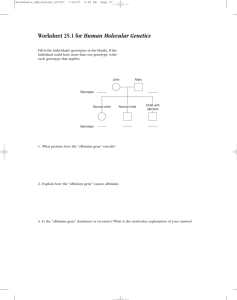
Genetics of Albinism: OCA Types & Melanin Production
advertisement
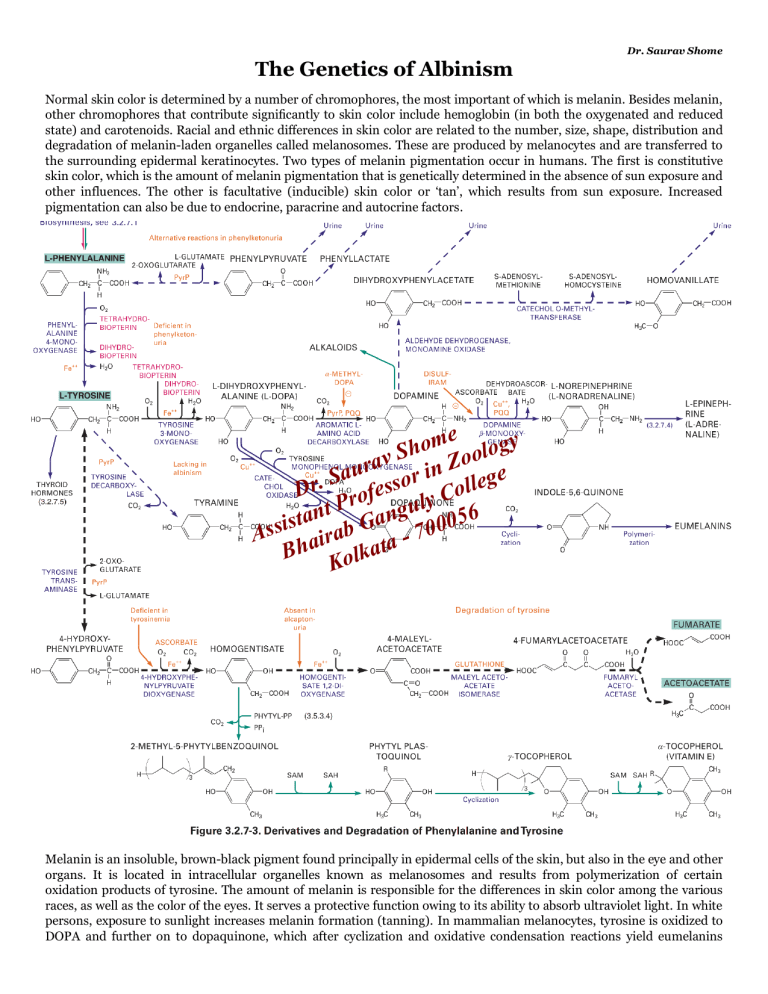
Dr. Saurav Shome The Genetics of Albinism Normal skin color is determined by a number of chromophores, the most important of which is melanin. Besides melanin, other chromophores that contribute significantly to skin color include hemoglobin (in both the oxygenated and reduced state) and carotenoids. Racial and ethnic differences in skin color are related to the number, size, shape, distribution and degradation of melanin-laden organelles called melanosomes. These are produced by melanocytes and are transferred to the surrounding epidermal keratinocytes. Two types of melanin pigmentation occur in humans. The first is constitutive skin color, which is the amount of melanin pigmentation that is genetically determined in the absence of sun exposure and other influences. The other is facultative (inducible) skin color or ‘tan’, which results from sun exposure. Increased pigmentation can also be due to endocrine, paracrine and autocrine factors. ome ology h S rav in Zo e u a r S g Dr. rofesso y Colle t P angul 56 n a t 0 s Assi airab G ta - 700 a Bh Kolk Melanin is an insoluble, brown-black pigment found principally in epidermal cells of the skin, but also in the eye and other organs. It is located in intracellular organelles known as melanosomes and results from polymerization of certain oxidation products of tyrosine. The amount of melanin is responsible for the differences in skin color among the various races, as well as the color of the eyes. It serves a protective function owing to its ability to absorb ultraviolet light. In white persons, exposure to sunlight increases melanin formation (tanning). In mammalian melanocytes, tyrosine is oxidized to DOPA and further on to dopaquinone, which after cyclization and oxidative condensation reactions yield eumelanins (dark pigments of the skin). This reaction is activated by irradiation. If the enzyme is deficient, albinism occurs. Similar DOPA oxidations in plants (by phenoloxidases) lead to darkening of cut fruits or branches. Decarboxylation of tyrosine without previous hydroxylation (e.g., by intestinal bacteria) yields tyramine, which elevates blood pressure. The hereditary inability to produce melanin is known as albinism. The presence of excessive melanin is also a marker of cancers that arise from melanocytes (melanoma). A related series of red polymers, the phaeomelanins found in red hair and feathers, are formed by addition of cysteine to dopaquinone. Albinism is a heterogeneous group of inherited disorders in which absent or reduced biosynthesis of melanin causes hypopigmentation in the hair, skin or eyes. It is found throughout the animal kingdom (from insects to humans). The conditions are broadly classified either as oculocutaneous albinism (OCA) which is more common and affects the eyes, hair and skin, and ocular albinism (OA), which is much less common, involves only the eyes, while skin and hair may appear similar or slightly lighter than that of other family members. Because albinism is a genetic disorder, it can't be cured. Treatment focuses on getting proper eye care and monitoring skin for signs of abnormalities. ➢ The most common type is oculocutaneous albinism (OCA), a family of closely related diseases that are autosomal recessive traits. In OCA, melanin pigment is absent or reduced in the skin, hair follicles and eyes. The frequency of OCA in whites is 1 per 18,000 in the United States and 1 in 10,000 in Ireland. Blacks have the same high frequency of OCA as the Irish. Two major forms of OCA are distinguished by the presence or absence of tyrosinase, the first enzyme in the biosynthetic pathway that converts tyrosine to melanin. Tyrosinase-positive OCA is the most common type of albinism in whites and blacks. Patients typically begin life with complete albinism, but with age, a small amount of clinically detectable pigment accumulates. The skin of all types of albinos is strikingly sensitive to sunlight. Exposed skin areas require strong sunscreens. These patients have greatly increased risk for squamous cell carcinomas of sun-exposed skin. In fact, among a group of more than 500 albinos in equatorial Africa, nearly all succumbed to cancer before age 40. Interestingly, albinos seem to have a below-normal frequency of malignant melanoma. Over the years, researchers have used various systems for classifying oculocutaneous albinism. Seven forms of oculocutaneous albinism are now recognized – OCA1, OCA2, OCA3, OCA4, OCA5, OCA6 and OCA7. Some are further divided into subtypes. • ome ology h S o in an enzyme called tyrosinase (11q14av a genetic Zdefect OCA1, or tyrosinase-negative albinism, results rfrom n u i a r melanosomes. S unpigmented ge Affected people have snow-white 21) and melanocytes are present but contain o e . s l r l s o to an absence of retinal pigment. They Dprominent hair, pale pink skin, blue irises and rofered gpupils, y Cowing l P u typically have severe ophthalmic problems, including photophobia, nystagmus and po or visual t 6 isstrabismus, n the enzyme an In OCA1A, 5 t a acuity. There are two subtypessof OCA1. inactive and melanin is produced, 0 s i G 00 OCA) and OCA-1MP no b OCA1B (yellow 7 a Aslight skin. leading to white hair and very In (minimal pigment OCA), r i ataof melanin is produced, leading to hair that may darken the enzyme is minimally active and Bha asmall oamount k l to blond, yellow/orange or even light brown, K as well as slightly more pigment in the skin. One subtype of OCA1B is called OCA1b TS (temperature sensitive), where the tyrosinase enzyme is with limited activity below 37°C (98°F) and no activity above this temperature and causes the body hair in cooler body regions to develop pigment (i.e. get darker). (An equivalent mutation produces the coat pattern in Siamese cats.) Yellow mutant type albinism is more common among the Amish than in other populations, and results in blonde hair and the eventual development of skin pigmentation during infancy, though at birth is difficult to distinguish from other types. About 1 in 40,000 people have some form of OCA1. Visual acuity is not as severely affected in OCA1B. • OCA2, or P gene albinism, results from a genetic defect in the P protein that helps the tyrosinase enzyme to function. A defect in the OCA2 gene (15q11.2-13), which encodes a melanocyte-specific transporter protein, prevents melanin synthesis. People with OCA2 mutations make a minimal amount of melanin pigment and can have hair color ranging from very light blond to brown. It is the most common form of OCA, accounting for approximately 50% of OCA worldwide. OCA2 was formerly called “tyrosinase -positive” albinism, or “brown OCA.” Inheritance is autosomal recessive. The OCA2 gene encodes an integral melanosomal protein that is important for normal biogenesis of melanosomes and normal processing and transport of melanosomal proteins such as tyrosinase and tyrosinase-related protein 1 (TYRP1). The cutaneous phenotype of OCA2 patients is broad, ranging from near-normal pigmentation to virtually no pigment. Newborns have pigmented hair. Pink irises are usually not seen. Visual defects are not as severe as in OCA1. Pigmentation increases with age, and visual acuity improves from infancy to adolescence. • OCA3 is caused by mutations in the TYRP1 gene. TYRP1 is a melanocyte-specific gene product involved in melanin synthesis, maintenance of melanosome structure and affects melanocyte proliferation and cell death. It also is an essential cofactor for tyrosinase activity. This form of OCA has been most frequently found in African patients and was called “rufous” or red OCA. Patients have red hair and reddish-brown skin. Visual abnormalities may not be detectable. People with OCA3 can have substantial pigment. ➢ • Membrane-associated transporter protein (MATP) also known as solute carrier family 45 member 2 (SLC45A2) or melanoma antigen AIM1 is a protein that in humans is encoded by the SLC45A2 gene. OCA4 is caused by mutations in the MATP (also known as SLC45A2) gene encoding a membrane-associated protein, predicted to span the membrane 12 times and to function as a transporter. Patients are hypopigmented to a variable degree and are phenotypically identical to patients with OCA2. Visual acuity is decreased, and nystagmus is found in many but not all patients. This has also been reported in a Turkish patient, as well as German, Japanese, and Korean OCA patients. People with OCA4 make a minimal amount of melanin pigment similar to people with OCA2. • OCA5 has been mapped to the 4q24. It has been described in a Pakistani family with golden hair, white skin, nystagmus, photophobia, and impaired visual acuity. • OCA6 is caused by mutations in SLC24A5, the gene product of which is a solute carrier protein importan t in melanosomal architecture, linking closely the structure of the melanosome to melanin synthesis. This form of OCA is found in diverse ethnicities, and the phenotype is heterogeneous with hair color from white to blond to dark brown. Most mutations occur in position 111 of the gene, with a Thr111 mutation in European or American OCA6 and Ala111 in African or Asian OCA6. • OCA7 is caused by mutation in the C10 or LRMDA gene (10q22.2-q22.3), the gene product of which is a member of the leucine-rich repeat proteins. The encoded protein is thought to play a role in melanocyte differentiation. Mutations in this gene have been associated with autosomal recessive oculocutaneous albinism 7 (OCA7). Ocular albinism (OA1) is caused by a change in the GPR143 gene that plays a signaling role that is especially important to pigmentation in the eye. This condition reduces the coloring (pigmentation) of the iris, which is the colored part of the eye, and the retina, which is the light -sensitive tissue at the back of the eye. Pigmentation in the eye is essential for normal vision. Ocular albinism is characterized by severely impaired sharpness of vision (visual acuity) and problems with combining vision from both eyes to perceive depth (stereoscopic vis ion). Although the vision loss is permanent, it does not worsen over time. Other eye abnormalities associated with this condition include rapid, involuntary eye movements (nystagmus); eyes that do not look in the same direction (strabismus); and increased sensitivity to light (photophobia). Many affected individuals also have abnormalities involving the optic nerves, which carry visual information from the eye to the brain. Unlike some other forms of albinism, ocular albinism does not significantly affect the color of the skin and hair. People with this condition may have a somewhat lighter complexion than other members of their family, but these differences are usually minor. The most common form of ocular albinism is known as the Nettleship-Falls type or type 1. Other forms of ocular albinism are much rarer and may be associated with additional signs and symptoms, such as hearing loss. OA1 follows a simpler pattern of inheritance because the gene for OA1 is on the X chromosome . The most common form of this disorder, ocular albinism type 1, affects at least 1 in 60,000 males. The classic signs and symptoms of this condition are much less common in females. ome ology h S rav in Zo e u a r S g Dr. rofesso y Colle t P angul 56 n a t 0 s Assi airab G ta - 700 a Bh Kolk Ocular albinism type 1 results from mutations in the GPR143 gene. This gen e provides instructions for making a protein that plays a role in pigmentation of the eyes and skin. It helps control the growth of melanosomes, which are cellular structures that produce and store a pigment called melanin. Melanin is the substance that gi ves skin, hair, and eyes their color. In the retina, this pigment also plays a role in normal vision. Most mutations in the GPR143 gene alter the size or shape of the GPR143 protein. Many of these genetic changes prevent the protein from reaching melanosomes to control their growth. In other cases, the protein reaches melanosomes normally but mutations disrupt the protein's function. As a result of these changes, melanosomes in skin cells and the retina can grow abnormally large. Researchers are uncertain how these giant melanosomes are related to vision loss and other eye abnormalities in people with ocular albinism. Rare cases of ocular albinism are not caused by mutations in the GPR143 gene. In these cases, the genetic cause of the condition is often unknown. Ocular albinism type 1 is inherited in an X-linked pattern. A condition is considered X-linked if the mutated gene that causes the disorder is located on the X chromosome, one of the two sex chromosomes. In males (who have only one X chromosome), one altered copy of the GPR143 gene in each cell is sufficient to cause the characteristic features of ocular albinism. Because females have two copies of the X chromosome, women with only one copy of a GPR143 mutation in each cell usually do not experience vision loss or other significant eye abnormalities. They may have mild changes in retinal pigmentation that can be detected during an eye examination. Researchers have also identified several other genes that result in albinism with other features. Hermansky -Pudlak syndrome (HPS) is a form of oculocutaneous albinism caused by recessive mutations in various autosomal genes [nine human genes (for HPS1, AP3B1 gene, and for HPS 3–9)]. Depending on the affected gene, it can occur with a bleeding disorder, immunodeficiency and/or lung and bowel diseases. Other complex diseases may lead to loss of coloring in only a certain area (localized albinism). These conditions include Chediak-Higashi syndrome (autosomal recessive disorder; mutations in the LYST or CHS1 gene; lack of coloring all over the skin, but not complete); tuberous sclerosis (an autosomal dominant disease; mutations in TSC1 and TSC2 tumor suppressor genes; small areas without skin coloring); Waardenburg syndrome (often a lock of hair that grows on the forehead, or no coloring in one or both irises). Four types of Waardenburg syndrome (WS) exist, with overlapping phenotypic features; three are autosomal dominant, and type IV is autosomal recessive. Six genes are associated with WS. Types I and III are caused by mutations in the PAX3 gene, encoding a transcription factor. Most cases of WS type II are caused by mutations in the MITF gene; however, some, more mildly affected patients with mutations in SOX10, EDN3, EDNRB, and SNA12 may present as WS type II. WS type IV is caused either by a heterozygous mutation in the SOX10 gene or by homozygous mutations in the endothelin-3 (EDN3) or the endothelin B receptor (EDNR3) gene. These mutations impair the ability of melanoblasts to reach their final target sites (inner ear, eye, skin) during embryogenesis. Patients with WS have features of piebaldism, with a white forelock, hypopigmentation, and premature graying, caused by absence of melanocytes in affected areas. ome ology h S rav in Zo e u a r S g Dr. rofesso y Colle t P angul 56 n a t 0 s Assi airab G ta - 700 a Bh Kolk