Endothermic & Exothermic Reactions: Notes & Examples
advertisement

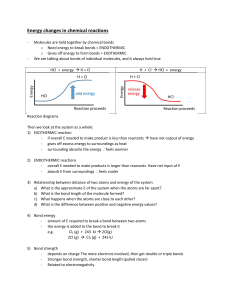
Endothermic and Exothermic Reactions Notes PWC Objective: PS-5 The student will investigate and understand changes in matter and the relationship of these changes to the Law of Conservation of Mass and Energy. Key concepts include: • chemical changes (reaction types, reactants and products, balanced equations) (SOL PS.5c) Essential Question: What are endothermic and exothermic reactions? In all chemical reactions, energy is either released or absorbed. This energy can take many forms: light, sound or electricity. Where does the energy to be released or absorbed come from? When most chemical reactions take place, some bonds in the reactants must be broken. To break chemical bonds, energy must be provided. In order for products to be produced, new bonds must be formed. Bond formation releases energy. One Way to Classify Reactions…All Reactions involve Energy. So, they are either… Endothermic reactions or Exothermic reactions An endothermic reaction is a chemical reaction that requires the addition of heat energy to proceed. The reaction absorbs heat from its surroundings. Takes in heat for reaction to occur (feels cold to the touch because it is taking away your heat). This causes a decrease in temperature. Example: Obtaining metal from its ore. (Aluminum metal is obtained by passing an electric current through molten aluminum ore). Grilling a hamburger, Liquid Icepacks (reaction between water and ammonium nitrate absorbs heat) Photosynthesis – plants make their own food (chemical energy) An exothermic is a chemical reaction that releases energy. Gives off heat for reaction to occur (feels hot to the touch because it is giving additional heat to you). This causes an increase in temperature, Example: The burning of wood, the explosion of dynamite and fireworks, and heat packs. Rusting (proceeds so slowly that it is hard to detect a temperature change. Activation Energy: the minimum amount of energy that must be applied to start a chemical reaction. Without this energy being applied, a reaction will not occur. Examples: Must apply enough energy to strike a match to start the match tip to burn. Gasoline won't burn in air, unless apply heat (spark). Factors affecting How Fast a Chemical Reaction Occurs (Rate of Reaction) 1. Temperature – as temperature increases, amount of molecular motion also increases, increasing the number of molecular collisions and the amount of energy in the collisions. As tempt increases, the speed of the reaction also increases. (Direct relationship) Example: Dissolving sugar in hot vs cold water. Every10oC increase in temp roughly doubles most chemical reactions. 2. Catalyst or Inhibitor – Catalyst lowers the amount of activation E needed to start a reaction, allowing the reaction to occur more easily. Many enzymes (big molecules) in our digestive system down food break down food in about 5 hours rather than the 3-7 days required by the food. Inhibitor slows down a reaction. Food preservatives (salted or smoked foods, chemicals, etc.) 3. Concentration – as the concentration of reactants increases, the distance between reactant molecules decreases and the number of molecular collisions increases. Putting the magnesium strips into stronger HCl (meaning greater # of H3O+ ions) would cause the magnesium to be corroded faster. 4. Amount of Surface Area – decreasing the particle size increases the number of reactant molecules, which increases the number of collisions between reactant molecules. Powdered sugar dissolves much faster than a lump of sugar.