 M 2. A 6.0 M solution of salt has a volume of 500 mL. Later, 275 mL of water is added. Confirm that the molarity of the resulting is approximately 3.87 M. (Hint: first find the moles of salt present before the additional 275 mL of water was added by using equation #2 and then find the new molarity using equation #1.) moles of salt = 6.0M x 0.5L = 3.0mol M = 3.0mol ÷ (0.500 + 0.275)L = 3.87 M 3. Calculate the molarity of the solution formed by taking 350 mL of 2.25 M HCl and adding 420 mL of water. mol solute = 2.25M x 0.350L = 0.788 mol M = 0.788mol ÷ (0.350 + 0.420) = 1.02 M 4. Imagine that you have 300 mL of a stock solution of 2.8 M HCl solution. Describe how I could prepare 50 mL of 1.2 M HCl solution by using some of stock solution and diluting it with water. Be specific. mol HCl needed = 1.2M x 0.050L = 0.060 mol (2.8M)(xL) = 0.060 x = 0.0214 L = 21.4 mL Take 21.4 mL of the stock solution and dilute it to 50 mL. 155 Information: Mixing Strong Acids and Strong Bases Usually when we speak of “salt” we mean table salt, which is sodium chloride (NaCl). A salt is a general term for an ionic compound formed when an acid and a base mix. Whenever an acid and a base react, water and a salt are formed. For example consider the following reactions in which nitric acid (HNO3) and hydrochloric acid (HCl) react with the base sodium hydroxide (NaOH): HCl + NaOH NaCl + H2O HNO3 + NaOH NaNO3 + H2O Notice that in each reaction water and a salt (sodium chloride one reaction and sodium nitrate in another) were formed. If equal moles of strong acid and strong base react, then they neutralize each other and form a solution of salt water. If there are more moles of acid than base then the resulting solution will be acidic. If there are more moles of base than acid, then the resulting solution will be basic. Critical Thinking Questions 5. Consider the reaction of 2.5 moles of hydrochloric acid with 1.9 moles of sodium hydroxide. a) If this reaction took place in 2.0 L of solution, what is the concentration of leftover hydrochloric acid after the reaction? mol leftover = 2.5 – 1.9 = 0.6 mol ÷ 2.0L = 0.3 M b) From your answer to part a, verify that the pH of the solution after the reaction is approximately 0.52. pH = -log(0.3) = 0.52 6. Question 5 could be rewritten like this: Consider the reaction of 1.0 L of _2.5_ M hydrochloric acid with 1.0 L of _1.9_ M sodium hydroxide. Fill in the blanks with the appropriate numbers indicating the molarity. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 156 7. 320 mL of 3.1 M HCl is mixed with 240 mL of 4.1 M NaOH. Use the following steps to find the pH of the resulting solution. a) Calculate the moles of HCl and the moles of NaOH that are reacting using Equation #2. mol HCl = (3.1M)(0.320L) = 0.992 mol mol NaOH = (4.1M)(0.240L) = 0.984 mol b) Find out which substance is left over and find out how many moles of this substance is left over. mol HCl leftover = 0.992 – 0.984 = 0.008 mol c) Divide the moles left over by the total volume in liters to get the concentration of the left over substance. 0.008 mol ÷ (0.320+0.240) = 0.014 M d) Your answer to part c is also the concentration of H+ (if the acid is left over) or the concentration of OH- (if the base is left over). From this information calculate the pH of the solution. You should get approximately 1.85 for your answer. [H+] = [HCl] = 0.014M pH = -log(0.014) = 1.85 8. Calculate the pH of a solution formed by mixing 450 mL of 0.79 M HCl with 430 mL of 1.2 M NaOH. Hint: this is very similar to question 7. 13.26 mol HCl = (0.79M)(0.450L) = 0.356mol; mol NaOH = (1.2M)(0.430L) = 0.516mol mol NaOH leftover = 0.516 – 0.356 = 0.16 mol ÷ (0.45+0.43) = 0.182 M = [OH-] pOH = -log(0.182) = 0.74 pH = 14 – 0.74 = 13.26 9. Calculate the pH of a solution formed by mixing 820 mL of 1.2 M HNO3 with 700 mL of 0.9 M NaOH. 0.633 mol HNO3 = (1.2)(0.820) = 0.984 mol; mol NaOH = (0.9)(0.700) = 0.630 mol mol HNO3 leftover = 0.984 – 0.630 = 0.354 mol ÷ (0.700+0.820) = 0.233 M = [H+] pH = -log(.233) = 0.633 10. Consider 400 mL of a 2.5 M HCl solution. How many milliliters of 1.25 M NaOH will be needed to neutralize the HCl? 800 mL mol NaOH must equal mol HCl = (2.5M)(0.400L) = 1.00 mol L = mol ÷ M = 1.00 ÷ 1.25 = 0.800 L 800 mL 157 ChemQuest 52 Name: ____________________________ Date: _______________ Hour: _____ Information: Review Definitions of Acids and Bases Recall the definitions of acids and bases according to Bronsted-Lowry: 1) acid: substance that donates a proton, H+, in a reaction 2) base: substance that accepts a proton, H+, in a reaction Recall also, that we have seen that it is possible for ions to act as acids or bases. We will now examine the acidity or basicity of ions. Critical Thinking Questions 1. If the chloride ion (Cl-) or the cyanide ion (CN-) were dissolved in water, would you expect the ions act as acids or bases? Ask yourself: could it accept a H+? Does it have a H+ to give away? Explain. Since they don’t have a H+ to give away, we can guess that they would act like bases and receive an H+. 2. If the ammonium ion (NH4+) were dissolved in water, would you expect it to act as an acid or base? Explain. We can expect NH4+ to act like an acid since it appears to have at least one H+ to give away. 3. Although not all ions act as acids or bases, we can generalize from the above two questions that IF an ion acts as an acid or a base the following rules will be observed: a) When in water, positive ions may act like ____acids_______. b) When in water, negative ions may act like ____bases_______. Information: Determining Whether an Ion Affects the pH It was mentioned above that not all ions will act as an acid or a base. Some, in fact, will not affect the pH of a solution at all. We are now going to look at what determines whether an ion affects the pH or not. Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 158 Consider the chloride ion. If it affects the pH, it will react with water in the following way: Equation 1: Cl- + H2O HCl + OHNow consider the cyanide ion. If it affects the pH, it will react with water in the following way: Equation 2: CN- + H2O HCN + OHHopefully, these equations confirm what you wrote for the questions above: negative ions, if they affect the pH, will act like bases because they produce OH- ions. In fact, the cyanide ion (CN-) is the conjugate base of the weak acid HCN. The base ionization constants (Kb) for equations 1 and 2 are shown below: K b1 = [ HCl ][OH − ] [Cl − ] K b2 = [ HCN ][OH − ] [CN − ] Critical Thinking Questions 4. The expression for Kb1 assumes that HCl exists in the solution without breaking into ions completely. Similarly, the expression for Kb2 assumes that HCN exists in solution without breaking into ions completely. However, one of these—HCl or HCN—completely breaks up into ions when dissolved. Which substance breaks up completely into ions? Explain. HCl completely breaks up into ions and exists as H+ and Cl- in water. We know this because HCl is a strong acid. 5. One of the ions—Cl- or CN-—does not affect the pH because it will not react as depicted in equation 1 or 2 above. Given your answer to question 4, which ion do you think will not affect the pH of a solution? Cl- will not affect the pH because it will not accept a H+ to form HCl. 6. Complete the following sentence: The negative ions formed from a _____strong________ acid strong/weak will not affect the pH, but the negative ion formed from a _____weak_______ acid will raise the strong/weak pH and act like a(n) _____base________. acid/base 7. Similar reasoning applies to the positive ions formed from strong and weak bases. Using question six as a pattern, complete the following sentence: The positive ions formed from a ______strong______ base will not affect the pH, but the strong/weak positive ion formed from a ____weak______ base will lower the pH and act like a(n) strong/weak _____acid______. acid/base 159 8. Determine whether each of the following will act like an acid (A) or a base (B). If the ion will not affect the pH, place an X in the blank. __X__ a) Na+ (from the strong base NaOH) __ X__ b) NO3- (from the strong acid HNO3) __A__ c) NH4+ (from the weak base NH3) __ X__ d) K+ (from the strong base KOH) __B__ e) NO2- (from the weak acid HNO2) __ B_ f) C2H3O2- (from the weak acid HC2H3O2) 9. Write the chemical equation for acetic acid dissociating. HC2H3O2 H+ + C2H3O2- or HC2H3O2 + H2O H3O+ + C2H3O210. From the Ka of acetic acid (1.7x10-5), calculate the Kb for the acetate ion. Kb = 1x10-14÷1.7x10-5 = 5.88x10-10 11. When ammonia (NH3) reacts with water, the ammonium ion is formed (NH4+). Write the chemical equation for this process. NH3 + H2O NH4+ + OH12. Given the Kb for ammonia (1.8x10-5), calculate the Ka for the ammonium ion. Ka = 1x10-14÷1.8x10-5 = 5.56x10-10 Information: Salts Salts are ionic compounds. They are similar to strong acids and bases because they dissociate completely in water. However, not all salts affect the pH. Consider the salt sodium acetate (NaC2H3O2). If you place 2.0 moles of sodium acetate in 1.0 L of water, it will dissociate completely as follows: NaC2H3O2 Na+ + C2H3O2Each mole of NaC2H3O2 breaks up into a mole of Na+ and a mole of C2H3O2-. Therefore, because you started out with 2.0 moles of NaC2H3O2 in 1.0 L of water, the concentration of Na+ is 2.0 M and the concentration of C2H3O2- is 2.0 M. We have seen already that Na+ will not affect the pH since it is derived from the strong base NaOH. C2H3O2- (derived from the weak acid HC2H3O2) will affect the pH by producing OH- ions: C2H3O2- + H2O HC2H3O2 + OH-. Critical Thinking Questions 13. Determine whether each of the following salts will act like an acid (A) or a base (B). If the salt will not affect the pH, place an X in the blank. __X__ a) NaCl __A__ b) NH4Cl __B__ c) NaCN __X__ d) KNO3 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 160 14. What is the pH of a 0.45 M solution of NaC2H3O2? (Follow the following steps.) a) Will Na+ or will C2H3O2- affect the pH? C2H3O2b) Hopefully your answer to part a is C2H3O2-. Find the concentration of C2H3O2- in the solution. (Note: remember that salts dissociate completely.) [C2H3O2-] = [NaC2H3O2] = 0.45 M c) Now that you know [C2H3O2-]initial complete the necessary equilibrium calculations using the Kb for C2H3O2- (see question 10) to calculate [OH-]. [HC2H3O2][OH-] (x)(x) x2 -10 Kb =5.88x10 = = = x = 1.63x10-5 M C2H3O2(0.45 – x) 0.45 d) Calculate the pOH of the solution. pOH = -log(1.63x10-5) = 4.79 e) From the pOH, verify that the pH is 9.2. pH = 14 – 4.79 = 9.21 ~ 9.2 15. Find the pH of a 0.32 M solution of NH4NO3. 4.88 NH4+ affects pH: NH4+ H+ + NH3 ; Ka = 5.56x10-10 (see question 12) [H+][NH3] (x)(x) x2 -10 Ka = 5.56x10 = = = x = 1.33x10-5 = [H+] [NH4+] 0.32-x 0.32 pH = -log(1.33x10-5) = 4.88 161 ChemQuest 53 Name: ____________________________ Date: _______________ Hour: _____ Information: Internal Energy Thermodynamics involves the study of the energy and disorder of a system. Every system has “internal energy”. Internal energy is the total amount of all the potential and kinetic energy that a system has. For an example of a “system” let’s take a car. A car has kinetic energy (the energy of motion) if it is moving and it has potential energy (chemical energy of the gasoline) if it has gas in the tank. A car always has a certain amount of energy associated with it. A car has more total energy (chemical potential energy) with a full tank of gas than with a half full tank. Therefore, after driving a car for a while the total amount of energy (called the internal energy) that the car has decreases. Critical Thinking Questions 1. If we use KE to symbolize kinetic energy and PE to symbolize potential energy, and U to symbolize internal energy, then write an equation for internal energy. U = KE + PE 2. Consider a car as described in the table below. Fill in the blanks in the table with the hypothetical values for kinetic energy, potential energy, and internal energy. (Assume that the overall mass of the car stays the same even though the amount of gasoline in the tank decreases.) Kinetic Energy Potential Energy Internal Energy A car is setting in the driveway before a long road trip. The gas tank is full. 0 The car has set out on the trip and has been driving for a while. Cruise control is set for a constant speed. 20 100 100 The car is still driving at the same speed. The car ran out of gas and is parked along the highway. 20 0 70 30 0 90 50 0 3. The Law of the Conservation of Energy states that energy isn’t created or destroyed. The car in question 2, lost energy. If the energy was not destroyed, hypothesize what happened to it. The energy changed forms into sound energy, friction, heat, etc. and was lost to the surroundings. 4. What would be the internal energy of the car in question 2 if someone brought enough gas to fill the tank back up completely while the car was stranded at the side of the highway? 100 Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 162 Information: First Law of Thermodynamics As you saw in question 2 above, the internal energy of a system can change. What happens to the energy? The energy merely changes form. In the car example, the internal energy was changed into heat energy as the gasoline burned. Also, some of the internal energy was used to do work by moving the car. The First Law of Thermodynamics states that the change in internal energy of a system equals the sum of the heat and the work. Internal energy of a system can increase or decrease. If a system is heated, it gains internal energy, but if the system loses heat then it decreases in internal energy. If a system does work it loses internal energy, but if work is done on the system then it gains internal energy. Critical Thinking Questions 5. For each of the following, state what the “system” is and also state whether the internal energy of the system would increase or decrease in each situation. a) Lifting a ball from the ground and holding it six feet off the ground. System: ball; internal energy increases b) Heating up a piece of pizza in the microwave. System: pizza; internal energy increases c) The logs in the fireplace are burning. System: logs; internal energy decreases d) An ice cube is melting while it sets on the kitchen counter. System: ice cube; internal energy increases e) Two molecules bond together and heat energy is released (exothermic). System: molecules; internal energy decreases Information: Enthalpy Heat energy is a large factor in how much the internal energy of a system changes. We will now turn our attention to chemical reactions. In your answer to question 5e above you should have identified the two molecules as the “system” and you should have noted that their overall internal energy decreased since they lost heat. Verify that this is correct. The heat energy involved in a chemical reaction is given the name “enthalpy”. The change in heat energy for a reaction is called the change in enthalpy and it is given the symbol ∆H and it has units of kilojoules (kJ). An example of 5e taking place is when one mole of hydrogen molecules reacts with ½ mole of oxygen molecules: Example Reaction 1: H2 (g) + ½ O2 (g) H2O (g) ; ∆Hf = -241.8 kJ In the following reaction, energy is absorbed by the system (endothermic) instead of being released. Example Reaction 2: C (s) + 2 S (s) CS2 (g) ; ∆Hf = 117 kJ Critical Thinking Questions 6. Give the definition for the following terms. a) endothermic: when energy is absorbed by a system b) exothermic: when energy is released by a system 163 7. In the above examples, Example Reaction 1 ∆H is negative and in Example Reaction 2 ∆H is positive. What does the sign on ∆H tell you? If ∆H is negative, the reaction is exothermic because heat is subtracted from the system. If ∆H is positive, the reaction is endothermic because heat is added to the system. 8. In Example Reaction 1, compare the amount of energy that the product has compared to the reactants. The products have less energy because energy was “lost” during the reaction in the form of heat. 9. In Example Reaction 2, compare the amount of energy that the product has compared to the reactants. The products have more energy because energy was “gained” during the reaction in the form of heat. Information: Enthalpy of Formation In Example Reaction 1 and 2, one substance was formed out of two. Whenever one substance is made from elements in their standard states, the accompanying enthalpy change is called the enthalpy of formation, given the symbol ∆Hf. Note the following two example reactions: Example Reaction 3: H2 (g) + S (s) H2S (g); ∆Hf = -20 kJ Example Reaction 4: C (s) + O2 (g) CO2 (g); ∆Hf = -393.5 kJ Notice that in the previous 4 example reactions, each substance formed is involved in the following reaction: Example Reaction 5: 2 H2O (g) + CS2 (g) 2 H2S (g) + CO2 (g); ∆H = ? The ∆H for Example Reaction 5 can be calculated from the data for Example Reactions 1-4 as follows: ∆H = [2(-20 kJ) + (-393.5 kJ)] – [2(-241.8 kJ) + (117 kJ)] = -66.9 kJ Critical Thinking Questions 10. In Example Reactions 1-4, the enthalpy value was given the symbol ∆Hf, but in Example Reaction 5, the enthalpy value was given the symbol ∆H. Why the difference? In reactions 1-4 one substance is made from several substances, which is called a formation reaction. 11. In calculating the ∆H for Example Reaction 5, why was –20kJ multiplied by 2? Why was the – 241.8kJ multiplied by 2? Forming H2O releases 20kJ (see Example Reaction 3) and since there are two H2O molecules present in Example Reaction 5, the -20kJ is multiplied by two. 12. Using a table for standard enthalpies of formation (∆Hf), verify that ∆H for the following reaction is approximately –91.4 kJ. 2 NaF + CaCl2 CaF2 + 2 NaCl -575.4 kJ -795 kJ -1215 kJ -411.1 kJ ∆H = [2(-411.1) + (-1215)] – [2(-575.4) + (-795)] = -91.4 kJ Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 164 13. Again using a table of standard enthalpy values calculate ∆H for each of the following reactions. a) LiCl + NaBr NaCl + LiBr -408 kJ -361 kJ -411.1 kJ -351 kJ ∆H = [-411.1 + (-351)] – [-361 + (-408)] = 6.9 kJ b) MgCO3 + 2 NaCl Na2CO3 + MgCl2 -1112 kJ -411.1 kJ -1130.8 kJ -641.6 kJ ∆H = [-641.6 + (-1130.8)] – [2(-411.1) + (-1112)] = 161.8 kJ c) CH4 + Cl2 HCl (g) + CH3Cl -74.87 kJ 0 kJ 92.31 kJ -83.7 kJ ∆H = [-83.7 + 92.31] – [-74.87 + 0] = -83.48 14. In 13c, you should have noted that ∆Hf for Cl2 is zero. ∆Hf is also zero for O2, Na (s), Mg (s), and other substances. Why do you think ∆Hf is zero for these substances? Cl2, O2, Na, and Mg are in their natural state. We do not speak of forming a sodium atom, for example, because the sodium atom is already formed. 15. Compare the ∆Hf for O2 (g) and for O (g). Why is ∆Hf equal to zero for O2, but not for O? O2 is its natural state because oxygen exists as diatomic molecule. Monatomic oxygen, however, must be formed. 16. Calculate ∆H for the following reaction using a table for standard enthalpy values: 2CO(g)+O2(g)2CO2(g). ∆Hf for CO = -110.5 kJ; ∆Hf for O2 = 0 kJ; ∆Hf for CO2 = -393.5 kJ ∆Hrxn = 2(-393.5) – [2(-110.5)] = -566 kJ 17. Consider the following reactions and ∆H values C + O2 CO2 ; ∆Hf = -393.5 kJ 2 CO CO2 + C ; ∆H = -172.5 a) Using only the above two ∆H values, how can you calculate ∆H for the reaction in question 16? Add the two ∆H together. b) What is the relationship between the two reactions above and the reaction in question 16? If you add the two reactions together you get the reaction from question 16. 18. In question 17a, you used Hess’s Law to find the ∆H for a reaction even though you didn’t know what Hess’s Law is. In your own words, state what you believe is Hess’s Law. When a reaction is formed by adding several reactions together, then the enthalpy change can be found by adding the enthalpy changes for the individual reactions. 165 19. Consider the following reaction: N2 + 2 O2 + 2 NO 2 NO + 2 NO2. Use Hess’s Law along with the following information to find the ∆H for this reaction. reaction #1 N2 + O2 2 NO ; ∆H = 181 kJ reaction #2 2 NO + O2 2 NO2 ; ∆H = -113 kJ Since the reaction is formed by adding reaction #1 and reaction #2, the enthalpy change for the reaction can be found by adding the enthalpy change for reactions #1 and #2. ∆Hrxn = 181 + (-113) = 68 kJ 20. Consider the following reaction: 2 NO N2 + O2 ; ∆H = -181 kJ. What relationship exists between this reaction and reaction #1 in question 19? How could the ∆H for this reaction be determined? The reaction is the reverse of reaction #1 from question 19. Thus, the ∆H value is also reversed in sign. 21. Using only the information presented in question 12, find the ∆H for the following reaction. CaF2 + 2 NaCl 2 NaF + CaCl2 This reaction is the reverse of the reaction in question 12, so we reverse the sign of the enthalpy change from question 12 to get: 91.4 kJ Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 166 ChemQuest 54 Name: ____________________________ Date: _______________ Hour: _____ Information: Spontaneity It is often desirable to know if a process will occur all by itself or if you need to supply energy for a process to occur. If a process occurs naturally, all by itself without outside help, it is said to be spontaneous. Examples of spontaneous processes include iron rusting and a ball rolling down a hill. Critical Thinking Questions 1. Which of the following processes are spontaneous? a) a rock rolling uphill b) a leaf falling c) water boiling at 100oC Information: Entropy It is important to consider the amount of disorder in a system when determining whether a reaction will occur spontaneously. Just as the energy change is given a special name and symbol (change in enthalpy, ∆H), the change in the amount disorder or randomness is also given a special name, “change in entropy”, which has the symbol ∆S. Entropy, S, is a measure of the amount of disorder in a system. When disorder increases, ∆S is positive. Critical Thinking Questions 2. When ∆Hreaction is negative that means that the reaction loses energy to become less energetic. When ∆Sreaction is negative, what is that telling us? The amount of disorder (entropy) of a system decreases when ∆Sreaction is negative. 167 3. Does disorder (entropy) increase or decrease for the formation of gaseous water? 2 H2 (g) + O2 (g) 2 H2O (g) Note the diagrams below in which every molecule is moving randomly. Explain your answer using the diagrams. = O2 molecule = H2O molecule = H2 molecule Entropy (the amount of disorder) decreases when water is formed. As can be seen in the above diagrams, the H2O has fewer individual molecules that are moving randomly. 4. Indicate whether for each of the following processes ∆S will be positive or negative? Explain each answer. (Hint: think in terms of the disorder or randomness of molecules and molecular motion.) a) an ice cube melting ∆S will be positive; entropy increases because the liquid molecules are not organized into a crystalline solid after it melts. b) water vaporizing ∆S will be positive; gases are more disorderly than liquids. c) cleaning your room ∆S will be negative; cleaning a room involves organizing the room, thus decreasing the disorder. d) folding paper into a paper airplane ∆S will be negative; the paper becomes more ordered by folding it in an ordered manner. Information: Comparing Entropy and Enthalpy It was once thought that for a chemical reaction to be spontaneous that it also had to be exothermic (∆H<0). Now, however, we recognize that some endothermic reactions are spontaneous. When deciding whether a reaction is spontaneous, you need to consider both entropy and enthalpy. Note that for any reaction you will have the reaction itself and its surroundings. Both the reaction and its surroundings together are called the universe so that ∆Suniverse = ∆Sreaction + ∆Ssurroundings. Consider the following table: sign of Sign of Sign of Sign of Spontaneous? Reaction ∆Hreaction ∆Sreaction ∆Ssurroundings ∆Suniverse #1 exothermic, + + + yes entropy increase #2 endothermic, + + + or depends entropy increase #3 exothermic, + + or depends entropy decrease #4 endothermic, + no entropy decrease Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 168 Critical Thinking Questions 5. For reaction #2, according to the table, it says that there is an entropy increase. What entropy is it referring to—∆Sreaction, ∆Ssurroundings, or ∆Suniverse? ∆Sreaction 6. Why can ∆Suniverse be + or – for reactions two or three? ∆Suniverse depends on both ∆Sreaction and ∆Ssurroundings, so whichever one is larger in magnitude determines the sign on ∆Suniverse. 7. What is the correlation between the sign of ∆Hreaction and the sign of ∆Ssurroundings? They always have opposite signs. 8. The sign of ∆Hreaction determines the sign of ∆Ssurroundings. Explain why this is true. (Hint: think about what happens to the motion of molecules in the surroundings when ∆Hreaction is positive and when it is negative.) If more heat is sent into the surroundings ( negative ∆Hreaction) then the molecules in the surrounding will move faster and more chaotically. 9. On the table above, the sign of one value determines whether the reactions are spontaneous or not. Which value’s sign determines the spontaneity of a reaction? ∆Suniverse must be positive for a reaction to be spontaneous. 10. Use your answer to question 9 to fill in the blanks. This is a statement of the Second Law of Thermodynamics! For any spontaneous reaction, the amount of _____entropy______ in the universe must enthalpy or entropy ____increase______. increase or decrease 11. In the above information section, the statement was made that “When deciding whether a reaction is spontaneous, you need to consider both entropy and enthalpy.” If only one value (answer to question 9) is needed to determine spontaneity, then why do you still need to consider the enthalpy? The enthalpy helps to determine ∆Ssurroundings and the ∆Ssurroundings helps to determine the ∆Suniverse. Information: Predicting and Calculating Entropy Changes Consider question 3 along with the diagrams of oxygen, hydrogen and water molecules. 2 H2 (g) + O2 (g) 2 H2O (g) When the two gaseous reactants (oxygen and hydrogen) combined to form gaseous water, hopefully you concluded that entropy decreased. Notice that in the balanced chemical equation, there are a total of three moles of gaseous reactants (2 H2 + O2) and only two moles of gaseous products (2H2O). In general, then, whenever the number of moles of gaseous products is less than the number of moles of gaseous reactants entropy will decrease. If there are more moles of gaseous products than gaseous reactants, entropy has increased. 169 Critical Thinking Questions 12. Explain why ∆S is negative for the following reaction. 2 Ca (s) + O2 (g) 2 CaO (s) The disorder decreases because the moles of gas decreases and the gas state is the most disorderly state of matter. 13. For each of the following reactions, predict if the entropy change is positive or negative. If you cannot tell, explain why. A) C2H2 (g) + 2 H2 (g) C2H6 (g) Negative; the total moles of gas is decreasing (moles of gaseous product is less than the moles of gaseous reactants). B) CH3NH2 (g) HCN (g) + 2 H2 (g) Positive; the total moles of gas is increased. C) N2 (g) + O2 (g) 2 NO (g) Since the moles of gas are equal on both sides, we cannot predict this one. 14. Entropy values can be calculated in much the same was as enthalpy values. Using a table of standard entropies, calculate the entropy change for the following reactions. A) N2 (g) + O2 (g) 2 NO (g) 191.5 J 205.0 J 210.65 J ∆S = 210.65 – [205.0 + 191.5] = -185.85 J B) CS2 (g) + 4 H2 (g) CH4 (g) + 2 H2S (g) 186.1 J 205.6 J 237.79 J 130.6 J ∆S = [2(205.6) + 186.1] – [4(130.6) + 237.79] = -162.89 J C) 2 H2 (g) + O2 (g) 2 H2O (g) 205.0 J 188.72 J 130.6 J ∆S = [2(188.72)] – [2(130.6 + 205.0] = -88.76 J Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 170 ChemQuest 55 Name: ____________________________ Date: _______________ Hour: _____ Information: Free Energy So far in our study of thermodynamics, we have seen that in order to determine if a reaction is spontaneous we must examine the entropy change (∆S) and the enthalpy change (∆H) associated with the reaction. A single quantity that combines both of these ideas is called the Gibbs Free Energy, which is given the symbol G. The change in free energy is related to the change in ethalpy for a reaction and the change in entropy for a reaction according to the following equation: ∆G = ∆H - T∆S • T is the temperature in units of Kelvin • ∆H is ∆Hreaction (in Joules or kilojoules) • ∆S is ∆Sreaction (in Joules or kilojoules) • Note: ∆Hreaction and ∆Sreaction must either both be Joules or both kilojoules Recall the following table: Reaction #1 exothermic, entropy increase #2 endothermic, entropy increase #3 exothermic, entropy decrease #4 endothermic, entropy decrease sign of ∆Hreaction - Sign of ∆Sreaction + Sign of ∆Ssurroundings + Sign of ∆Suniverse + Spontaneous? + + - + or - depends - - + + or - depends + - - - no yes Critical Thinking Questions 1. What units must ∆S have if it is to be used in the above equation for free energy? ∆S must have units of kJ/K because T is in units of Kelvin and ∆H is in units of kJ. 2. For a spontaneous reaction, what is the sign of ∆G—positive or negative? Negative 3. For a nonspontaneous reaction, what is the sign of ∆G—positive or negative? Positive 171 4. In the table above, under “Spontaneous” for reactions #2 and #3 it says, “depends”. What does this mean? In other words, what does the spontaneity depend on in reaction #2 and reaction #3? It depends on the magnitude of ∆S and ∆H. ∆G must be a negative value. 5. You have seen that ∆Hreaction can be calculated by the following: ∆Hreaction = [sum of ∆Hf of products] – [sum of ∆Hf of reactants] The ∆S and ∆G for reactions can be calculated in the same way using values from a table of standard values. Use the information in a table of standard values and the following balanced equation to prove that ∆G = ∆H - T∆S. Pay special attention to units and note that each value in the table was determined at 25oC (298 K). CaCO3 (s) CaO (s) + CO2 (g) ∆H = -1206.9 kJ -635.1 kJ -393.5 kJ ∆Hrxn = 178.3 kJ 38.2 J 213.7 J ∆Srxn = 159 J ∆S = 92.9 J ∆G = -1128.8 kJ -603.5 kJ -394.4 kJ ∆Grxn = 130.9 kJ proof ∆G = ∆H - T∆S = 178.3 – 298(0.159) = 130.9 kJ 6. Is the reaction from question 5 spontaneous at 25oC? Explain. No, ∆G is a positive number. 7. By varying the temperature, is it possible to change the spontaneity of the reaction from question 5? Explain. Yes, if the temperature is large enough then T∆S would be larger than ∆H and ∆G would therefore be negative. Information: Coupling Reactions Sometimes in industry we need to carry out nonspontaneous reactions. An example of a nonspontaneous reaction is isolating iron from iron ore. The equation and free energy are given below: 2 Fe2O3 (g) 4 Fe (s) + 3 O2 (g) ; ∆G = 1487 kJ To get this reaction to proceed spontaneously, you can “couple” it with another reaction. One such reaction would be the conversion of carbon monoxide to carbon dioxide. This equation and its free energy is given below: 6 CO (g) + 3 O2 (g) 6 CO2 (g) ; ∆G = -1543 kJ By “coupling” the reactions, we essentially carry out both reactions at the same time and use the spontaneous reaction to drive the nonspontaneous one. The overall ∆G for the process then becomes -56 kJ (found by adding -1543 + 1487). The overall final balanced equation is then: 2 Fe2O3 (g) + 6 CO (g) 4 Fe (s) + 6 CO2 (g) ; ∆G = -56 kJ Copyright 2002-2004 by Jason Neil. All rights reserved. To make copies permission must be obtained from www.ChemistryInquiry.com. 172 Critical Thinking Questions 8. In the overall final balanced equation above, why isn’t O2 written? O2 appears on both sides of the reaction so it is produced and then used up so there is no need to write it in the overall equation. 9. Consider the following reaction to obtain pure aluminum from aluminum ore: Al2O3 (s) + 2 Fe (s) Fe2O3 (s) 2 Al (s) Which of the following two reactions would prove beneficial when coupled with the above reaction? a) CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g) OR b) 2 CH3OH (l) + 3 O2 (g) 2 CO2 (g) + 4 H2O (l) First, calculate the ∆G value for the aluminum reaction: Al2O3 (s) + 2 Fe (s) Fe2O3 (s) 2 Al (s); ∆G = 838 kJ We need to find out which reaction (a or b) has a ∆G greater than -838 kJ so that when the reactions are added, the overall reaction will have a negative ∆G and be spontaneous. a. CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g); ∆G = -800.8 kJ b. 2 CH3OH (l) + 3 O2 (g) 2 CO2 (g) + 4 H2O (l); ∆G = -1404.9 kJ Reaction b should be coupled with the aluminum ore reaction so that the overall ∆G will be negative. 10. Given your answer to question 9, write the overall equation (after coupling) and calculate the ∆G for this overall reaction. Al2O3 (s) + 2 Fe (s) + 2 CH3OH (l) + 3 O2 (g) Fe2O3 (s) 2 Al (s) + 2 CO2 (g) + 4 H2O (l) ∆G = 838 + (-1404.9) = -566.9 kJ

