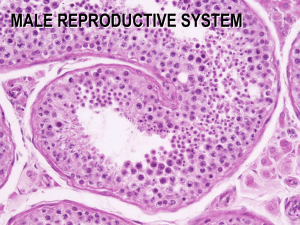
Cell-Cell Interactions in the Testis
advertisement

0163-769X/91/1201-0045$03.00/0 Endocrine Reviews Copyright © 1991 by The Endocrine Society Vol. 12, No. 1 Printed in U.S.A. Cell-Cell Interactions in the Testis* MICHAEL K. SKINNERf Department of Pharmacology, Vanderbilt University School of Medicine, Nashville, Tennessee 37232-6600 genesis, organogenesis, and determination of cell lineage (reviews in Refs. 8-12). Alterations in these cell-cell interactions, therefore, may cause abnormal tissue physiology associated with disease states. Disorganization of normal cell-cell and cell-basement membrane interactions is associated with the process of metastasis and malignancy (13-16). Alterations in cellular differentiation associated with cell-cell interactions will also result in abnormal tissue functions (17, 18). Further examination of the cellular and molecular biology of abnormal tissue physiology is speculated to reveal that many cellular oncogenes (19) and tumor suppressor genes (20) may be associated with cell-cell interactions (21). The increasing awareness of the importance of cellcell interactions has become critical in disciplines such as endocrinology, cell biology, and pharmacology. This is illustrated in the more than 700 literature citations during the past year on the topic of cell-cell interactions. The cellular interactions identified have also increased dramatically in diversity. A categorization of cell-cell interactions is proposed to help classify the different types of interactions as well as understand the interrelationships between the different interactions in the control of tissue function (Table 1 and Ref. 22). Cellular interactions are classified into three general categories of environmental, nutritional, and regulatory interactions. The categorization of cell-cell interactions presented provides a vocabulary with which to distinguish the various types of interactions as well as classify the effects of specific interactions on cellular physiology. The classification of cell-cell interactions (22) shown in Table 1 will be used to more thoroughly discuss the specific cell-cell interactions in the testis. I. Introduction A. Concept and categorization of cell-cell interactions B. Testis cell biology and function C. Analysis of cell-cell interactions II. Specific Cell-Cell Interactions A. Sertoli cell-germinal cell interactions B. Peritubular cell-Sertoli cell interactions C. Sertoli cell-Leydig cell interactions D. Leydig cell-peritubular cell interactions E. Additional cell-cell interactions III. Summary A. Testicular cell-cell interactions B. Endocrine regulation of testis function C. Future directions I. Introduction A. Concept and categorization of cell-cell interactions T HE ability of cells to interact and communicate is a biological phenomenon that developed with the evolution of multicellular organisms. The cell-cell interactions that have evolved are essential for the survival of both simple organisms such as dictyostelium and complex organisms such as mammals. These cellular associations allow an organism to develop capacities that are greater than the simple sum of their individual parts. As early as 1878, Claude Bernard proposed that the "milieu in.terieur" (i.e. internally produced fluid environment) and a cybernetic-like control system (i.e. cell-cell interactions and communication) in multicellular organisms are needed to adaptively regulate the growth, development, and maintenance of normal tissue function (1, 2). The experimental analysis of cellular interactions was initiated with the investigation of cell aggregation in simple organisms such as sponges (3, 4) and progressed into the areas of embryology (5) and cell biology (6, 7). Analysis of cell biology on a molecular level has revealed the importance of cell-cell interactions during embryo- B. Testis cell biology and function The observation that early man domesticated animals through castration implies that the testis is likely one of the first organs investigated on a physiological level. This is supported by reference to the testis in ancient Greek, Roman, Egyptian, and Assyrian writings (23-26). Scientists engaged in the developing discipline of anatomy retained this interest in reproduction and Address requests for reprints to: Dr. Michael K. Skinner, Department of Pharmacology, Vanderbilt University School of Medicine, Nashville, Tennessee 37232-6600. * Experiments presented in this paper were supported in part by NIH Grant HD-20583. f PEW Scholar. 45 SKINNER 46 TABLE 1. Categorization of cell-cell interactions Classification Definition Examples/mediators Environmental Interactions that influence the extracellular environment of the cell to affect cell contacts and cytoarchitecture Extracellular matrix: cell adhesion molecules Nutritional Interactions involved in the delivery of essential nutrients between cells Transfer of energy metabolites, metals, or vitamins Regulatory Agents provided by a cell that through a signal transduction event regulates another cell's function on a molecular level Paracrine/autocrine factors: growth factors; differentiation factors; cytokines investigated the details of testis and sperm cell structure, which played a role in the development of the cell theory (27-30). The advent of the light microscope facilitated anatomical analysis of the testis with often surprising detail (31-33). More recently, investigators (34-39) have explored the ultrastructure of the testis by means of the electron microscope. Comparative anatomy of the testis of a number of species indicates that although differences can be observed, the process of spermatogenesis and cellular associations are similar in many animals (40, 41). In most mammals the process of spermatogenesis occurs within seminiferous tubules that release spermato- Vol. 12, No. 1 zoa into a rete testis which is connected to the epididymis (Fig. 1). The seminiferous tubules are formed by the Sertoli cells that provide structural support for the developing germinal cells. Peritubular myoid cells surround the tubule and are in contact with the basal surface of the Sertoli cells. In the interstitium of the testis between tubules are the Leydig cells responsible for the production of androgen. The majority of research on the cell biology of the testis has focused on Sertoli, peritubular myoid, Leydig and/or developing germinal cells. Cell-cell interactions between all three of the somatic cells are possible; however, Sertoli cells appear to be the primary somatic cell to directly interact with the developing germinal cells. The cellular associations and interactions illustrated in Fig. 2 will be reviewed. In addition to investigation of the anatomy of the testis, the observation of Smith in 1930 that removal of the pituitary inhibited the process of spermatogenesis (42, 43) initiated an intense investigation of the endocrine regulation of testis function that continues today (44, 45). Initial speculation was that hormones acted directly on germinal cells (46-49). Subsequent research and consideration of testis physiology, however, demonstrated that the actions of hormones are primarily confined to the somatic cells (review in Refs. 50 and 51). The appreciation that the endocrine hormones, particularly androgens, acted directly on the somatic cells of the testis, and not the germinal cells, led to initial speculations reviewed by Fritz in 1978 (50) regarding the potential importance of cell-cell interactions in the endocrine regulation of testis function. Recent studies have identified numerous secretory products of specific testicular cell types. These products range from steroids to specific secreted proteins. Inter- Seminiferous Tubules Leydig ^ ~ ^ L Peritubular Sertoli Testis FIG. 1. Cell biology of the testis. CELL-CELL INTERACTIONS IN THE TESTIS February, 1991 47 TABLE 2. Major Sertoli cell secretory products Peritubulor Secretory product Transport/binding proteins ABP (60-62) Transferrin (63) Ceruloplasmin (64) Sulfated glycoprotein-1 (65-67) Function and/or characteristics Androgen transport/localization Iron transport Copper transport Sphingolipid binding (68) Germinal FIG. 2. Cell-cell interactions in the testis. estingly, most of the secretory products identified appear to be involved directly or indirectly in cell-cell interactions. For this reason, the major secretory products of Sertoli cells, Leydig cells, and peritubular cells will be categorized and tabulated for subsequent reference. The secretory products of Sertoli cells and their proposed functions have previously been reviewed (37, 50, 52-59). The major Sertoli cell products (60-102) are shown in Table 2 and include transport/binding proteins, proteases, extracellular matrix components, growth factors, and cellular metabolites. These Sertoli cell secretory products will subsequently be discussed in reference to specific cell-cell interactions. The critical secretory role of the Leydig cell was established with the early observations that Leydig cells are the primary site of androgen production (103,104) which is under endocrine control (reviews in Ref. 105-107) and essential for the maintenance of spermatogenesis and the endocrine status of the male. Nonsteroidal Leydig cell secretory products have not been thoroughly investigated. Although few secretory products have been identified and/or characterized, the major secretory products known (Table 3) appear to be involved in cell-cell interactions. The peritubular myoid cell has also recently been shown to secrete components that may be important for the maintenance and control of testicular function. As found with Sertoli cells and Leydig cells, the secretory products of peritubular cells identified (Table 3) also appear to be involved in cell-cell interactions. Proteases/inhibitors Plasminogen activator (72, 73) Cyclic protein-2 (75-76) a2-Macroglobulin (78) Extracellular matrix components Laminin (79) Collagen IV and I (79) Proteoglycans (80) Growth factors TGFa (81) TGF/3 (82) IGF-I (83-86) IL-1 (88) Regulatory proteins Inhibin (93) Mullerian duct inhibitory agent (94) Sulfated glycoprotein-2 (95, 96) Metabolites Lactate/pyruvate (89-91) Estrogen (92) Potential correlates S70 (69) Band 4 (70) Testibumin (71) Activate plasminogen (74) Cathepsin activity (77) Protease inhibitor Extracellular matrix component Extracellular matrix component Extracellular matrix component Growth stimulation Growth inhibition Maintenance growth/differentiation Growth regulation SC-EGF (87) Endocrine/paracrine agent Fetal Sertoli cells development Sperm coating (66) DAG-protein (65-66) Immunosuppressant Clusterin (99-101) (97, 98) S45:S35 (69) CMB-21 (102) Energy metabolites Steroid hormone C. Analysis of cell-cell interactions A number of different experimental approaches can be taken to investigate cellular interactions and identify potential autocrine and paracrine agents (Table 4). A thorough examination requires a number of criteria to be considered including localization, production, secretion, characterization, action, and physiology. Few testicular cell interactions examined have considered all the criteria listed in Table 4. Therefore, examination of cellcell interactions in the testis requires a critical consideration of the data available to determine which cellular interactions are simply speculated to exist us. those more thoroughly established as important physiologically. As demonstrated with many organs and tissues (121— 123), the advent of cell culture procedures has provided 48 SKINNER TABLE 3. Major Leydig cell and peritubular cell secretory products Function and/or characteristic Secretory product Leydig cells: Androgen (103, 104) POMC peptides (108-112) Inhibin (113) IGF-I (114) Peritubular cells: PModS (117-119) Plasminogen activator inhibitor (120) Fibronectin (115-116) Collagen I (79) Proteoglycans (80) TGFa (81) TGF|3 (82) IGF-I (114) Steroid/endocrine/paracrine agent Opiods/POMC regulatory agents Endocrine/paracrine regulatory agents Maintenance growth/differentiation Paracrine regulatory agent Inhibition of plasminogen activator activity Extracellular matrix component Extracellular matrix component Extracellular matrix component Growth stimulation/EGF-like Growth inhibition Maintenance growth/differentiation a significant advance in an understanding of the molecular and cellular aspects of testis function. Initially organ culture of the testis was utilized (124-126); however, individual cell types were subsequently isolated and cultured to provide more definitive information regarding their functions and hormone responses. Methods have been developed to isolate purified preparations of Leydig cells (127-134), germinal cell populations at various stages of development (135-146), Sertoli cell populations (147-160), and peritubular myoid cells (161-165). Histochemical and immunocytochemical procedures have been developed to assess the purity of cell preparations (161, 162, 165, 166). Several cell lines have been developed for Sertoli cells and Leydig cells (167-169) which TABLE 4. Considerations for potential agents involved in cell-cell interactions Criteria Localization Production Secretion Characterization Action Physiology Method Immunohistochemistry Gene expression/protein synthesis Presence in conditioned medium/biological fluids Protein structure/purification RNA/DNA sequence Bioactivity/pharmacology In vivo analysis Observations/ information Presence of agent Local site production Extracellular actions/soluble agent Novelty of agent Site action/function Function/relevance Vol. 12, No. 1 are useful for investigation of parameters such as signal transduction events or when a product of interest is produced by the cells. The majority of experiments regarding cell-cell interactions in the testis have used primary cell cultures. When an in vitro experimental approach is utilized it is inevitable and appropriate that questions regarding physiological relevance be raised (170). Eagle (171) provided insight early in the establishment of in vitro cell culture procedures when he stated "the study of discrete cells will not suffice to explain the interaction of many different cell types associated into organs which in turn exercise a mutual control." Therefore, a combination of an in vitro analysis to investigate molecular parameters and an in vivo analysis to investigate physiological parameters will be most useful to elucidate cell-cell interactions. II. Specific Cell-Cell Interactions Consideration of cell-cell interactions in the testis began with the identification of multiple testicular cell types which initiated studies that have continued for the past 100 yr. The increased amount of research in the area of cellular interactions in the testis over the past 20 yr can be illustrated by the number of literature citations obtained from a National Library of Medicine literature search on the general topic of cell-cell interaction in the testis (Fig. 3). A significant and continuous rise in the number of citations per year is apparent. For this reason there have been a number of informative reviews (22, 50, 51, 172-191) that address selected cellular interactions, individual agents involved in specific cellular interactions, or the research associated with a given laboratory. A. Sertoli cell-germinal cell interactions The early microscopic examination of spermatogenesis and the development of the spermatozoa within the seminiferous tubules were active areas of research between 1830 and 1860 (192). Although the concept of a support cell for the developing germinal cells was discussed, this idea was not seriously considered by figures such as Koelliker who suggested the presence of polygonal cells as a paved epithelium. In 1865, however, Enrico Sertoli (193) described the existence of special branched cells in the seminiferous tubules of the human testis. Sertoli postulated "the function of the branched cells is linked to the formation of spermatozoa." Further analysis of the Sertoli cell and its association with the developing germinal cells led to the concept reviewed by Peter in 1899 (31) that this "nurse cell" is essential for the germinal cell and the process of spermatogenesis. The research that has progressed since that time has refined these observations and provided insight into the molec- February, 1991 CELL-CELL I CELL-CELL TESTIS CITATIONS 60 | o> 50 / / 40 - o / £ 30 »— « 2° • UJ / CQ | 10 0 0 70 75 80 85 YEAR 90 FIG. 3. Number of citations per year derived from a National Library of Medicine literature search on the topic of cell-cell interactions in the testis. ular mechanisms that Sertoli cells utilize to influence germinal cell development. Environmental. The cytoarchitectural arrangements between Sertoli cells and the developing germinal cells provide one of the most complex examples of an environmental cell-cell interaction. The columnar and convoluted structure of the Sertoli cells, which extends from the basal to the apical surface of the seminiferous tubule (review in Ref. 37), provides the physical support for the spermatogonia undergoing mitosis, the spermatocytes undergoing meiosis, and the spermatids undergoing the process of spermiogenesis to become spermatozoa (reviews in Refs. 194-196). The Sertoli cell is also required to maintain the germ cell syncitium that connects all the cells derived from an initial clone of cells. One of the initial environmental interactions postulated to be important was identified between Sertoli cells near the base of the epithelium as tight and gap junctions referred to as junctional specializations (197-199). These tight junctions were found to exclude the passage of macromolecules from the interstitial space to the lumen of the seminiferous tubules (200-202). These observations provided support for the existence of a blood-testis barrier initially postulated from an examination of the ionic, amino acid, and protein content of testicular fluids from the tubule, lymph, and plasma (203-206). The environmental interactions between Sertoli cells mediated by this tight junction, therefore, are essential in the creation of the blood-testis barrier and the maintenance of the unique microenvironment within the seminiferous tubule required for germinal cell development (207). Specific types of environmental interaction identified between Sertoli cells and germinal cells include the ec- 49 toplasmic specializations (208-210). These are flattened cisternae of the Sertoli cell endoplasmic reticulum that appear in Sertoli cell membranes facing spermatocytes and the acrosomes of the spermatids (197-199, 211-214). These junctional specializations have generally been considered attachment devices (198,199, 208, 215). Another specific environmental interaction identified between Sertoli cells and germinal cells is referred to as the tubulobulbar complex (209, 216, 217). This is a narrow tubular projection of plasma membrane from the heads of maturation-phase spermatids that invaginate the adjacent plasma membrane of Sertoli cells (218, 219). The functional significance of these structures remains to be determined (220). In an elegant set of experiments designed to investigate the complexity of the structural interactions between Sertoli cells and germinal cells, a three-dimensional structure of an individual Sertoli cell was determined (212, 221, 222). These studies of Russell and colleagues demonstrated that an individual Sertoli cell can be in contact with 5 adjacent Sertoli cells at the basal surface of the cell and 47 adjacent germinal cells at various stages of development. The morphology of the Sertoli cell, therefore, is exceedingly complex, and the ability of an individual cell to be in contact with greater than 50 adjacent cells indicates the importance of environmental interactions between the cells. The presence of various generations of spermatocytes and spermatids derived from individual spermatogonia (223) initiates waves of spermatogenesis that are not random but instead occur in a specific cyclic manner referred to as stages (224). Progression of these stages of germinal cells is referred to as the cycle of the seminiferous epithelium and suggests potential alterations in cellular function during this cycle (187, 224-228). Changes that occur in Sertoli cell size (229, 230) and lipid content (225, 231, 232) during the cycle have been examined. Transillumination procedures to microdissect different stages have been developed (233, 234) and used to investigate biochemical aspects of the cycle (review in Ref. 187). Comparison of stages in rodents us. humans (235) implies that the dynamics of the cycle can vary between species. Experiments recently designed to manipulate the cycle of the seminiferous tubule were based on the dependence of spermatogenesis on retinoids (236239). Vitamin A deficiency causes an arrest at the preleptotene stage of spermatogenesis (238, 240). Morales and Griswold (241) found that replacement of vitamin A to deficient animals restores spermatogenesis in a synchronized stage-specific manner. All the seminiferous tubules in a synchronized testis appear to be at the same stage of the cycle, which provides a technical advance to study the cycle of the seminiferous epithelium as well as 50 SKINNER environmental interactions between the Sertoli cells and germinal cells (241-243). The continuous nature of germinal cell development and the presence of the cycle of the seminiferous epithelium indicate that environmental interactions between Sertoli cells and germ cells are dynamic and will require a rapid rate of tissue remodeling. The initial aspect of tubule remodeling considered was the need to translocate early stage spermatocytes from the basal compartment to the apical compartment through the tight junctional complex (244-248). The production of plasminogen activator and other protease activities by Sertoli cells (72, 77, 249) may be needed in several aspects of tissue remodeling associated with translocation of spermatocytes, degradation of junction complexes, and release of mature spermatozoa into the lumen of the tubule. The function and control of the proteolytic activities in the tubule requires further investigation. The presence of junctional contacts between Sertoli cells and germinal cells observed microscopically suggested that these environmental interactions may be needed for cell attachment and association (250, 251). Germinal cell shape and junctional interactions can be maintained in vitro (252), and the viability of spermatogenic cells is facilitated by coculture with Sertoli cells (253). The binding of germ cells to Sertoli cells is specific and temperature dependent (254). Proteins on the surface of spermatogenic cells potentially involved in the environmental interaction with Sertoli cells have been identified (255, 256), and specific Sertoli cell-secreted proteins have also been speculated to be involved in this attachment process (257). Sertoli cells also must adhere firmly to the membrane of the residual bodies of released spermatozoa to prevent their loss from the surface of the seminiferous epithelium (215). These residual bodies must be freed from the released spermatozoa, remain attached to the Sertoli cells (209, 258), and subsequently be phagocytized and metabolized by the Sertoli cells (227, 259). Nutritional. The transport of essential nutritional components between Sertoli cells and germinal cells is critical for germ cell survival and metabolism. The earliest reference to the Sertoli cell was with regard to its probable "nurse cell" function for the developing spermatogenic cells (31, 193). A number of Sertoli cell functions have been identified on a molecular level that are directly or indirectly involved in the nutritional support of the germinal cells. These nutritional interactions between Sertoli cells and germinal cells are required due to the presence of the blood-testis barrier (207, 260, 261). One of the major functions of the Sertoli cells that has evolved is the ability to transport essential nutritional compo- Vol. 12, No. 1 nents to the spermatogenic cells sequestered in a serumfree unique microenvironment. Early during fetal development, junctional interactions between the germ cells and immature Sertoli cells may facilitate transfer of nutritional agents between the cells (262-265). In the adult testis a complex interaction, referred to as a desmosome-gap junction, forms between Sertoli cells and germ cells (198, 212, 263, 266, 267). These junctions appear to form primarily between Sertoli cells and pachytene spermatocytes and are less frequent with spermatids (212). These junctional complexes are permeable to agents with mol wt less than 600-700 (263) and appear to transfer metabolic substances such as choline between Sertoli cells and germ cells (268). Due to the existence of the germ cell syncitium and cytoplasmic bridges between germinal cells, junctional contacts may not be necessary between each spermatogenic cell and Sertoli cell. Further examination of the functional importance and the components transported by gap junctions between the Sertoli cells and germinal cells is required. Developing spermatogenic cells sequestered within the blood-testes barrier are not exposed to the high glucose levels in the interstitial fluid. Localization of metabolic enzymes such as ATPase in the tubule (269) and demonstration that Sertoli cells produce glucose metabolites such as inositol (270) suggested that Sertoli cells may provide energy metabolites to germinal cells. Sertoli cells have been shown to metabolize glucose to lactate and pyruvate resulting in high levels of extracellular metabolites (89-91, 271) that increase in response to FSH (90, 91, 272-274). Both pyruvate and lactate were found to support germinal cell metabolism and appear to provide an efficient energy source for the cells (90, 91, 272, 275277). Analysis of metabolic enzymes, such as lactate and malate dehydrogenases (278), and the actions of FSH on glucose transport by Sertoli cells (279) also suggest that Sertoli cells may provide energy metabolites to spermatogenic cells. Whether the lactate and pyruvate can be transported by gap junctions between the cells or by active transport across the plasma membranes remains to be determined. Although the physiological significance of pyruvate and lactate production by Sertoli cells remains to be elucidated, the sequestration of the developing germinal cells within the blood-testis barrier suggests that the Sertoli cells will likely be required to provide an energy source for spermatogenic cells. Other cellular metabolites, such as coenzymes, nucleotides, and amino acids, may also require a nutritional interaction between these cells to support the process of spermatogenesis. Although many nutritional components are small soluble molecules that can be transported by junctional interactions or secreted, a large number of substances February, 1991 CELL-CELL INTERACTIONS IN THE TESTIS FlG. 4. Binding protein-mediated nutritional interaction between Sertoli cells and germinal cells in the seminiferous tubule. Serum-derived binding protein (sBP) with a nutritional component (*) bound (BP-*) and transfer with a Sertoli cell-derived testicular binding protein (tBP). require binding proteins to facilitate transport due to low solubility or reactive chemical properties. Functional identification of Sertoli cell-secreted proteins has revealed the production of a number of different transport proteins (reviews in Refs. 53-56). The Sertoli cells appear to acquire essential nutritional components at the basal surface of the cell from specific binding proteins present in the interstitial fluid. The serum binding proteins can enter the cell and transfer the nutritional component to a newly synthesized binding protein. The testicular binding protein can then be secreted by the Sertoli cell and transport the nutritional component to developing germinal cells sequestered within the blood-testis barrier (Fig. 4). The first Sertoli cell transport protein identified was androgen binding protein (ABP) (Refs. 60+62; review Ref. 53). Although ABP is a useful marker of Sertoli cell function (280), the transport of androgen to germinal cells is not required (50). The postulated function for ABP is to localize the transport androgens throughout the male reproductive tract; therefore, ABP does not appear to mediate a nutritional interaction between Sertoli cells and germinal cells. An abundant Sertoli cell secretory product functionally identified is testicular transferrin (63). Transferrin is an iron-binding protein, previously thought to be primarily produced by the liver, that binds and transports iron in the circulatory system. Iron is bound by transferrin with an extremely high affinity due to the low solubility of iron. All cells require 51 iron for cytochrome activity and respiration (281). The transferrin produced by Sertoli cells is the same gene product as serum transferrin produced by the liver with differences in glycosylation of the two proteins (282, 283). Transferrin delivers iron to the cell through a receptor-mediated process. Transferrin and the receptor complex are endocytosed to an acidified vesicle where iron is released, and the transferrin and receptor complex is recycled to the cell surface (284, 285). Transferrin receptors are present on all cells, and high concentrations of transferrin receptors have been localized on pachytene spermatocytes (286, 287). The observations from a number of laboratories support this proposed transferrinmediated nutritional interaction between Sertoli cells and germinal cells (63, 287-292). Another metal binding and transport protein found to be synthesized and secreted by Sertoli cells is ceruloplasmin (64). Ceruloplasmin is a copper transport protein, also thought to be primarily produced by the liver, that binds and transports copper to cells that require copper as a coenzyme for proteins and feroxidase (293, 294). A nutritional interaction is proposed for ceruloplasmin that is similar to transferrin with the exception that the ceruloplasmin receptor complex appears to be degraded upon internalization. The nutritional interaction between Sertoli cells and germinal cells involving ceruloplasmin is not as thoroughly characterized as the nutritional interaction mediated by transferrin. Lipids are another nutritional component that generally require a binding protein for transport. Several unique fatty acids have been identified in Sertoli cells and germinal cells, and the potential transport of these fatty acids from Sertoli cells to germinal cells has been postulated (295, 296). Although the production of general lipid transport proteins by Sertoli cells has not been thoroughly investigated, one specific lipid binding protein has been identified. A major Sertoli cell-secreted protein referred to as sulfated glycoprotein-1 (SGP-1) (66, 67) has been identified as a unique form of a sphingolipid-binding protein involved in sphingolipid solubilization (67, 68). The precursor form of sphingolipid activator protein that is involved in sphingolipid binding and metabolism (68) has been shown to have a high degree of homology with SGP-1 (67, 68). The lipid specificity of SGP-1 is unclear, and the possibility that SGP1 may be involved in more general lipid binding remains to be investigated. The rapid expansion of the germ cell population during spermatogenesis is anticipated to require the transport of lipid precursors and specific fatty acids between Sertoli cells and germinal cells (295). In addition, the metabolism and degradation of the residual body by Sertoli cells are also anticipated to require lipidbinding proteins. Further investigation of lipid transport and the binding proteins involved is required. SKINNER 52 Sertoli cells will also likely have developed a mechanism with which to transport vitamins to spermatogenic cells. Many vitamins utilize binding proteins in the circulatory system to facilitate their transport and localization. Preliminary evidence exists for the production of vitamin-binding proteins by Sertoli cells, such as folatebinding protein and biotin-binding protein, that may transport these vitamins to germinal cells (296). Further characterization and investigation of potential Sertoli cell derived vitamin-binding proteins are required. As discussed previously, one vitamin that has been shown to be essential for testis function and spermatogenesis is vitamin A, retinoids (236-239, 298). Cellular retinoidbinding proteins have been localized in Sertoli cells (299-301) and germinal cells (300, 302). Sertoli cells respond directly to retinol and retinoic acid with an increase in several functional parameters (303-307), including transferrin production (Fig. 5). Vitamin A deficiency also directly influences Sertoli cell functions (SOSSI 1). Retinoids appear to act on Sertoli cells through the nuclear retinoid receptor to directly regulate Sertoli cell functions. Information regarding the actions of vitamin A on spermatogenesis, however, imply that retinoids also directly influence germ cell development (236-243, 312). Due to the low solubility of retinoids, binding proteins in the serum, retinol-binding protein, and the cell, cellular retinol and retinoic acid binding proteins exist to facilitate retinoid transport. The majority of retinol accumulation by Sertoli cells is esterified with lipids and apparently stored as compounds such as retinol-palmitate esters (313, 314). Retinol-binding protein facilitates the uptake of retinol by Sertoli cells (315) and whether retinol, retinoic acid and/or retinol-esters are transported to germinal cells remains to be investigated. Although several studies have implied that Sertoli cells TRANSFERRIN PRODUCTION BY SERTOLI CELLS .5 8 0 - F I R T TREATMENT FIRT PMODS FIG. 5. Transferrin production by Sertoli cells isolated from 20 dayold rats and cultured for 5 days in the absence, control (C), or presence of 100 ng/ml FSH (F), 5 Mg/ml insulin (I), 0.35 fiU retinol (R), 1 fiM testosterone (T), a combination of FSH, insulin, retinol, and testosterone (FIRT), or 20 ng/ml PModS (PMODS). Transferrin levels were determined with a RIA and expressed as (nanograms per ^g Sertoli cell DNA) and presented as the mean ± SEM of three different experiments performed in triplicate. Vol. 12, No. 1 may be capable of producing a retinoid binding protein (316, 317), the detection and characterization of a Sertoli cell-derived retinoid binding protein that may facilitate retinoid transport to germinal cells remain to be elucidated. Regulatory. Regulatory interactions between Sertoli cells and germinal cells were proposed as the concepts of paracrine factors and local trophic agents developed. Initial morphological observations regarding alterations of Sertoli cell-germinal cell associations led several laboratories to postulate the potential presence of such interactions. Disruption of testis cell associations and morphology by heat (318), irradiation (319-321), cytotoxic agents (322), or disease states (323) suggested that regulatory interactions may exist between Sertoli cells and germinal cells. Analysis of the expression of specific genes in germ cells and Sertoli cells at different stages of the seminiferous cycle (324) and the effects of removal of germ cells on Sertoli cell products in vivo also suggested that germ cells may regulate Sertoli cell function (325, 326). In addition, the ability of hormones to indirectly influence germinal cell development through actions on the Sertoli cells supports the presence of interactions between the cells (50, 327, 328). Although these experiments and observations imply that interactions appear to occur between Sertoli cells and germinal cells, the specific type of interaction involved can not be distinguished. As previously discussed, alterations in critical environmental or nutritional interactions may have profound effects on spermatogenic cells and Sertoli cells. Further investigation of potential regulatory interactions between Sertoli cells and germinal cells requires the elucidation of the paracrine factors involved. Initial experiments to investigate the effects of Sertoli cells on germinal cells utilized coculture of the cells. The presence of Sertoli cells in culture with spermatogenic cells has been shown to stimulate germ cell RNA and DNA synthesis (329), induce the appearance of germ cell surface antigens (330), and maintain spermatogenic cell glutathione synthesis (331). Although these observations do not demonstrate the presence of a regulatory interaction, these experimental systems may be used in the future to detect the potential presence of Sertoli cellderived paracrine factor(s). Several Sertoli cell secretory products have been identified that may mediate regulatory interactions between Sertoli cells and germinal cells. Insulin growth factor-I (IGF-I) has been shown to be produced by Sertoli cells (83-86) and may act on meiotic spermatogenic cells at IGF-I receptors (85, 332). IGF-I has been shown to act on most cell types to maintain a number of cellular functions and regulate DNA synthesis. Since IGF-I is a ubiquitous paracrine factor, actions on germinal cells will likely be similar to those on somatic February, 1991 CELL-CELL INTERACTIONS IN THE TESTIS cells. Further investigation of IGF-I and IGF-I receptor gene expression and production by cells of the seminiferous tubule is required. Examination of direct actions of IGF-I on germinal cells is also needed to further elucidate IGF-I mediated regulatory interactions between Sertoli cells and spermatogenic cells. Two other Sertoli cell-derived growth factors that have not been fully characterized, but are speculated to act on germ cells, are the seminiferous growth factor (SGF) (333335) and Sertoli cell-secreted growth factor (SCSGF) (336). The mitogenic properties of SGF and SCSGF, however, have been demonstrated on somatic cells, but not spermatogenic cells. Further investigation is required to determine whether SGF or SCSGF may mediate regulatory interactions between these cells and promote growth of developing germinal cells. Transforming growth factors-a (TGFa) and 0 (TGF/3) have also recently been shown to be produced by Sertoli cells (81, 82) and potentially could influence germinal cells. Initial studies imply that developing germinal cells do not contain the epidermal growth factor (EGF) receptor (81, 337). The actions of TGF« on spermatogenic cells, therefore, appear unlikely but remain to be thoroughly investigated. TGF0 is similar to IGF-I in that it is a ubiquitous paracrine factor that acts on many cell types. TGF/? may mediate a regulatory interaction between Sertoli cells and germinal cells; however, the actions of TGF/3 on spermatogenic cells remain to be elucidated. Other regulatory agents postulated to be produced by Sertoli cells and act on germinal cells, such as interleukin-1 (IL-1) that may stimulate germ cell growth (88, 190, 338) and insulin growth factor-II (IGF-II) that may influence germ cell metabolism (339), also require further examination of both the sites of production and action of these potential paracrine factors. Initial experiments to investigate the effects of germinal cells on Sertoli cells also utilized coculture of the cells. The presence of germinal cells in culture with Sertoli cells has been shown to influence Sertoli cell protein glycosylation (340), increase basal and FSHstimulated ABP production by Sertoli cells (341-343), inhibit estradiol production by Sertoli cells (343), and influence the vectorial secretion of ABP by Sertoli cells (344). Pachytene spermatocytes generally were found to have more dramatic effects than late stage spermatids, and the effects of germ cells on Sertoli cells may be age dependent (343, 345). These observations have been extended through analysis of the effects of conditioned medium obtained from spermatogenic cell cultures on Sertoli cells. Germ cell-conditioned medium was found to stimulate the phosphorylation of specific proteins in Sertoli cells (346); stimulate 7-glutamyl transpeptidase activity in Sertoli cells (347); increase ABP production and decrease estradiol synthesis by Sertoli cells (348, 53 349); decrease RNA synthesis in Sertoli cells (350); increase transferrin production (351, 352) and transferrin gene expression (353); and influence vectorial secretion of proteins by Sertoli cells (352). Conditioned medium from cultures of pachytene spermatocytes and early stage round spermatids generally had the most dramatic effects. Initial characterization of the active component(s) in the conditioned medium indicate the activity is heat and trypsin sensitive (348,349). Partial purification demonstrated that the activity was present in a fraction containing three polypeptides between 10 and 30 kilodaltons (353). Further purification and characterization of the factors in germ cell conditioned medium are required to determine the number of potential factors responsible for the biological activities and potential identification of the paracrine factor. Due to the relatively poor viability of germ cells in culture, an important consideration that will require investigation is whether the paracrine factor(s) is actively secreted by germ cells or simply present in germinal cell cytosol and derived from cell lysis. In addition to the above analysis of germinal cell-derived paracrine factors, nerve growth factor (NGF) has also been postulated to be a paracrine factor in the seminiferous tubule (354). /3-NGF gene expression and NGF immunoreactivity have been identified in spermatocytes and early spermatids (355, 356). NGF receptor gene expression has recently been identified in Sertoli cells under androgen regulation (354). The observations that germ cells produce NGF and Sertoli cells contain the NGF receptor have led these investigators to postulate that NGF may mediate regulatory interactions between germinal cells and Sertoli cells. Demonstration that these and other potential factors can be secreted by germinal cells and subsequently act on Sertoli cells remains to be elucidated. The physiological significance of the regulatory interactions between Sertoli cells and germinal cells requires further consideration. General factors required for the maintenance of cellular function, such as IGF-I and possibly TGF/3, are likely needed by all cell types. Therefore, developing germinal cells may also require these same factors. Whether unique paracrine factors mediate Sertoli-germinal cell interactions is unclear at present. Spermatogenesis and meiosis are among the most evolutionarily conserved biological processes to assure propagation of the species. For this reason, the process of germinal cell development and cellular associations has been shown to be similar in a large number of different animals (41). To maintain a high degree of stability and conservation, the majority of regulatory elements required for spermatogenesis will likely be associated with the genome of the germinal cell and require minimal external regulatory interactions with other cell types. Therefore, spermatogenesis may be a passive process, 54 SKINNER and the primary Sertoli cell-germinal cell interactions will be environmental and nutritional. Alternatively, spermatogenesis may be an active process and require a complex network of regulatory interactions to instruct germinal cells to proceed from one stage of development to the next. Further elucidation of Sertoli cell-germinal cell interactions will elucidate the degree to which germinal cell development is an active or passive process. B. Peritubular cell-Sertoli cell interactions The stromal cells that surround the seminiferous tubule and are in contact with the basal surface of the Sertoli cells are referred to as the peritubular myoid cells (357-366). The widespread occurrence of peritubular myoid cells among various species suggests that they are an integral functional component of the mammalian testis (357, 361-363, 365, 367). Differences in the thickness of the peritubular cell layers that surround the tubule in different species, however, have been observed (357, 362). The peritubular cells are thought to provide structural integrity for the tubule and also appear to be involved in contraction of the tubule (357, 362, 368, 369). A peritubular cell sheath is present early in development (370); however, myoid cells do not differentiate until the early stages of puberty (359, 363, 371, 372) in response in part to the actions of androgens (366,373). Alterations in the peritubular cell layers have been shown to occur in pathological conditions of the testis generally associated with an increased thickness of the tissue (374-380). Peritubular cell-Sertoli cell interactions are postulated to play an integral role in the maintenance of testis function (reviews in Refs. 22 and 178). Environmental. One of the primary environmental interactions between peritubular cells and Sertoli cells is mediated by the complex extracellular matrix (i.e. basement membrane) between the two cell types. This extracellular matrix provides structural integrity for the tubule and assists in the maintenance of an efficient bloodtestis barrier (200). The junctional interactions between peritubular cells and the extracellular matrix provide a permeability barrier or prefilter while the tight junctional interactions between Sertoli cells create a functional blood-testis barrier. The extracellular matrix between the peritubular cells and Sertoli cells appears to be produced cooperatively by the two cell types (79, 381, 382). Sertoli cells in culture produce laminin and collagen types I and IV (79) and unique proteoglycans (80). Peritubular cells in culture produce collagen type I (79), unique proteoglycans (80), and fibronectin (79, 115). Therefore, peritubular cells and Sertoli cells appear to produce individual components of the basement membrane, and the presence of both cell types appears necessary to generate an organized deposited extracellular Vol. 12, No. 1 matrix (79). The ability of peritubular cells and Sertoli cells to cooperate in the production of this extracellular matrix is postulated to be an essential environmental interaction between the cells to form the basement membrane of the seminiferous tubule (79). Coculture of the two cell types increased the attachment and viability of Sertoli cells (383, 384) and altered the pattern and rate of Sertoli cell migration (385, 386). Similar results have been observed when Sertoli cells are cultured on a cell line of peritubular cells (387). Since the primary environmental interactions between peritubular cells and Sertoli cells are mediated by the extracellular matrix, a number of laboratories have examined the effects of an extracellular matrix on Sertoli cells (388-392). The presence of an extracellular matrix in culture was found to increase cell attachment and viability, as well as promote a histotype that appears similar to that found in vivo with a columnar shape cell, nucleus near the basal surface of the cell, and tight junctions between Sertoli cells. Although the presence of an extracellular matrix can promote the structural differentiation of the Sertoli cell (388, 389, 392), the extracellular matrix may not influence Sertoli cell functions on a molecular level (392); that will require a regulatory type interaction. The presence of an extracellular matrix has also been shown to promote peritubular myoid cells in culture to express a morphology that is similar to that observed in the seminiferous tubule (393). Therefore, peritubular cells and Sertoli cells cooperate in the production of the basement membrane of the seminiferous tubule that, in turn, promotes structural differentiation of the peritubular cells and Sertoli cells. The observations that the presence of an extracellular matrix promoted a columnar shaped cell in vitro with tight junctions between Sertoli cells led several laboratories to develop dual-chamber culture systems to investigate polarized secretion by Sertoli cells (394-397). A monolayer of Sertoli cells is placed on a permeable membrane to create an apical and basal chamber. The presence of an extracellular matrix was found to facilitate the polarization of the cell and create a permeable barrier (394). Sertoli cells secrete ABP, transferrin, plasminogen activator, and inhibin in a polarized manner dependent on the functional marker examined. Peritubular cells were found to increase the efficiency of the permeability barrier and altered the polarized secretion of Sertoli cell products (398-402). Therefore, an additional environmental interaction between peritubular cells and Sertoli cells is to promote and maintain the polarity of the Sertoli cell and vectorial secretion of cellular products. Proteases and antiproteases are additional cellular products that can influence the environmental interactions between peritubular cells and Sertoli cells. Sertoli cells produce plasminogen activator (72), which could be • February, 1991 CELL-CELL INTERACTIONS IN THE TESTIS involved in the degradation and turnover of the basal lamina of the tubule and influence the junctional interaction between Sertoli cells. Peritubular cells have been shown to produce a protease inhibitor that inhibits plasminogen activator activity (120). The production of the antiprotease by peritubular cells, therefore, might regulate the level of protease activity present to control degradation of the extracellular matrix between Sertoli cells and peritubular cells, as well as maintain the bloodtestis barrier (120). This hypothesis is supported by an investigation of the effects of proteases and antiproteases on the permeability barrier of Sertoli cells in a dualchamber culture system (400). Nutritional. Both Sertoli cells and peritubular cells are in contact with interstitial fluid such that essential components from the circulatory system can readily be obtained by both cell types. Junctional interactions between Sertoli cells and gap junctions between peritubular cells (200) can facilitate nutritional interaction between the individual cell types. Sertoli cells in vitro have been shown to metabolically cooperate with peritubular cells (403) and fibroblasts (404) through junctional contacts and transport metabolites such as nucleotide precursors. The presence of the basal lamina of the seminiferous tubule, which separates peritubular cells and Sertoli cells, however, prevents junctional contacts to form between the cells (200). Therefore, the metabolic cooperation observed in vitro between the cells is likely not present in vivo. Nutritional interactions between peritubular cells and Sertoli cells may not be important due to the availability of serum components to both cell types and lack of junctional interactions between the cells in vivo. Regulatory. Regulatory interactions between peritubular cells and Sertoli cells were first postulated when it was observed that androgens alone could not promote peritubular cell differentiation, but gonadotropin action on Sertoli cells was also required to indirectly effect peritubular cell differentiation (366). Coculture of Sertoli cells and peritubular cells indicates that the presence of peritubular cells increases the production of ABP by Sertoli cells (383, 405); stimulates transferrin production by human Sertoli cells (406); alters the enzyme histochemistry of Sertoli cells (407); and influences the vectorial secretion of proteins by Sertoli cells (398, 401, 408). These observations imply that peritubular cells may influence Sertoli cell functions through a regulatory type interaction. Peritubular cells were subsequently shown to produce a nonmitogenic paracrine factor that modulates Sertoli cell function and has been termed PModS (117, 409). Serum-free peritubular cell-conditioned medium was found to stimulate the production of a number of proteins by Sertoli cells including ABP and transferrin (117, 409, 410). PModS has been purified and 55 characterized and found to have a more dramatic effect on Sertoli cell functions in vitro than any individual regulatory agent previously identified, including FSH (118). An illustration of the effect of PModS on Sertoli cell function in comparison with other hormones is shown in Fig. 5. PModS is shown to stimulate transferrin production by Sertoli cells in culture to the same extent as a mixture of FSH, insulin, and retinol. A combination of PModS and these hormones results in an additive response with an 8- to 10-fold stimulation of Sertoli cell function. The actions of PModS on transferrin and ABP production correlate with an increase in steady state levels of messenger RNA, and the dramatic effects of PModS appear to be due to the signal transduction system utilized (119). Although PModS stimulates most functions associated with the differentiation of the cell, cellular functions apparently independent of Sertoli cell differentiation, such as aromatase activity, may be suppressed by PModS (411). The hypothesis has developed that PModS may have an important role in the induction and maintenance of Sertoli cell differentiation (22, 118, 119,188). The in vivo actions of PModS, however, remain to be investigated. Additional paracrine factors that have been identified to potentially mediate regulatory interactions between peritubular cells and Sertoli cells are growth factors. The EGF-like substance, TGFa has been shown to be synthesized and secreted by Sertoli cells and peritubular cells (81). The EGF-like growth factor previously shown to be produced by Sertoli cells (87) is thought to be TGFa (81). Functional EGF/TGFa receptors are expressed on peritubular cells but not Sertoli cells or germinal cells (81). Immunolocalization of the EGF receptor, however, indicated that the EGF receptor may be on Sertoli cells (337). TGFa/EGF stimulates peritubular cell growth but not Sertoli cell growth (81). EGF has been shown to influence Sertoli cell functions (412, 413); however, several Sertoli cell functions were not found to be influenced by TGFa/EGF in highly purified preparations of Sertoli cells (81). Further examination of the cellular localization of EGF receptor expression and the actions of EGF/ TGFa in the seminiferous tubule is required to elucidate the role TGFa may have in regulatory interactions between peritubular cells and Sertoli cells. TGF/3 is a growth inhibitor that has also been shown to be produced by Sertoli cells and peritubular cells (82). TGF/3 can inhibit TGFa/EGF-stimulated peritubular cell growth and promote peritubular cell migration and colony formation (82). TGF/3 appears to promote the differentiation of peritubular cells and can increase the production of specific proteins (82) such as the plasminogen activator inhibitor antiprotease (414). TGF/3 may also promote peritubular cell chemotaxis to assist in the morphogenesis of the seminiferous tubule (82). TGF/3 56 SKINNER does not appear to have general effects on major Sertoli cell functions (82); however, TGF/3 may increase lactate production by Sertoli cells (415). Further examination of the production and action of TGF/3 in the tubule is required to determine the importance TGF/3 may have in mediating peritubular cell-Sertoli cell interactions. Another growth factor produced by peritubular cells and Sertoli cells is IGF-I, which is a somewhat ubiquitously expressed growth factor that maintains cellular metabolism and acts as a progression factor in the growth of most cell types. IGF-I has been shown to directly stimulate a number of Sertoli cell functions and facilitate the growth of peritubular cells and prepubertal Sertoli cells. Examination of the cellular localization of IGF-I and IGF-I receptor gene expression in the seminiferous tubule (83-86, 416-419) and the actions of IGF-I is required to elucidate whether locally produced IGF-I may mediate peritubular cell-Sertoli cell interactions. The high concentration of IGF-I in the interstitial fluid derived from the circulatory system is readily available to both cell types; therefore, the physiological need for actions of a locally produced IGF to mediate peritubular cell-Sertoli cell interactions needs to be questioned. C. Sertoli cell-Leydig cell interactions The specialized cells within the interstitial tissue of the testis referred to as Leydig cells (420) were distinguished morphologically by a number of early investigators (31-33). The functional importance of this cell was realized with the demonstration that Leydig cells are the site of androgen production (421-426) and that LH through the actions of cAMP directly stimulates androgen production (427-431). The local action of androgen on testis function was initially demonstrated when testosterone alone in the absence of gonadotropins was shown to support spermatogenesis (432) at significantly reduced androgen levels as observed in vivo (433, 434). Androgens, therefore, were the first paracrine agents involved in cell-cell interactions in the testis to be investigated. These observations initiated a relatively intense analysis of Sertoli cell-Leydig cell interactions. Environmental. The location of Leydig cells in the interstitium and the presence of peritubular myoid cells and the basement membrane of the seminiferous tubule do not allow for physical contact between Sertoli cells and Leydig cells. The inability of these cells to physically interact indicates that direct environmental interactions between Sertoli cells and Leydig cells are not possible in vivo. Nutritional. Both Sertoli cells and Leydig cells have ready access to essential nutritional components in the interstitial fluid derived from the circulatory system. For Vol. 12, No. 1 this reason, and because the cells are unable to form junctional contacts, nutritional interactions between Sertoli cells and Leydig cells appear minimal. Leydig cells, however, have been shown to have junctional interactions between themselves (435-439) that can mediate nutritional interactions. Cooperativity between Leydig cells mediated by steroids (440-442) and nonsteroidal substances (443, 444) has been observed. Regulatory. The identification of androgen production by Leydig cells (421-431) and the ability of androgens to maintain the process of spermatogenesis (432-434) led a number of laboratories to investigate the actions of androgens on Sertoli cells. Sertoli cells have been shown to contain and express the androgen receptor (445-451), which is influenced by hormones, sexual maturation, and the cycle of the seminiferous epithelium (452-457). The actions of androgens on Sertoli cell function have been investigated, and the effects observed in vitro are generally negligible or less than the actions of FSH (303, 458462) as shown in Fig. 5. Although the androgen receptor is present in Sertoli cells, a complete understanding of the role of androgen actions on Sertoli cells requires further investigation. The ability of peritubular cells to enhance the actions of androgen on Sertoli cells, however, suggests that androgens may indirectly regulate Sertoli cell function and points out the importance of using a purified preparation of Sertoli cells to investigate the actions of androgens (463). Current data imply that an important regulatory interaction between Leydig cells and Sertoli cells is mediated through the local production and action of androgens, but the physiological significance of this regulatory interaction remains to be fully elucidated. In addition to androgens, Leydig cells can potentially influence Sertoli cells through nonsteroidal factors. A number of peptides and proteins have been shown to be produced or localized in Leydig cells that can potentially have a regulatory action on Sertoli cells and the seminiferous tubule including renin (464-467), prodymorphin (468), and oxytocin (469-471). The functional and physiological roles of these peptides, however, remain to be investigated. Another peptide produced by Leydig cells that may regulate Sertoli cell and seminiferous tubule function is POMC and related peptides (108-112, 472474). Several peptides that are derived from the POMC protein and appear to be produced by Leydig cells include 0-endorphin, aMSH, and ACTH (108, 475). These peptides have been postulated to be involved in the regulation of Sertoli cell function (476-478). The actions of /?endorphin, aMSH, and ACTH on Sertoli cell function have been examined (479-485). The stimulating effects of aMSH and ACTH on Sertoli cell function are small and appear to involve in vitro alterations in cAMP levels. February, 1991 CELL-CELL INTERACTIONS IN THE TESTIS The j8-endorphin receptor has been shown to be present on Sertoli cells (479). Although /?-endorphin alone was found to have little effect, /3-endorphin can partially decrease the ability of FSH to stimulate Sertoli cell functions. The concentrations of these POMC peptides required to obtain effects on Sertoli cells in vitro were relatively high, and these peptides have not been shown to have dramatic effects on Sertoli cell function in comparison with factors such as FSH. Perfusion of the testis with these peptides revealed that ACTH, but not aMSH or /3-endorphin, can influence androgen production by Leydig cells (486, 487). Further work is required to determine the physiological significance of POMC-derived peptides and their importance in mediating regulatory interactions between Leydig cells and Sertoli cells. The ability of Sertoli cells and the seminiferous tubules to affect Leydig cell function was initially hypothesized from observations that Leydig cell morphology was altered by tubules with abnormal function and spermatogenesis (488, 489). Damage of seminiferous tubule function with cytotoxic agents, vitamin A deficiency, or fetal irradiation was found to cause abnormal cytological features and function in adjacent Leydig cells (490). Analysis of testis morphology under pathological conditions (49i-493) and in aged man (494) supports the interrelationship of the seminiferous tubules and Leydig cells. Cryptorchidism has been extensively utilized to inhibit seminiferous tubule and Sertoli cell function to examine subsequent effects on Leydig cell function and morphology (495-502). In addition to studies with damaged or abnormal testis, examination of normal testis has revealed that Leydig cell morphology changes with the seminiferous cycle being most dramatic at stages VII and VIII (503-505). These morphological studies of both abnormal and normal testis implied that a regulatory interaction may exist between Sertoli cells and Leydig cells. To extend these observations, several laboratories cocultured Leydig cells with Sertoli cells or seminiferous tubules. Sertoli cells were found to generally increase basal and hormone-stimulated Leydig cell functions, and FSH was found to indirectly, through the Sertoli cell, stimulate Leydig cell steroidogenesis (506-512). Seminiferous tubules in coculture, particularly at stages VI and VIII, were also found to influence Leydig cell function (513, 514). Further examination of this potential regulatory interaction between Sertoli cells or seminiferous tubules utilized conditioned culture medium. A number of laboratories have identified a stimulatory activity in Sertoli cell-conditioned medium that increases both basal and hormone-stimulated Leydig cell function (515-527). Both FSH-dependent and -independent activities have been identified. Inhibitory activities in the conditioned medium that can decrease basal and hormone-stimulated 57 Leydig cell steroidogenesis have also been identified (528-535). An interesting study with the use of spent media from seminiferous tubules isolated from normal and cryptorchid testis revealed that under normal conditions predominantly inhibitory activity was present whereas cryptorchidism induced the appearance of stimulatory activity (536). Although the contributions of germinal cells and peritubular myoid cells to the activities in conditioned medium from seminiferous tubules remain to be elucidated, observations imply that Sertoli cells may produce multiple factors that can influence Leydig cell function and size. Purification and characterization of these Sertoli cell products are required to determine the novelty of the substances investigated and to address the physiological significance of this proposed regulatory interaction between Sertoli cells and Leydig cells. Additional indirect support of the presence of local factor(s) in the testis that can regulate Leydig cell function is provided with the observations that testicular interstitial fluid has the ability to stimulate Leydig cell steroidogenesis (537-546). The stimulatory activity present in interstitial fluid is speculated to be derived from Sertoli cells; however, further analysis of the sites of action, sites of production, and biochemical properties of the active components is required. Several Sertoli cell-secretory products have been identified that can potentially mediate regulatory interactions between Sertoli cells and Leydig cells. One of the first paracrine factors considered was estrogen. Sertoli cells can produce estrogen from androgens (92, 547), and estrogen generally has inhibitory effects on Leydig cell androgen production (548, 549). The proposal has been made that Sertoli cell-derived estrogen may mediate inhibitory actions on Leydig cell testosterone production; however, Leydig cells can also produce relatively high levels of estrogen (550), which could act as an autocrine factor for the cell. The physiological significance of estrogen-mediated regulatory interactions between Sertoli cells and Leydig cells, therefore, remains to be elucidated. Another factor postulated to be produced by Sertoli cells that can act on Leydig cells is a LHRH-like substance (551-557). Leydig cells from some species have been shown to contain receptors for LHRH (551, 558-561), and LHRH generally has long term inhibitory effects on Leydig cell steroidogenesis (562, 563). The production of an LHRH-like substance by Sertoli cells, however, has been questioned (564) and appears to be somewhat species specific in both the production and action of an LHRH-like substance. Although altered molecular forms of an LHRH molecule may be present in the testis (565567), further analysis of species specificity, sites of production, sites of action, and biochemical characterization of such substances will be required. Sertoli cells also produce a number of growth factors 58 SKINNER that potentially mediate Sertoli cell-Leydig cell interactions. IGF-I is produced by Sertoli cells (83-86, 568-570) and can act on Leydig cells to regulate steroidogenesis (571-576). The ability of Sertoli cells to stimulate Leydig cell steroidogenesis in coculture of the two cell types can be partially inhibited with immunoneutrilization with an IGF-I antibody (577). Leydig cells, however, can produce IGF-I (114) that can act as an autocrine factor for the cell. In addition, the concentration of liver-derived IGFI in the circulatory system and interstitial fluid is approximately 2 orders of magnitude higher than the concentrations required to influence Leydig cell steroidogenesis. Therefore, the physiological relevance of an IGF-I mediated regulatory interaction between Sertoli cells and Leydig cells is questioned. Two other growth factors produced by Sertoli cells are TGFa and TGF/? (81, 82). Leydig cells contain the EGF/TGFa receptor and can respond to EGF or TGF« to influence steroidogenesis and cell growth (578-581). The potential production of TGFa/EGF by Leydig cells (582) or interstitial cells would question the importance of a paracrine interaction between Sertoli cells and Leydig cells. Leydig cell steroidogenesis can also be influenced by the actions of the growth inhibitor TGF/? (583-586). The TGF0 produced by Sertoli cells (82, 587) could act as a paracrine factor to regulate Leydig cell function and growth, but further investigation of additional sites of TGF0 production and action is required to elucidate the physiological relevance of this potential cell-cell interaction. Inhibin (588) is a peptide hormone produced by Sertoli cells (reviews in Refs. 93, 589, and 590) under the control of FSH or agents that alter cAMP levels (591-596). Although a major function for inhibin is to act on the pituitary to regulate FSH production, potential local actions of inhibin have been postulated. Inhibin and its related protein activin both can influence Leydig cell steroidogenesis (597,598). Therefore, inhibin may potentially mediate a regulatory interaction between Sertoli cells and Leydig cells. Leydig cells have been postulated to be involved in the regulation of Sertoli cell inhibin production (599, 600); however, Leydig cells have also been shown to produce both inhibin and activin (113, 601-603). The ability of both Sertoli cells and Leydig cells to produce inhibin and related peptides questions the relevance of a paracrine interaction between the cells mediated by inhibin. The observation that other cell types, such as germinal cells (604), may provide additional sites of action for inhibin or activin suggests that an understanding of the role inhibin/activin has in testicular cell-cell interactions will require further investigation of the sites of action and sites of production of inhibin/activin. Potential alternate or new biological activities of forms of inhibin such as the pro-a-c region of the inhibin subunits also needs to be examined. The role Vol. 12, No. 1 TABLE 5. Sertoli cell-Leydig cell regulatory interactions Potential paracrine factor Site production Site action Actions/ proposed function Androgen Leydig Sertoli Regulate/maintain function and differentiation POMC peptides /?-endorphin Leydig Sertoli MSH, ACTH Stimulatory factor (?) (FSH dependent or independent) Inhibitory factor (?) LHRH-like factor Sertoli Leydig Sertoli Leydig Sertoli Leydig Estrogen Sertoli Leydig IGF-I Sertoli Leydig TGFa Sertoli Leydig TGFjf? Sertoli Leydig IL-1 Sertoli Leydig Inhibin Sertoli Leydig Decrease FSH actions Increase FSH actions/cAMP Increase steroidogenesis Decrease steroidogenesis Decrease steroidogenesis Decrease steroidogenesis Increase steroidogenesis Decrease steroidogenesis/increase growth Increase steroidogenesis Decrease steroidogenesis Increase steroidogenesis inhibin and its related peptides may have in the local regulation of testis function remains to be elucidated. A number of potential paracrine factors may mediate regulatory interactions between Sertoli cells and Leydig cells (Table 5). Additional regulatory agents that can influence Leydig cell function, such as IL-1 (605, 606) and vasopressin-like peptides (607), may also be involved in this interaction but require further investigation. The biological activities present in conditioned medium will require biochemical characterization in order that the potential role these agents may have in cell-cell interactions may be understood. Many of the stimulatory and inhibitory effects observed with materials such as Sertoli cell-conditioned medium may potentially be attributed to the known regulatory agents investigated, such as IGF-I, IL-1, TGFa, or TGF0. Although a list of potential paracrine factors is developing, further analysis is necessary to elucidate their involvement in cell-cell interactions and physiological relevance. Although the production of androgen by Leydig cells and subsequent action on the seminiferous tubule is an essential regulatory interaction to maintain testis function, the physiological requirement for Sertoli cell-Leydig cell regulatory interactions requires further considera- i A 1 A February, 1991 CELL-CELL INTERACTIONS IN THE TESTIS tion. The concentration of androgen present in the adult testis is significantly higher than the concentrations needed to maintain germinal cell development (608-611). Local transient alterations in androgen production by 80-90% may not have major effects on Sertoli cell or germinal cell development. A stimulation in androgen production would not appear to be important for adult testis function. Although an active regulation of Leydig cell androgen production may be needed during embryonic, postnatal, and pubertal development, in the adult testis regulatory interactions between Sertoli cells and Leydig cells to influence Leydig cell steroidogenesis may not be physiologically important. Additional functions of the Leydig cells, however, may need to be activity regulated. D. Leydig cell-peritubular cell interactions The Leydig cell and peritubular myoid cell are both thought to be derived from the pluripotential urogenital mesenchyme. Although peritubular myoid cell differentiation occurs postnatally, Leydig cells appear to develop from precursor mesenchymal cells located in the peritubular region during fetal development (612, 613), and this development is thought to be similar to the pattern of Leydig cell development during postnatal life (438, 614). The origins of the Leydig cells and peritubular myoid cells, therefore, are closely associated. These cell types are not terminally differentiated, and both peritubular cells and Leydig cells proliferate in the adult and have a defined turnover time (615). The development and proliferation of these cell types require a continuous association between Leydig cells and peritubular myoid cells. Environmental. In the adult tissue Leydig cells in the interstitium do not generally form a physical contact with differentiated peritubular myoid cells surrounding the tubule and in contact with the basal surface of the Sertoli cell. Outer layers of less differentiated peritubular myoid cells and undifferentiated fibroblasts or stromal cells and lymphatic endothelium generally separate Leydig cells from myoid cells. The inability of these cells to physically interact indicates that direct environmental interactions between Leydig cells and peritubular myoid cells are generally not present in the adult testis. During development, however, less mature or differentiated Leydig cells and peritubular cells may potentially form environmental interactions. Nutritional. Both Leydig cells and peritubular myoid cells have ready access to essential nutritional components in the interstitial fluid derived from the circulatory system. The lack of apparent junctional interactions between these cell types and ready access to nutritional 59 components implies that minimal nutritonal interactions between Leydig cells and peritubular myoid cells are needed. Although Leydig cells appear to interact among themselves (435-444), no apparent nutritional interactions between Leydig cells and peritubular myoid cells have been observed. Regulatory. The primary regulatory interaction identified between Leydig cells and peritubular myoid cells is mediated by androgens. Peritubular myoid cell development is postulated to be dependent on androgens (366, 373). Inhibition of the androgen production by Leydig cells or the presence of antiandrogens will interfere with peritubular myoid cell development. The peritubular cells accumulate androgen (46) and contain the androgen receptor (456, 616, 617). The level of androgen receptor present in peritubular cells is approximately the same as present in Sertoli cells (617). Peritubular cells in organ culture (373) and isolated peritubular cells in culture respond to androgens (117, 161, 411, 463). The production of the testicular paracrine factor PModS by peritubular cells appears to be under androgen control (117, 463). Therefore, a cascade of cellular interactions is speculated to occur in response to LH and androgen. LH acts on Leydig cells to stimulate the production of androgen that subsequently acts on peritubular myoid cells to stimulate PModS production that modulates Sertoli cell functions and nutritional interactions essential for germinal cell development (117-119). This androgenmediated regulatory interaction between Leydig cells and peritubular cells involving PModS is postulated to be important for the maintenance of testis function and the process of spermatogenesis (22, 118, 119, 188). Further analysis of the in vivo actions of PModS is required to determine the physiological importance of this cell-cell interaction. Additional Leydig cell products that influence peritubular myoid cells have not been identified. Further analysis of biochemical markers for peritubular cell function, such as PModS, will be needed to elucidate potential nonsteroid-mediated regulatory interactions between Leydig cells and peritubular cells. Peritubular cell products that may act as paracrine factors and influence Leydig cell function include IGF-I (114), the EGF-like substance TGF« (81), and TGF/3 (81). IGF-I and TGF0 may potentially stimulate Leydig cell steroidogenesis (571-576, 583-586) whereas TGFa may regulate Leydig cell growth and steroidogenesis (578-581). Although these growth factors are produced by peritubular cells and can act on Leydig cells, the role of these growth factors in mediating cell-cell interactions requires further investigation. The high concentration of serum-derived IGF-I in the interstitial fluid makes questionable the role of IGF-I as a mediator of regulatory interactions between 60 SKINNER the cells. Further analysis of TGFa- and TGF0 production and action in the interstitial tissue is also needed before speculations regarding their involvement in cellular interactions between Leydig cells and peritubular myoid cells can be postulated. The androgen-mediated regulatory interactions between Leydig cells and peritubular myoid cells provide a potentially important indirect mode of androgen action in the testis. Therefore, the peritubular cells may play a critical role in the endocrine regulation of the process of spermatogenesis. The dramatic effects of PModS on Sertoli cell function and inability of purified Sertoli cells to respond to androgens in vitro support these speculations of the importance of this indirect mode of androgen action. Peritubular cell-Sertoli cell interactions provide an example of mesenchymal-epithelial cell interactions present in a variety of tissues. Steroid actions on tissues have been postulated to, in part, be mediated through mesenchymal-epithelial cell interactions (review in Ref. 11). The prostate stromal/mesenchymal cells, in response to androgens, appear to produce an inducer substance that regulates the functions and differentiation of the adjacent prostatic-epithelial cells (618-620). This proposed cell-cell interaction is similar to that proposed between Leydig cell cells, peritubular myoid cells, and Sertoli cells. The possibility that PModS may be a general mediator of androgen actions in other organs in addition to the testis has previously been postulated (22, 118), and preliminary evidence was provided with prostate cell culture-conditioned medium (621, 622). Further characterization of PModS and its potential expression and action in androgen-responsive tissues will be required to determine its tissue specificity. E. Additional cell-cell interactions Although the Leydig cells, peritubular myoid cells, Sertoli cells, and germinal cells are major functional cell types in the testis, several additional existing cell populations include the vasculature and lymphatic endothelium, lymphocytes, macrophages, and the stromal fibroblast cell population. Several studies have been initiated to investigate the physiology of these cell populations and their potential involvement in cell-cell interactions. The stromal fibroblast population of cells in the interstitium and surrounding the outer layers of the seminiferous tubules is thought to contribute to the structural integrity of the tissue as well as contain a stromal/ mesenchymal cell population from which Leydig cells and peritubular myoid cells have been speculated to be derived. Because Leydig cells and peritubular myoid cells proliferate and have a limited turn over time, a less differentiated population of precursor cells or a stem cell population of stromal/mesenchymal cells appears nec- Vol. 12, No. 1 essary to repopulate the cell types. Whether the Leydig cells and peritubular myoid cells are derived from the same precursor population of cells or separate precursor cell types is unclear. Studies to investigate the potential involvement of these cells in cellular interactions have focused on Leydig cells. Treatment of rats with the cytotoxic agent ethane dimethanesulfonate (EDS) has been found to destroy the mature Leydig cell population (623-625). Although EDS does influence Sertoli cell and peritubular myoid cell functions (626, 627), EDS has been utilized to examine Leydig cell regeneration and interactions with other cell types (628-632). Repopulation of Leydig cells after removal of EDS suggests that the Leydig cells are derived from the stromal fibroblast population of cells often associated with the outer layer of cells of the seminiferous tubule (628, 630-632). The regeneration of the Leydig cells from this stromal fibroblast population is speculated to be influenced by the seminiferous tubules and Sertoli cells for growth and possibly differentiation (629, 631, 632). Analysis of cell proliferation and pulse-chase radiolabeling experiments of the stromal/mesenchymal cell population, in comparison to other testicular cell types, support the hypothesis that Leydig cells may be derived from this cell population (633). These stromal/mesenchymal precursor cells can be isolated and cultured and appear to be responsive to the combined actions of LH and androgen (634). Further analysis of cellular interaction with this Leydig cell precursor population of stromal fibroblast cells requires characterization of the cell population and identification of functional parameters of the cells. Another cell population in the testis is derived from the immune system. Macrophages have been shown to reside in the interstitial compartment of the testis (635637). These testicular macrophages have been isolated and characterized (638). Testicular macrophages often physically associate with Leydig cells, and speculations of potential cellular interactions between the cells have been made (639). Interestingly, the conditioned medium from testicular macrophage cultures has been shown to stimulate Leydig cell steroidogenesis (640). Although testicular macrophages will likely have traditional bactericidal and phagocytic activities in the testis (641-647), potential environmental and regulatory interactions between testicular macrophages and other testis cell types, particularly Leydig cells, may also exist. Lymphocytes are also present in the testis and participate in the normal immunology of the animal. The testis, however, has been shown to be an immunologically protected tissue (648-651). Potential cellular interactions between testis cell types and these lymphocytes remain to be thoroughly investigated. Several testis cell products may interact with the lymphocyte population present. Sertoli cells appear to produce protein(s) that can inhibit lym- February, 1991 CELL-CELL INTERACTIONS IN THE TESTIS phocyte proliferation (98, 652). The apparent Sertoli cell product IL-1 (88) may also potentially act as a paracrine factor to influence lymphocytes. Products of testis cell types may be needed to regulate local lymphocyte functions important for immunological protection of the tissue (653-655). In contrast, lymphocyte products, such as interferon and tumor necrosis factor, may be needed to influence testis cell functions (656-658). Further investigation of the reproductive immunology of the testis (652) is required to determine specific regulatory interactions between immune system cells, such as macrophages and lymphocytes, with other cell types in the testis. Vascular and lymphatic endothelial cells are also relatively abundant cell populations in the testis. The arteries, veins, and capillaries that vascularize the testis at puberty are generally associated with the tubules (659662). The permeability of this vasculature is relatively high such that substances introduced into the circulation equilibrate with testicular lymph (207, 260, 663). Potential interactions between this vasculature and other testicular cells, particularly the seminiferous tubule, may be mediated by angiogenic factors. Fibroblast growth factor (FGF) is one of the most potent and well characterized angiogenic factors that promotes the vascularization of tissues (664). FGF has been shown to be present in testis extracts (665, 666); however, the site of synthesis of testicular FGF remains to be elucidated. The ability of testis cells to produce FGF as an angiogenic factor to promote and maintain vascularization of the testis constitutes a potentially important regulatory cell-cell interaction. Other paracrine factors that may mediate regulatory interactions between the vasculature and other testis cell types, such as the renin-angiotensin or atrial natriuretic peptides, remain to be investigated. In addition to the vasculature, lymphatic endothelium often associates with the outer peritubular wall and envelops the Leydig cells (667-669). Although the association of lymphatic endothelium with tubules is variable among species, this cell population is thought to be important in the formation of the interstitium compartment and support of testicular lymph. 61 III. Summary A. Testicular cell-cell interactions A general summary of the major categories of cell-cell interactions among the primary testicular cell types considered are illustrated in Table 6. The major environmental interactions occur between Sertoli cells and spermatogenic cells and between peritubular cells and Sertoli cells. The lack of physical contact between Leydig cells and Sertoli cells or peritubular myoid cells indicates that environmental interactions between these cells will not likely be an essential cell-cell interaction. The primary nutritional interaction in the testis is between Sertoli cells and germinal cells. Ready access to serum-derived nutritional components in interstitial fluid by the other cell types indicates that major nutritional interactions between Leydig, peritubular, and Sertoli cells will not be required. Regulatory interactions occur among all the cell types considered. Although further investigation of regulatory interactions between Sertoli cells and germinal cells is needed, initial studies suggest the presence of such interactions. Observations imply that the cellular anatomy of the testis and factors such as the blood-testis barrier play a major role in the types of cell-cell interactions that have evolved and are needed to maintain testis function. The cellular associations and physiology of the testis, however, differ depending on the stage of development. Therefore, the cell-cell interactions required and present in the prenatal testis, prepubertal testis, pubertal testis, and adult testis will be altered depending on the specific physiological requirements. As specific cellular interactions are more thoroughly characterized in the more mature stages of development, their role in the developing testis can be examined. Several aspects of testis cell biology that are integrally associated with local cellular interactions involve growth and differentiation. All testicular cell types prepubertally require cell proliferation, which terminates for Sertoli cells at the early stages of puberty. All other testicular cell types, except Sertoli cells, require varying degrees of cell proliferation in the adult testis. Therefore, the local production and action of growth factors are required to regulate testis cell growth (review in Ref. 670). These TABLE 6. Classification of testicular cell-cell interactions Interaction Sertoli-germinal Peritubular-Sertoli Sertoli-Leydig Leydig-peritubular Environmental Nutritional Regulatory Yes (e.g. cytoarchitecture) Yes (e.g. basement membrane) No Yes (e.g. transport proteins) No Yes (e.g. IGF-I) Yes (e.g. PModS) Yes (e.g. POMC peptides) Yes (e.g. androgen) No No No SKINNER 62 growth factors can act as autocrine and paracrine factors to mediate regulatory interactions among various testis cell types. The majority of proposed potential growth factor-mediated cell-cell interactions are outlined in Fig. 6. All of these proposed interactions require further investigation to elucidate their physiological relevance. IGF-I can potentially mediate interactions between all the cell types; however, due to the high concentration of serum-derived IGF-I present in the interstitium, only Sertoli cell-germinal cell IGF-I-mediated interaction may be important. TGFa and TGF/3 have antagonist growth effects and may also mediate interactions between all the cell types, but further analysis of the sites of production and action of TGF« and TGF/3 is required. IL-1 has been postulated to be produced by Sertoli cells and potentially interact with Leydig cells and germinal cells. Interactions of IL-1 with other testicular cell types such as macrophages and lymphocytes may be of equal or greater physiological importance for testis function. FGF has been shown to be present in the testis (665, 666); however, the site of synthesis of testicular FGF remains to be determined. FGF has the ability to influence Leydig cell (671-674) and Sertoli cell function (675); however, potential actions on lymphatic and vascular endothelial cells may be of equal or greater physiological relevance. Other potential testicular growth factors that require further characterization such as SGF (333, 334) and SCSGF (336) may also mediate cell-cell interactions. Clearly, locally produced growth factors in the testis will be important mediators of regulatory interactions between various cell types; however, further investigation is needed to elucidate the specific interactions and growth factors that will be physiologically relevant in the regulation of testis cell growth. Classical growth factors such as FGF or EGF/TGFa act to promote a cell into the growth cycle, and subsequently a progression factor such as IGF-I will facilitate DNA synthesis followed by the proliferation of the cell. Vol. 12, No. 1 The function and pharmacology of these growth factors are directed toward putting the cell into the cell cycle to allow cell proliferation. Differentiation of a cell is generally independent of cell growth and may require a separate class of regulatory agents to promote and maintain the differentiated state of the cell. Optimal differentiation of the cell often requires the cell to be at a specific stage of the cell growth cycle. Alteration of the cell cycle by growth factors, therefore, may indirectly affect cellular differentiation. As with most organs, the testis requires a set of differentiation type regulatory agents to promote development, control function, and maintain cellular differentiation. Although identification and analysis of differentiation type factors is generally more difficult than analysis of growth factors, a summary of the regulatory agents involved in cell-cell interactions is shown in Fig. 7. The endocrine hormones LH and FSH play an integral role in promoting and maintaining Leydig cell and Sertoli cell function, respectively. Androgen production by Leydig cells promotes and maintains the differentiation and function of peritubular myoid cells and Sertoli cells. PModS derived from peritubular cells appears to promote and control Sertoli cell function and differentiation. TGF/3 is generally a growth inhibitor that stops cell growth and puts the cell into a stage in the cell cycle more responsive for optimal differentiation. Therefore, TGF/3 can indirectly act as a differentiation factor by inhibiting growth. TGF/3 has been shown to stimulate Leydig cell and peritubular cell function, and its potential role on germinal cells remains to be investigated. Inhibin/activin are also regulatory agents with hormone-like differentiation properties that are likely independent of growth similar to TGF/3. Inhibin has been postulated to be involved in Sertoli cell-Leydig cell interactions; however, further analysis of the sites of production and action of inhibin/actin is needed to fully elucidate involvement in cell-cell interactions. Further investigation of these and additional differentiation fac- Leydig TGF0 Peritubular Sertoli Germinal FIG. 6. Potential growth factor-mediated cell-cell interactions in the testis. Peritubular Sertoli Germinal FlG. 7. Potential cell-cell interactions that regulate cellular differentiation. February, 1991 63 CELL-CELL INTERACTIONS IN THE TESTIS tors is needed to fully understand the specific regulatory interactions involved in the control and maintenance of cellular differentiation in the testis. B. Endocrine regulation of testis function Analysis of cell-cell interactions in the testis has revealed that the actions of the endocrine system are often indirectly mediated through local cellular interactions. Leydig cells respond to the gonadotropin LH to increase androgen production. The response to LH, however, is the initiation of a cascade of events in which androgens can act on peritubular cells to promote PModS production that acts on Sertoli cells to influence functions essential for germ cell development (Fig. 7). This androgen-mediated mesenchymal (stromal)-epithelial cell interaction may be similar in other androgen-dependent organs during fetal development and in the adult (11, 22, 676). It is speculated that differentiation type factors such as PModS may mediate steroid- or hormone-induced interactions in a wide variety of organs throughout development. In addition to LH-induced cell-cell interactions, FSH actions on Sertoli cells also promote indirect effects on other testicular cell types. FSH will indirectly effect germinal cell development by altering Sertoli cell-germinal cell interactions. Sertoli cells also appear to produce FSH-dependent factors that can influence Leydig cell function. Therefore, the two major endocrine agents known to influence testis physiology act, in large part, indirectly through alterations in local cellular interactions. Further investigation of the effects of endocrine agents on cell-cell interactions in the testis will be required to elucidate male reproductive endocrinology. The evolution of local cellular interactions that influence tissue function under the control of the endocrine system provides an efficient mechanism to regulate tissue physiology. The next era for the field of endocrinology will be to elucidate how endocrine agents affect tissue function on a molecular level through local cell-cell interactions. C. Future directions Information is needed on the paracrine and autocrine factors that mediate testicular cell-cell interactions. Factors such as the peritubular cell-produced PModS, growth factors such as SGF and SCSGF, and the Sertoli cell-produced factors that influence Leydig cells need to be more thoroughly purified and characterized on the protein and molecular level. This is needed to identify the unique or common properties of the factors as well as provide reagents to further investigate the specific cellular interactions. Two initial parameters to be considered in the analysis of cell-cell interactions are demonstration of the sites of production and action of regulatory agents. The expression of a specific gene and the capacity of the cell to secrete the factor is one prerequisite for an agent to be involved in a local cellular interaction. Of equal importance is the demonstration of the site of action of paracrine or autocrine factors. Regulatory factors must have defined and significant actions on target cells to effect cellular growth, function, or differentiation. The majority of proposed cell-cell interactions in the testis require further analysis on the cellular and molecular level. Elucidation of the biochemical properties of a potential paracrine/autocrine agent and analysis of its site(s) of production and actions in vitro, however, do not directly address the physiological relevance of the proposed cellular interaction. The only testicular cell-cell interaction that is known to be essential for testis function is the need for the actions of androgen. Although androgens are needed, the mode(s) of androgen actions remain to be investigated. Few of the proposed cell-cell interactions can be investigated thoroughly on a physiological level at this time. As specific cell-cell interactions are elucidated it will be critical to determine the physiological relevance and importance of these cellular interactions. Acknowledgments I thank Mary Couey, Karen Kerce, Lisa Halburnt, Jeff Parrott, and Loretta Noisin for assistance in preparation of the manuscript, and Dr. Bill Kovacs, Cathy Anthony, Andy Roberts, Brian Mullaney, and John Norton for helpful comments on the manuscript. References 1. Bernard C 1878 Sur les phenomenes de la vie. J. B. Balliere et Fils, Paris 2. Bernard C 1927 An introduction to experimental medicine. Translation Green HC, introduction Henderson LJ, Macmillan, New York, pp 1-226 3. Wilson HV 1907 On some phenomena of coalescence and regeneration in sponges. J Exp Zool 5:245 4. Steinberg MS 1963 Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 141:401 5. Spemann H 1938 Embryonic development and induction. Yale University Press, New Haven, CT. 6. Tyler A 1946 An auto-antibody concept of cell structure, growth and differentiation. Growth 10 (symposium 6):7 7. Weiss P 1947 The problem of specificity in growth and development. Yale J Biol Med 19:235 8. Grobstein C1967 Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr 26:279 9. Roth S 1973 A molecular model for cell interactions. Q Rev Biol 48:541 10. Hood L, Huang HV, Dreyer WJ 1977 The area-code hypothesis: the immune system provides clues to understanding the genetic and molecular basis of cell recognition during development. J Supramol Struct Cell Biochem 7:531 11. Cunha GR, Chung LWK, Shannon JM, Taguchi 0, Fujii H 1983 Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog Horm Res 39:559 12. Trosko JE, Chang C, Madhukaar BV, Oh SY 1990 Chemical, oncogene and growth regulatory modulation of extracellular, intracellular and intercellular communication. In: DeMello, WC (ed) Cell Intercommunication. CRC Press, Cleveland, OH, pp 111 64 SKINNER 13. Pienta KJ, Partin AW, Coffey DS 1989 Cancer as a disease of DNA organization and dynamic cell structure. Cancer Res 49:2525 14. Fidler IJ, Hart IR 1982 Biological diversity in metastatic neoplasms: origins and implications. Science 217:998 15. Greenberg ME, Brackenberry R, Edelman GM 1984 Alteration of neural cell adhesion molecule (N-CAM) expression after neuronal cell transformation by Rous sarcoma virus. Proc Natl Acad Sci USA 81:969 16. Todaro GJ, Green H 1963 Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol 17:299 17. Pignatelli M, Bodmer WF 1988 Genetics and biochemistry of collagen binding-triggered glandular differentiation in a human colon carcinoma cell line. Proc Natl Acad Sci USA 85:5561 18. Marks PA, Sheffery M, Rifkind RA1987 Induction of transformed cells to terminal differentiation and the modulation of gene expression. Cancer Res 47:659 19. Bishop JM 1987 The molecular genetics of cancer. Science 235:305 20. Murphee AL, Benedict WF 1984 Retinoblastoma: clues to human oncogenesis. Science 223:1028 21. Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzler KW, Vogelstein B 1990 Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 247:49 22. Skinner MK 1987 Cell-cell interactions in the testis. Ann NY Acad Sci 513:158 23. Spencer RF 1946 The cultural aspects of eunuchism. Ciba Symp 8:406 24. Derbes VJ 1970 The keepers of the bed. Castration and religion. JAMA 212:97 25. Jocelyn HD, Setchell BP 1972 A treatise concerning the generative organs of men. An annotated translation of "Tractatus de virorum organis generation inservientibus" 1668. J Reprod Fertil [Suppl]17:l 26. Bremner WJ 1981 Historical aspects of the study of the testis. In: Burger H and deKretser D (eds) The Testis. Raven, New York, ppl-5 27. Baer CE von 1827 Beitrage zur kenntnis der niederen thiere. Nova Acta Leopold Carol 13:640 28. Wagner R 1836 Die genesis der samenthierchen. Miiller's Arch Anat Physiol 225 29. Wagner R 1839 Erlauterungstafeln zur physiologie und entwicklungsgeschichte. Voss, Leipzig. 30. Koelliker RA von 1841 Beitrage zur kenntris der geschlechtsverhaltnisse und der samenflussigkeit wirbelloser thiere. Berlin 31. Peter K1899 Die bedeutung der nahrzelle im hoden. Arch Mikrosk Anat 53:180 32. Branca A 1923 Les can alicules testiculaures et al spematogenese d l'homme. Etudes cytologiques. Arch Zool Exp Gen 62:53 33. Stieve H 1930 Mannliche genitalorgane. In: Mollendorf WV (ed) Handbuch der mikro skopischen Anatomie des Menschen. J. Springer, Berlin, 7(2) pp 1-142 34. Fawcett DW 1958 The structure of the mammalian spermatozoon. Int Rev Cytol 7:195 35. de Krester DM 1969 Ultrastructural features of human spermiogenesis. Z Zellforsh Mikrosk Anat 98:477 36. Rowley MJ, Berlin JD, Heller CG 1971 The ultrastructure of four types of human spermatogonia. Z Zellforsh Mikrosk Anat 112:461 37. Fawcett DW 1975 The ultrastructure and functions of the Sertoli cell. In: Greep R, Hamilton E, Washington DC (eds) Handbook of Physiology, sect 7, vol, 5, American Physiological Society, Washington DC, pp 21 38. Russell LD, Clermont Y 1977 Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat Rec 187:3477 39. Holstein AF, Roosen-Runge EC 1981 Atlas of Human Spermatogenesis. Grosse Verlag, Berlin, pp 1-224 40. Rossen-Runge EC 1973 Germinal-cell loss in normal metazoan spermatogenesis. J Reprod Fertil 35:339 41. Rossen-Runge EC 1977 The process of spermatogenesis in animals. Cambridge University Press, Cambridge, U.K. Vol. 12, No. 1 42. Smith PE, Engle ET 1927 The disabilities caused by hypophysectomy and their repair. JAMA 88:158 43. Smith PE 1930 Hypophysectomy and a replacement therapy in the rat. Am J Anat 45:205 44. Steinberger E, Steinberger A 1989 Hormonal control of spermatogenesis. In: DeGroot LJ (ed) Endocrinology. Saunders Co, Philadelphia, vol 3:2132 45. Griffin JE, Wilson JD 1985 Disorders of the testis and male reproductive tract. In: Wilson JD, Foster DW (eds) Williams Textbook of Endocrinology, ed. 7 Saunders Co, Philadelphia, p 259 46. Sar M, Stumpf WE, McLean WS, Smith AA, Hanson V, Nayfen SN, French F 1975 Localization of androgen target cells in the rat testis. Autoradiographic studies In: French FS, Hansson V, Ritzen EM, Nayfon SN (eds) Hormonal Regulation of Spermatogenesis. Plenum Press, New York, p 311 47. Mulder E, Peters MJ, Van Beurden WMO, Galdieri M, Rommerts FFG, Janzen FHA, vander Molen HJ 1976 Androgen receptors in isolated cell preparations obtained from rat testicular tissue. J Endocrinol 70:331 48. Sanborn BM, Steinberger A, Meistrich ML, Steinberger E 1975 Androgen binding sites in testis cell fractions as measured by a nuclear exchange assay. J Steroid Biochem 6:1459 49. Hansson V, Djoseland O, Torgersen O, Ritzen EW, French FS, Nayfeh SN 1976 Hormones and hormonal target cells in the testis. Andrologia 8:195 50. Fritz IB 1978 Sites of action of androgens and follicle stimulating hormone on cells of the seminiferous tubule. In: Litwack G (ed) Biochemical Actions of Hormones. Academic Press, New York, vol 5:249 51. Fritz IB 1984 Past, present and future of molecular and cellular, endocrinology of the testis. INSERM Symp 123:15 52. Steinberger A, Steinberger E 1977 The Sertoli cell. In: Johnson A, Gomes W (eds) The Testis. Academic Press, New York, vol 4:371-394 53. Tindall D, Rowley D, Murthy L, Lipschultz L, Chang C 1985 Structure and biochemistry of the Sertoli cell. Int Rev Cytol 94:127 54. Griswold MD 1988 Protein secretions of Sertoli cells. Int Rev Cytol 110:133 55. Griswold MD, Morales C, Sylvester SR 1988 Molecular biology of the Sertoli cell. Oxford Rev Reprod Biol 10:124 56. Bardin CW, Cheng CY, Musto NA, Gunsalus GL1988 The Sertoli cell. In: Knobil E, Neill J (eds) The Physiology of Reproduction. Raven Press Ltd, New York, vol 1:933-974 57. Setchell BP 1978 The Mammalian Testis. Cornell University Press, Ithaca NY 58. Ritzen EM, Hansson V, French F 1989 The Sertoli cell. In: Burger H, deKretser D (eds) The Testis, ed 2. Raven Press, New York, P 269 59. Schulze C 1984 Sertoli cells and Leydig cells in man. Adv Anat Embryol Cell Biol 88:1-104 60. Fritz IB, Rommerts FG, Louis BG, Dorrington JH 1976 Regulation of FSH and dibutyryl cyclic AMP of the formation of androgen-binding protein in Sertoli cell-enriched cultures. J Reprod Fertil 46:17 61. Steinberger A, Heindel JJ, Lindsey JN, Elkington JSH, Sanborn BM, Steinberger E 1975 Isolation and culture of FSH responsive Sertoli cells. Endocr Res Commun 2:261 62. Hagenas L, Ritzen EM, Ploen L, Hansson V, French FS, Neyfeh SN 1975 Sertoli cell origin of testicular androgen-binding protein (ABP). Mol Cel Endocrinol 2:339 63. Skinner MK, Griswold MD 1980 Sertoli cells synthesize and secrete a transferrin-like protein. J Biol Chem 255:9523 64. Skinner MK, Griswold MD 1983 Sertoli cells synthesize and secrete a ceruloplasmin-like protein. Biol Reprod 28:1225 65. Kissinger C, Skinner MK, Griswold MD 1982 Analysis of Sertoli cell secreted proteins by two-dimensional gel electrophoresis. Biol Reprod 27:233 66. Sylvester SR, Skinner MK, Griswold MD 1984 A sulfated glycoprotein synthesized by Sertoli cells and by epididymal cells is a component of the sperm membrane. Biol Reprod 31:1087 February, 1991 CELL-CELL INTERACTIONS IN THE TESTIS 67. Collard MW, Sylvester SR, Tsuruta JK, Griswold MD 1988 Biosynthesis and molecular cloning of sulfated glycoprotein 1 secreted by rat Sertoli cells: sequence similarity with the 70kilodalton precursor of sulfatide/GMl activator. Biochemistry 27:4557 68. O'Brien JS, Kretz KA, Dewji N, Wenger DA, Esch F, Fluharty AL 1988 Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science 241:1098 69. Kierszenbaum AL, Crowell JA, Shabinowitz RB, DePhilip RM, Tres LL 1986 Protein secretory patterns of rat Sertoli and peritubular cells are influenced by culture conditions. Biol Reprod 35:239 70. Wilson RM, Griswold MD 1979 Secreted proteins from rat Sertoli cells. Exp Cells Res 123:127 71. Cheng CY, Bardin CW 1986 Rat testicular testibumin is a protein responsive to follicle stimulating hormone and testosterone that shares immunodeterminants with albumin. Biochemistry 25:5276 72. Lacroix M, Smith FE, Fritz IB 1977 Secretion of plasminogen activator by Sertoli cell enriched cultures. Mol Cell Endocrinol 9:227 73. Hettle JA, Waller EK, Fritz IB 1986 Hormonal stimulation alters the type of plasminogen activator produced by Sertoli cells. Biol Reprod 34:895 74. Dano K, Andreasen PA, Grondahl-Hansen J, Kristensen P, Nielsen LS, Skriver L 1985 Plasminogen activators, tissue degradation and cancer. Adv Cancer Res 44:139 75. Wright WW, Luzarraga ML 1986 Isolation of cyclic protein-2 from rat seminiferous tubule fluid and Sertoli cell culture medium. Biol Reprod 35:761 76. Wright WW, Parvinen M, Musto NA, Gunsalus GL, Phillips DM, Mather JP, Bardin CW 1983 Identification of stage-specific proteins synthesized by rat seminiferous tubules. Biol Reprod 29:257 77. Erickson-Lawrence M, Zabludoff SD, Wright WW 1990 Cyclic protein-2, a secretory product of rat Sertoli cells, is the proenzyme form of cathepsin-L. J Cell Biol 111:107a 78. Cheng CY, Grima J, Stahler MS, Guglielmotti A, Silvestrini B, Bardin CW 1990 Sertoli cell synthesizes and secretes a protease inhibitor, a2-macroglobulin. Biochemistry 29:1063 79. Skinner MK, Tung PS, Fritz IB 1985 Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol 100:1941 80. Skinner MK, Fritz IB 1985 Structural characterization of proteoglycans produced by testicular peritubular cells and Sertoli cells. J Biol Chem 260:11874 81. Skinner MK, Takacs K, Coffey RJ 1989 Cellular localization of transforming growth factor-alpha gene expression and action in the seminiferous tubule: peritubular cell-Sertoli cell interactions. Endocrinology 124:845 82. Skinner MK, Moses HL 1989 Transforming growth factor-beta gene expression and action in the seminiferous tubule: peritubular cell-Sertoli cell interactions. Mol Endocrinol 3:625 83. Hall K, Ritzen EM, Johnsonbaugh RE, Parvinen M 1983 Secretion of somatomedin-like compound from Sertoli cells in vitro. In: Spencer EM (ed) Insulin-like Growth Factors/Somatomedins. DeGruyter, New York, pp 611-614 84. Ritzen EM 1983 Chemical messengers between Sertoli cells and neighboring cells. J Steroid Biochem 19:499 85. Tres LL, Smith EP, Van Wyk JJ, Kierzenbaum AL 1986 Immunoreactive sites and accumulation of somatomedin-C in rat Sertoli-spermatogenic cell co-cultures. Exp Cell Res 162:33 86. Smith EP, Svoboda ME, Van Wyk JJ, Kierszenbaum AL, Tres LL 1987 Partial characterization of a somatomedin-like peptide from the medium of cultured rat Sertoli cells. Endocrinology 120:186 87. Holmes SD, Spotts G, Smith RG 1986 Rat Sertoli cells secrete a growth factor that blocks epidermal growth factor (EGF) binding to its receptor. J Biol Chem 261:4076 88. Khan SA, Soder O, Syed V, Gustafsson K, Lindh M, Ritzen EM 1987 The rat produces large amounts of an interleykin-1-like factor. Int J Androl 10:495 65 89. Robinson R, Fritz IB 1981 Metabolism of glucose by Sertoli cells in culture. Biol Reprod 24:1032 90. Jutte NHPM, Grootegoed JA, Rommerts FFG, van der Molen HJ 1981 Exogenous lactate is essential for metabolic activities in isolated rat spermatocytes and spermatids. J Reprod Fertil 62:399 91. Jutte NHPM, Jansen R, Grootegoed JA, Rommerts FFG, van der Molen HJ 1983 FSH stimulation of the production of pyruvate and lactate by rat Sertoli cells may be involved in hormonal regulation of spermatogenesis. J Reprod Fertil 68:219 92. Dorrington JH, Fritz IB, Armstrong DT 1978 Control of testicular estrogen synthesis. Biol Reprod 18:55 93. deJong FH, Robertson DM 1985 Inhibin: 1985 update on action and purification. Mol Cell Endocrinol 42:95 94. Josso N, Picard JY 1986 Anti-mullerian hormone. Physiol Rev 66:1038 95. Griswold MD, Roberts K, Bishop P 1986 Purification and characterization of a sulfated glycoprotein secreted by Sertoli cells. Biochemistry 25:7265 96. Collard MW, Griswold MD 1987 Biosynthesis and molecular cloning of sulfated glycoprotein-2 secreted by rat Sertoli cells. Biochemistry 26:3297 97. Jenne DE, Tschopp J 1989 Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc Natl Acad Sci USA 86:7123 98. Wyatt CR, Law L, Magnuson JA, Griswold MD, Magnuson NS 1988 Suppression of lymphocyte proliferation by proteins secreted by cultured Sertoli cells. J Reprod Immunol 14:27 99. Blaschuk O, Burdzy K, Fritz IB 1983 Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem 258:7714 100. Blaschuk O, Fritz IB 1984 Isoelectric forms of clusterin isolated from ram rete testis fluid and from secretions of primary cultures of ram and rat Sertoli cell enriched cultures. Can J Biochem Cell Biol 62:456 101. Cheng CY, Chen CL, Feng ZM, Marshall A, Bardin CW 1988 Rat clusterin isolated from primary Sertoli cell enriched culture medium is sulfated glycoprotein-2 (SGP-2). Biochem Biophys Res Commun 155:398 102. Cheng CY, Mather JP, Byer AL, Bardin CW 1986 Identification of hormonally responsive proteins in primary Sertoli cell-culture medium by anion exchange high performance liquid chromatography. Endocrinology 118:480 103. Walsh EL, Cuyler WK, McCullagh DR 1933 Effect of testicular hormone on hypophysectomized rats. Proc NY Soc Exp Biol Med 50:848 104. Walsh EL, Cuyler WK, McCullagh DR 1934 Physiologic maintenance of male sex glands: effect of androtin on hypophysectomized rats. Am J Physiol 107:508 105. Odell WD 1989 The Leydig cell. In: LJ DeGroot (ed) Endocrinology. Saunders Co, Philadelphia, p 2137 106. Ascoli M 1985 Leutinizing Hormone Receptors and Actions, CRC Press, Boca Raton FL 107. Odell WD 1976 Etiologies of sexual maturation: a model system based on the sexually maturing rat. Recent Prog Horm Res 32:245 108. Margioris AN, Liotta A, Vaudry H, Bardin CW, Krieger DT 1983 Characterization of immunoreactive proopiomelanocortin-related peptides in rat testis. Endocrinology 113:663 109. Melner MH, Puett D 1984 Evidence for synthesis of multiple proopiomelanocortin-like precursors in murine Leydig tumor cells. Arch Biochem Biophys 232:197 110. Tsong S-D, Phillips DM, Halmi N, Krieger D, Bardin CW 1982 /3-Endorphin is present in the male reproductive tract on five species. Biol Reprod 27:755 111. Chen C, Mather JP, Morris PL, Bardin CW 1984 Expression of pro-opiomelanocortin-like gene in the testis and epididymis. Proc Natl Acad Sci USA 81:5672 112. Pintar JE, Schachter BS, Herman AB, Durgerian S, Krieger DT 1984 Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science 225:632 113. Risbridger GP, Clements J, Robertson DM, Drummond AE, Muir J, Berger HG, de Kretser DM 1989 Immuno- and bioactive inhibin 66 114. 115. 116. 117. 118. 119. 120. 121. 122. 123. 124. 125. 126. 127. 128. 129. 130. 131. 132. 133. 134. 135. 136. 137. SKINNER and inhibin alpha-subunit expression in rat Leydig cell cultures. Mol Cell Endocrinol 66:119 Cailleau J, Vermeire S, Verhoeven G 1990 Independent control of the production of insulin-like growth factor I and its binding protein by cultured testicular cells. Mol Cell Endocrinol 69:79 Tung PS, Skinner MK, Fritz IB 1984 Fibronectin synthesis is a marker for peritubular cell contaminants in Seroli-enriched cultures. Biol Reprod 30:199 Skinner MK, Stallard B, Anthony CT, Griswold MD 1989 Cellular localization of fibronectin gene expression in the seminiferous tubule. Mol Cell Endocrinol 66:45 Skinner MK, Fritz IB 1985 Testicular peritubular cells secrete a protein under androgen control that modulates Sertoli cell function. Proc Natl Acad Sci USA 82:114 Skinner MK, Fetterolf PM, Anthony CT 1988 Purification of a paracrine factor, PModS, produced by testicular peritubular cells that modulates Sertoli cell function. J Biol Chem 263:2884 Norton JN, Skinner MK 1989 Regulation of Sertoli cell function and differentiation through the actions of a testicular paracrine factor PModS. Endocrinology 124:2711 Hettle JA, Balekjian E, Tung PS, Fritz IB 1988 Rat testicular peritubular cells in culture secrete an inhibitor of plasminogen activator activity. Biol Reprod 38:359 Sato G 1979 The growth of cells in germ-free hormone-supplemented medium. In: Jakoby WB, Pastan IH (eds) Methods in Enzymology. Academic Press, New York, pp 94-109 Ham RG, McKeehan WL 1979 Media and growth requirements. In: Jakoby WB, Pastan IH (eds) Methods in Enzymology. Academic Press, New York, pp 44-93 Hsueh AJW, Adashi EY, Jones PBC, Welsh TH 1984 Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev 5:76 Kodani M, Kodani K 1966 The in vitro culturation of mammalian Sertoli cells. Proc Natl Acad Sci USA 56:1200 Steinberger E, Steinberger A, Perloff WH 1964 Studies on growth in organ culture of testicular tissue from rats of various ages. Anat Rec 148:581 Steinberger E, Steinberger A, Ficher M 1970 Study of spermatogenesis and steroid metabolism in cultures of mammalian testes. Recent Prog Horm Res 26:547 Janszen FHA, Cooke BA, van Driel MJA, van der Molen HJ 1976 Purification and characterization of Leydig cells from rat testis. J Endocrinol 70:345 Conn PM, Tsuruhara T, Dafau ML, Catt KJ 1977 Isolation of highly purified Leydig cells by density gradient centrifugation. Endocrinology 101:639 Payne AH, Downing JR, Wong KL 1980 Luteinizing hormone receptors and testosterone synthesis in two distinct populations of Leydig cells. Endocrinology 106:1424 Browning JY, D'Agata R, Grotjan Jr HE 1981 Isolation of purified rat Leydig cells using continuous Percoll gradients. Endocrinology 109:667 Cooke BA, Magee-Brown R, Golding M, Dix CJ 1981 The heterogeneity of Leydig cells from mouse and rat testes: evidence for a Leydig cell cycle? Int J Androl 4:355 Gale JS, Wakefield J, Ford HC 1982 Isolation of rat Leydig cells by density gradient centrifugation. J Endocrinol 92:293 Schumacher M, Schafer G, Holstein AF, Hilz H 1978 Rapid isolation of mouse Leydig cells by centrifugation in Percoll density gradients with complete retention of morphological and biochemical integrity. FEBS Lett 91:333 Kerr JB, Robertson DM, deKretser DM 1985 Morphological and functional characterization of interstitial cells from mouse testes fractionated on Percoll density gradients. Endocrinology 116:1030 Lam DMK, Furrer R, Bruce WR 1970 The separation, physical characterization and differentiation kinetics of spermatogonial cells of the mouse. Proc Natl Acad Sci USA 65:192 Go VLM, Vernon RG, Fritz IB 1971 Studies on spermatogenesis in rats. I. Application of the sedimentation velocity technique to an investigation of spermatogenesis. Can J Biochem 49:753 Loir M, Lanneau M 1974 Separation of ram spermatids by sedimentation at unit gravity. Exp Cell Res 83:319 Vol. 12, No. 1 138. Meistrich ML 1972 Separation of mouse spermatogenic cells by sedimentation velocity. J Cell Physiol 80:299 139. Meistrich ML, Bruce WR, Clermont Y 1973 Cellular composition of mouse testis cells following velocity sedimentation separation. Exp Cell Res 79:213 140. Davis JC, Schuetz AW 1975 Separation of germinal cells from immature rat testes by sedimentation at unit gravity. Exp Cell Res 91:79 141. Romrell LJ, Bellve' AR, Fawcett DW 1976 Separation of mouse spermatogenic cells by sedimentation velocity: a morphological characterization. Dev Biol 49:119 142. Bellve' AR, Millette CF, Bhatnagar YM, O'Brien DA 1977 Dissociation of the mouse testis and characterization of isolated spermatogenic cells. J Histochem Cytochem 25:480 143. Bellve' AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M 1977 Spermatogenic cells of the prepuberal mouse (isolation and morphological characterization). J Cell Biol 74:68 144. Meistrich ML, Longtin J, Brock WA, Grimes SR, Mace ML 1981 Purification of rat spermatogenic cells and preliminary biochemical analysis of these cells. Biol Reprod 25:1065 145. Stern L, Gold B, Hecht NB 1983 Gene expression during mammalian spermatogenesis. I. Evidence for stage-specific synthesis of polypeptides in vivo. Biol Reprod 28:483 146. Risley M 1983 Spermatogenic cell differentiation in vitro. Gamete Res 4:331 147. Dorrington JH, Fritz IB 1975 Cellular localization of 5a-reductase and 3a-hydroxysteroid dehydrogenase in the seminiferous tubule of the rat testis. Endocrinology 96:879 148. Dorrington JH, Roller NF, Fritz IB 1975 Effects of folliclestimulating hormone on cultures of Sertoli cell preparations. Mol Cell Endocrinol 3:57 149. Steinberger A, Heindel JJ, Lindsey JN, Elkington JSH, Sanborn BM, Steinberger E 1975 Isolation and culture of FSH responsive Sertoli cells. Endocr Res Commun 2:261 150. Steinberger A, Elkington JSH, Sanborn BM, Steinberger E, Heindel JJ, Lindsey JN 1975 In: French FS, Hansson V, Ritzen EM, Nayfeh S (eds) Hormonal Regulation of Spermatogenesis. Plenum Press, New York, pp 399-411 151. Welsh MJ, Wiebe JP 1975 Rat Sertoli cells: a rapid method for obtaining viable cells. Endocrinology 96:618 152. Tung PS, Dorrington JH, Fritz IB 1975 Structural changes induced by follicle-stimulating hormone or dibutyryl cyclic AMP on presumptive Sertoli cells in culture. Proc Natl Acad Sci USA 72:1838 153. Fritz IB, Louis BG, Tung PS, Griswold MD, Rommerts FG, Dorrington JH 1975 In: French FS, Hansson V, Ritzen EM, Nayfeh S (eds) Hormonal Regulation of Spermatogenesis. Plenum Press, New York, pp 367-382 154. Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini MJ 1981 Pure Sertoli cell cultures: a new model for the study of somaticgerm cell interactions. J Androl 2:249 155. Wagle JR, Heindel JJ, Steinberger A, Sanborn BM 1986 The effect of hypotonic treatment on Sertoli cell purity and cell function in culture. In Vitro 22:325 156. Tung PS, Lacroix M, Fritz IB 1980 Effects of cytosine arabinoside on properties of testicular preparations in culture. Biol Reprod 22:1255 157. Gilmont RR, Coulter GH, Sylvester SR, Griswold MD 1990 Synthesis of transferrin and transferrin mRNA in bovine Sertoli cells in culture and in vivo: sequence and partial cDNA clone for bovine transferrin. Biol Reprod 43:139 158. Perrard-Sapori MH, Saez JM, Dazord A 1985 Hormonal regulation of proteins secreted by cultured pig Sertoli cells: characterization by two-dimensional polyacrylamide gel electrophoresis. Mol Cell Endocrinol 43:189 159. Keeping HS, Winters SJ, Troen P 1985 Identification of androgen-binding protein from testis cytosol and Sertoli cell culture medium of the Cynomolgus monkey, Macaca fascicularis. Endocrinology 117:1521 160. Lipschultz LI, Murthy L, Tindall DJ 1982 Characterization of human Sertoli cells in vitro. J Clin Endocrinol Metab 55:228 161. Anthony CT, Skinner MK 1989 Cytochemical and biochemical February, 1991 162. 163. 164. 165. 166. 167. 168. 169. 170. 171. 172. 173. 174. 175. 176. 177. 178. 179. 180. 181. 182. 183. 184. 185. 186. CELL-CELL INTERACTIONS IN THE TESTIS characterization of testicular peritubular (myoid) cells. Biol Reprod 40:811 Tung PS, Fritz IB 1990 Characterization of rat testicular peritubular myoid cells in culture: a-smooth muscle isoactin is a specific differentiation marker. Biol Reprod 42:351 Tung PS, Fritz IB 1977 Isolation and culture of testicular cells. A morphology at characterization. In: Hafez ESE (ed) Techniques of Human Andrology. North Holland, Amsterdam, pp 125-146 Polombi F, DiCarlo C 1988 Alkaline phosphatase is a marker for myoid cells in cultures of rat peritubular and tubular tissue. Biol Reprod 39:1101 Chapin RE, Phelps JL, Miller BE, Gray TJB 19$7 Alkaline phosphatase histochemistry discriminates peritubular cells in primary rat testicular cell culture. J Androl 8:155 Virtanen I, Kallajoki M, Narvanen 0, Paranko J, Thornell L-E, Miettinen M, Lento V-P 1986 Peritubular myoid cells of human and rat testis are smooth muscle cells that contain desmin-type intermediate filaments. Anat Rec 215:10 Mather JP 1980 Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol Reprod 23:243 Mather JP, Zhuang L, Perez-Infante V, Philips DM 1982 Culture of testicular cells in hormone-supplemented serum-free medium. Ann NY Acad Sci 383:44 Ascoli M 1981 Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology 108:88 Russell LD, Steinberger A 1989 Sertoli cells in culture: views from the perspectives of an in vivoist and an in vitroist. Biol Reprod 41:571 Eagle H 1965 Growth regulatory effects of cellular interactions. Israel J Med Sci 1:1220 Sharpe RM 1984 Intratesticular factors controlling testicular function. Biol Reprod 30:29 Sharpe RM 1986 Paracrine control of testis. Clin Endocrinol Metab 15:185 Findlay JK, Risbridger GP 1987 Intragonadal control mechanisms. Baillieres Clin Endocrinol Metab 1:223 Saez JM, Perrard-Sapori MH, Chatelain PG, Tabone E, Rivarola MA 1987 Paracrine regulation of testicular function. J Steroid Biochem 27:317 Demoulin A, Frenchimont P 1989 Paracrine regulation of testicular function. Acta Urol Belg 57:47 Fritz IB, Skinner MK, Tung PS 1986 The nature of somatic cell interactions in the seminiferous tubule. In: Eshkol A, Eckstein B, Dekel N, Peterson H, Tsafriri A (eds) Development and function of reproductive organs. Cristengraf Publishers, Rome, p 185 Fritz IB, Tung PS 1986 Role of interactions between peritubular cells and Sertoli cells in mammalian testicular function. Gametogenesis and Early Embryo. Alan Liss Inc, New York p 151 Fritz IB 1985 Reflections on the evolution of the regulation of spermatogenesis. In: Sato G, Serraro G, Hayashi J (eds) International Symposium on Cellular Endocrinology. Alan Liss Publishers, New York, p 1 Fritz IB, Tung PS 1986 Testicular peritubular cell-Sertoli cell interactions. In: Stefanini M, Conti M, Geremia R, Ziparo E (eds) Molecular and Cellular Endocrinology of the Testis. Excerpta Medica, Amsterdam NY, p 27 Closset J, Dombrowicz D, Vandenbroeck M, Scippo ML, Igout A, Gothot A, Sente B, Hennen G 1989 Endocrine, paracrine and autocrine factors in the maturation and functional development of the testis. Bull Mem Acad R Med Belg 144:188 Grootegoed JA, Den Boer PJ, Mackenbach P 1989 Sertoli cellgerm cell communication. Ann NY Acad Sci 564:232 Saez JM, Avallet O, Naville D, Perrard-Sapori MH, Chatelain PG 1989 Sertoli-Leydig cell communications. Ann Ny Acad Sci 564:210 Rommerts FF, Brinkman AO 1981 Modulation of steroidogenic activities in testis Leydig cells. Mol Cell Endocrinol 21:15 Sharpe RM, Fraser HM, Cooper I, Rommerts FF 1982 The secretion, measurement and function of a testicular LHRH-like factor. Ann NY Acad Sci 383:272 Risbridger GP, deKretser DM 1989 Paracrine regulation of the 187. 188. 189. 190. 191. 192. 193. 194. 195. 196. 197. 198. 199. 200. 201. 67 testis. In: Burger H, deKretser DM (eds) The Testis, 2 ed. Raven Press Ltd New York, p 255 Parvinen M, Vihko KK, Toppari J 1986 Cell interactions during the seminiferous epithelial cycle. Int Rev Cytol 104:115 Skinner MK 1989 Peritubular myoid cell-Sertoli cell interactions which regulate testis function and growth. Perspect Androl 53:175 Fabbri A, Ulisse S, Bolotti M, Ridolfi M, Spera G, Dufau ML, Isidori A 1989 Opioid regulation of testicular function. Perspect Androl 53:203 Soder O, Pollanen P, Syed U, Hoist M, Granholm K, Arver S, Euler M, Gustafsson K, Froysa B, Parvinen M, Ritzen EM 1989 Perspect Androl 53:215 de Kretser DM 1987 Local regulation of testicular function. Int Rev Cytol 109:89 Koelliker RA von 1899 Erinnerungen aus meinem Leben. W. Engelmann, Leipzig. Sertoli E 1865 On the existence of special branched cells in the seminiferous tubules of the human testis. Morgagni 7:31 Clermont 1972 Kinetics of spermatogenesis in mammals: seminiferous epithelial cycle and spermatogonial renewal. Physiol Rev 52:198 Bellve' AR 1979 The molecular biology of mammalian spermatogenesis. In: CA Finn (ed) Oxford Reviews of Reproductive Biology, vol 1. Calendron Press, Oxford, pp 159-261 Russell LD, Alger LE, Nequin LG 1987 Hormonal control of pubertal spermatogenesis. Endocrinology 120:1204 Brokelmann J 1963 Fine structure of germ cells and Sertoli cells during the cycle of the seminiferous epithelium in the rat. Z Zellforsch Mikrosk Anat 59:820 Nicander L 1967 An electron microscopical study of cell contacts in the seminiferous tubules of some mammals. Z Zellforsch Mikrosk Anat 83:375 Flickinger C, Fawcett DW 1967 The junctional specializations of Sertoli cells in the seminiferous epithelium. Anat Rec 158:207 Dym M, Fawcett DW 1970 The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 3:308 Dym M 1972 The fine structure of the monkey Sertoli cell and its role in establishing the blood-testis fluid barrier. Biol Reprod 7:129a 202. Aoki A, Fawcett DW 1975 Impermeability of Sertoli cell junctions to prolonged exposure to peroxidase. Andrologia 7:63 203. Setchell BP 1960 Do the Sertoli cells secrete the rete testis fluid? J Reprod Fertil 19:391 204. Setchell BP 1967 The blood-testicular fluid barrier in sheep. J Physiol (Lond) 189:63 205. Tuck RR, Setchell BP, Waites GM, Young JA 1970 The composition of fluid collected by micropuncture and catheterization from the seminiferous tubules and rete testis of rats. Pflueger Arch 318:225 206. Waites GMH 1977 Fluid secretion. In: Johnson A, Gomes W (eds) The Testis, vol 4. Academic Press, New York, 91-123 207. Setchell BP 1980 The functional significance of the blood-testis barrier. Andrology 1:3 208. Ross MH 1976 The Sertoli cell junctional specialization during spermiogenesis and at spermiation. Anat Rec 186:79 209. Russell LD, Clermont Y 1976 Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec 185:259 210. Russell L 1977 Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat 148:301 211. Franke WD, Grund C, Fink A, Weber K, Jockush BM, Zentgraf H, Osborn M 1978 Location of actin in the microfilament bundles associated with the junctional specializations between Sertoli cells and spermatids. Biol Cell 31:7 212. Russell LD, Tallon-Doran M, Weber JE, Wong V, Peterson RN 1983 Three-dimensional reconstruction of a rat stage V Sertoli cell. III. A study of specific cellular relationships. Am J Anat 167:181 213. Vogl AW, Soucy LJ 1985 Arrangement and possible function of actin filament bundles in ectoplasmic specializations of ground squirrel Sertoli cells. J Cell Biol 100:814 68 SKINNER 214. Grove BD, Vogl AW 1986 Sertoli cell ectoplasmic specializations: a type of actin-associated adhesion junction? J Cell Sci 93:309 215. Fawcett DW, Phillips DM 1969 Observations on the release of spermatozoa and on changes in the head during passage through the epididymis. J Reprod Fertil [Suppl]6:405 216. Russell LD 1979 Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat Rec 194:213 217. Malone J 1979 A study of Sertoli spermatid tubulobulbar complexes in selected mammals. Anat Rec 193:610 218. Russell LD 1978 Testosterone induced deformities in rat spermiogenesis: failure of tubulobulbar complexes to form in late spermatids. Anat Rec 190:527 219. Gravis CJ 1979 Inhibition of spermiation in the Syrian hamster using dibutyryl cAMP. Cell Tissue Res 192:241 220. Clermont Y, McCoshen J, Hermo L 1980 Evolution of the endoplastic reticulum in Sertoli cell cytoplasm encapsulating the head of late spermatids in the rat. Anat Rec 196:83 221. Wong V, Russell LD 1983 Three dimensional reconstruction of a rat stage V Sertoli cell. I. Methods, basic configuration and dimensions. Am J Anat 167:143 222. Weber JE, Russell LD, Wong V, Peterson RN 1983 Three dimensional reconstruction of a rat stage V Sertoli cell. II. Morphometry of Sertoli-Sertoli and Sertoli-germ-cell relationships. Am J Anat 167:163 223. Clermont Y 1972 Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 52:198 224. Leblond CP, Clermont Y 1952 Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci 55:548 225. Kerr JB, deKretser DM 1975 Cyclic variations in Sertoli cell lipid content throughout the spermatogenic cycle of the rat. J Reprod Fertil 43:1 226. Hilscher B, Passia D, Hilscher W 1979 Kinetics of the enzymatic pattern in the testis. I. Stage dependence of enzymatic activity and its relation to cellular interactions in the testis of the Wistar rat. Andrologia 11:169 227. Morales C, Clermont Y, Nadler NJ 1986 Cyclic endocytic activity and kinetics of lysosomes in Sertoli cells of the rat: a morphometric analysis. Biol Reprod 34:207 228. Johnson L, Amann RP, Pickett BW 1978 Scanning electrons and light microscopy of the equine seminiferous tubule. Fertil Steril 29:208 229. Cavicchia JC, Dym M 1977 Relative volume changes of Sertoli cells in monkey seminiferous epithelium: a stereological analysis. Am J Anat 150:501 230. Bugge HP, Ploen L 1986 Changes in the volume of Sertoli cells during the cycle of the seminiferous epithelium in the rat. J Reprod Fertil 76:39 231. Niemi M, Kormano M 1965 Cyclical changes in and significance of lipids and acid phosphatase activity in the seminiferous tubules of the rat testis. Anat Rec 151:159 232. Posalaki Z, Szabo D, Basci E, Okros I 1968 Hydrolytic enzymes during spermatogenesis in rat. An electron microscopic and histochemical study. J Histochem Cytochem 16:249 233. Parvinen M, Vanha-Perttula T 1972 Identification and enzyme quantitation of the stages of the seminiferous epithelial wave in the rat. Anat Rec 174:435 234. Parvinen M, Hecht NB 1981 Identification of living spermatogenic cells of the mouse by transillumination-phase contrast microscopic technique for 'in situ' analyses of DNA polymerase activities. Histochemistry 71:567 235. Nikkanen U, Soderstrom KO, Parvinen M 1978 Identification of the spermatogenic stages of living seminiferous tubules of man. J Reprod Fertil 53:255 236. Mason KE 1933 Differences in testes injury and repair after vitamin A deficiency, vitamin E deficiency and inanition. Am J Anat 52:153 237. Thompson JN, Howell JM, Pitt GA 1964 vitamin A and reproduction in rats. Proc R Soc Lond B Biol 159:510 238. Huang HF, Hembree WC 1979 Spermatogenic response to vitamin A in vitamin A deficient rats. Biol Reprod 21:891 Vol. 12, No. 1 239. Huang HF, Dyrenfurth I, Hembree WC 1983 Endocrine changes associated with germ cell loss during vitamin A induced recovery of spermatogenesis. Endocrinology 112:1163 240. Morales C, Hugly S, Griswold MD 1987 Stage dependent levels of specific mRNA transcripts in Sertoli cells. Biol Reprod 36:1035 241. Morales C, Griswold MD 1987 Retinol-induced stage synchronization in seminiferous tubules of the rat. Endocrinology 121:432 242. Bartlett JMS, Weinbauer GF, Nieschlag E 1990 Stability of spermatogenic synchromilization achieved by depletion and restoration of vitamin A in rats. Biol Reprod 42:603 243. VanPelt AMM, DeRooij DG 1990 The origin of the synchrounization of the seminiferous epithelium in vitamin A-deficient rats after vitamin A replacement. Biol Reprod 42:677 244. Russell L 1977 Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat 148:313 245. Russell LD 1978 The blood-testis barrier and its formation relative to spermatocyte maturation in the adult rat: a lanthanum tracer study. Anat Rec 190:99 246. Lacroix M, Parvinen M, Fritz IB 1981 Localization of testicular plasminogen activator in discrete proteins (stages VII and VIII) of the seminiferous tubule. Biol Reprod 25:143 247. Fritz IB, Karmally K 1983 Hormonal influences on formation of plasminogen activator by cultured testis tubule segments at defined stages of the cycle of the seminiferous epithelium. Can J Biochem Cell Biol 61:553 248. Ailenberg M, McCabe D, Fritz IB 1990 Androgens inhibit plasminogen activator activity secreted by Sertoli cells in culture in a two-chambered assembly. Endocrinology 126:1561 249. Wright WW, Zabludoff SD, Erickson-Lawrence M, Karzai AW 1989 Germ cell-Sertoli cell interactions. Studies of cyclic protein2 in the seminiferous tubule. Ann NY Acad Sci 564:173 250. Ross MH, Dobler J 1975 The Sertoli cell junctional specializations and their relationship to the germinal epithelium as observed after efferent ductule ligation. Anat Rec 183:267 251. Ulvik NM 1984 Lamellar germ cell processes: structures for possible interaction between germ cells and Sertoli cells during spermatogonial differentiation and early meiotic prophase. Int J Androl 7:129 252. Eddy EM, Kahri AI1976 Cell association and surface features in cultures of juvenile rat seminiferous tubules. Anat Rec 185:333 253. Tres LL, Kierszenbaum AL 1983 Viability of rat spermatogenic cells in vitro is facilitated by their coculture with Sertoli cells in serum-free hormone-supplemented medium. Proc Natl Acad Sci USA 80:3377 254. DePhilip RM, Danahey DG 1987 Germ cell binding to rat Sertoli cells in vitro. Biol Reprod 37:1271 255. D'Agostino A, Stefanini M 1987 A rat spermatocyte surface protein is involved in adhesion of pachytene spermatocytes to Sertoli cells in vitro. Mol Cell Biol 7:1250 256. D'Agostino A, Monaco L, Stefanini M, Geremia R 1984 Study of the interaction between germ cells and Sertoli cells in vitro. Exp Cell Res 150:430 257. Zani BM, Ziparo E, Russo MA, Filippini A, Stefanini M 1987 Membrane molecules involved in adhesion properties of cultured Sertoli cells. Gamete Res 18:301 258. Russell LD 1984 Spermiation-the sperm release process: ultrastructural observations and unresolved problems. In: Blerkom J Van, Motta PM (eds) Electron Microscopy in Biology and Medicine. Ultrastructure of Reproduction. Martinus Nijhoff Publishers, Boston, pp 46-65 259. Millette CF, Bellve' AR 1985 Selective partitioning of plasma membrane antigens during mouse spermatogenesis. Dev Biol 79:309 260. Waites GM, Gladwell RT 1982 Physiological significance of fluid secretion in the testis and blood-testis barrier. Physiol Rev 62:624 261. Christensen AK, Komorowski TE, Wilson B, Ma SF, Stevens RW 1985 The distribution of serum albumin in the rat testis, studied by electron microscope immunocytochemistry or ultrathin frozen sections. Endocrinology 116:1983 262. Nagano T, Suzuki F 1978 Cell to cell relationships in the seminiferous epithelium in the mouse embryo. Cell Tissue Res 189:389 263. Pelletier RM, Friend DS 1983 The Sertoli cell junctional complex: February, 1991 CELL-CELL INTERACTIONS IN THE TESTIS structure and permeability to filipin in the neonatal and adult guinea pig. Am J Anat 168:213 264. McGinley DM, Posalaky Z 1986 Gap junctions between gonocytes and epithelial cells in the sex cords of the one day old rat. A freeze fracture study. J Submicrosc Cytol 18:75 265. De Felici M, Dolci S, Siracusa G 1989 Fetal germ cells establish cell coupling with follicle cells in vitro. Cell Differ Dev 28:65 266. Russell LD 1977 Observations on rat Sertoli ectoplasmic ("junctional") specializations in their association with germ cells of the rat testis. Tissue Cell 9:475 267. McGinley DM, Posalaky Z, Porvaznik M, Russell LD 1979 Gap junctions between Sertoli cells and germ cells of rat seminiferous tubules. Tissue Cell 11:741 268. Ziparo E, Siracusa G, Palombi F, Russo MA, Stefanini M 1982 Formation in vitro of intercellular junctions between isolated germ cells and Sertoli cells in the rat. Ann NY Acad Sci 383:511 269. Gravis CJ, Yates RD, Chen IL 1976 Light and electron microscopic localization of ATPase in normal and degenerating testes of Syrian hamsters. Am J Anat 147:419 270. Robinson R, Fritz IB 1979 Myoinositol biosynthesis by Sertoli cells, and levels of myoinositol biosynthetic enzymes in testis and epididymis. Can J Biochem 57:962 271. Grootegoed JA, Oonk RB, Jansen R, van der Molen HJ 1986 Metabolism of radiolabelled energy-yielding substrates by rat Sertoli cells. J Reprod Fertil 77:109 272. Mita M, Price JM, Hall PF 1982 Stimulation by FSH of synthesis of lactate by Sertoli cells from rat testis. Endocrinology 110:1535 273. Yee JB, Hutson JC 1989 Follicle-stimulating hormone-induced lactate secretion by cultured Sertoli cells does not require extracellular calcium. Life Sci 44:1193 274. Oonk RB, Jansen R, Grootegoed JA 1989 Differential effects of follicle-stimulating hormone, insulin, and insulin-like growth factor I on hexose uptake and lactate production by rat Sertoli cells. J Cell Physiol 139:210 275. Boitani C, Palombi F, Stefanini M 1983 Influence of Sertoli cell products upon the in vitro survival of isolated spermatocytes and spermatids. Cell Biol Int Rep 7:383 276. Mita M, Hall PF 1982 Metabolism of round spermatids from rats lactate as the preferred substrate. Biol Reprod 26:445 277. Risley MS 1990 Support of Xenopus laevis spermatogenesis in vitro by different energy substrates. Biol Reprod 42:511 278. Santiemma V, Salfi V, Casasanta N, Fabbrini A 1987 Lactate dehydrogenase and malate dehydrogenase of Sertoli cells in rats. Arch Androl 19:59 279. Hall PF, Mita M 1984 Influence of FSH on glucose transport by cultured Sertoli cells. Biol Reprod 31:863 280. Gunsalus GL, Musto NA, Bardin W 1978 Immunoassay of androgen binding protein in blood: a new approach for study of the seminiferous tubule. Science 200:65 281. Aisen P, Listowsky I 1980 Iron transport and storage proteins. Annu Rev Biochem 49:357 282. Skinner MK, Cosand WL, Griswold MD 1984 Purification and characterization of testicular transferrin secreted by rat Sertoli cells. Biochem J 218:313 283. Huggenvik JI, Idzerda RL, Haywood L, Lee DC, McKnight GS, Griswold MD 1987 Transferrin messenger ribonucleic acid: molecular cloning and hormonal regulation in rat Sertoli Cells. Endocrinology 120:332 284. Ciechanover A, Schwartz AL, Dautry-Varsat A, Lodish HF 1983 Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. J Biol Chem 258:9681 285. Octave J-N, Schneider Y-J, Trouet A, Crichton RR 1983 Iron uptake and utilization by mammalian cells. I. Cellular uptake of transferrin and iron. Trends Biochem Sci 8:217 286. Holmes SD, Bucci LR, Lipshultz LI, Smith RG 1983 Transferrin binds specifically to pachytene spermatocytes. Endocrinology 113:1916 287. Sylvester SR, Griswold MD 1984 Localization of transferrin and transferrin receptors in rat testes. Biol Reprod 31:195 288. Huggenvik J, Sylvester SR, Griswold MD 1985 Control Of transferrin mRNA synthesis in Sertoli cells. Ann NY Acad Sci 438:1 69 289. Morales C, Sylvester SR, Griswold MD 1987 Transport of iron and transferrin synthesis by the seminiferous epithelium of the rat in vivo. Biol Reprod 37:995 290. Wauben-Penris PJ, Strous GJ, van der Donk HA 1986 Transferrin receptors of isolated rat seminiferous tubules bind both rat and human transferrin. Biol Reprod 35:1227 291. Wauben-Penris PJ, Veldscholte J, van der Ende A, van der Donk HA 1988 The release of iron by Sertoli cells in culture. Biol Reprod 38:1105 292. Roberts KP, Griswold MD 1990 Characterization of rat transferrin receptor cDNA: the regulation of transferrin receptor mRNA in testes and in Sertoli cells in culture. Mol Endocrinol 4:531 293. Frieden E, Hsieh HS 1976 Ceruloplasmin: the copper transport protein with essential oxidase activity. Adv Enzymol 44:187 294. Mareschal JC, Rama R, Crichton RR 1980 The role of ceruloplasmin in Fe(III)-transferrin formation in vitro. FEBS Lett 110:268 295. Beckman JK, Coniglio JG 1980 The metabolism of polyunsaturated fatty acids in rat Sertoli and germinal cells. Lipids 15:389 296. Marzouki AM, Coniglio JG 1982 Effect of essential fatty acid deficiency on lipids of rat Sertoli and germinal cells. Biol Reprod 27:312 297. Fetterolf PM, Skinner MK 1987 Sertoli cells produce vitamin binding proteins. Ann NY Acad Sci 513:480 298. Appling DR, Chytil F 1981 Evidence of a role for retinoic acid (vitamin A-acid) in the maintenance of testosterone production in male rats. Endocrinology 108:2120 299. Huggenvik J, Griswold MD 1981 Retinol binding protein in rat testicular cells. J Reprod Fertil 61:403 300. Porter SB, Ong DE, Chytil F, Orgebin-Crist MC 1985 Localization of cellular retinol-binding protein and cellular retinoic acid-binding protein in the rat testis and epididymis. J Androl 6:197 301. Galdieri M, Piantedosi R, Blaner WS 1989 Levels of binding proteins for retinoids in cultured Sertoli cells: effect of medium composition. Biochim Biophys Acta 1011:168 302. Blaner WS, Galdieri M, Goodman DS 1987 Distribution and levels of cellular retinol- and cellular retinoic acid-binding protein in various types of rat testis cells. Biol Reprod 36:130 303. Skinner MK, Griswold MD 1982 Secretion of testicular transferrin by cultured Sertoli cells is regulated by hormones and retinoids. Biol Reprod 27:211 304. Karl AF, Griswold MD 1980 Actions of insulin and vitamin A on Sertoli cells. Biochem J 186:1001 305. Galdieri M, Monaco L 1983 Retinol increases synthesis and secretion of Sertoli cell mannose-containing glycoproteins. Cell Biol Int Rep 7:219 306. Galdieri M, Caporale C, Adamo S 1986 Calcium-, phospholipiddependent protein kinase activity of cultured rat Sertoli cells and its modifications by vitamin A. Mol Cell Endocrinol 48:213 307. Galdieri M, Nistico L 1986 Vitamin A modifies the glycopeptide composition of cultured Sertoli cells. J Androl 7:303 308. Unni E, Rao MRS 1986 Androgen binding protein levels and FSH binding to testicular membranes in vitamin A deficient rats and during subsequent replenishment with vitamin A. J Steroid Biochem 25:579 309. Carson DD, Lennarz WJ 1983 Vitamin A deprivation selectively lowers uridine nucleotide pools in cultured Sertoli cells. J Biol Chem 258:1632 310. Chytil F 1988 Early effects of retinol and retinoic acid on protein synthesis in retinol deficient rat testes. Biochem Biophys Res Commun 151:53 311. Huang HF, Gould S, Boccabella AV 1989 Modification of Sertoli cell functions in vitamin A-deficient rats. J Reprod Fertil 85:273 312. Haneji T, Maekawa M, Nishimune Y 1984 Vitamin A and folliclestimulating hormone synergistically induce differentiation of type A spermatogonia in adult mouse cryptochid testis in vitro. Endocrinology 114:801 313. Bishop PD, Griswold MD 1987 Uptake and metabolism in retinol in cultured Sertoli cells: Evidence for a kinetic model. Biochemistry 26:7511 314. Shingleton JL, Skinner MK, Ong DE 1989 Retinol esterification 70 315. 316. 317. 318. 319. 320. 321. 322. 323. 324. 325. 326. 327. 328. 329. 330. 331. 332. 333. 334. 335. SKINNER in Sertoli cells by lecithin-retinol acyltransferase. Biochemistry 28:9647 Shingleton JL, Skinner MK, Ong DE 1989 Characteristics of retinol accumulation from serum retinol-binding protein by cultured Sertoli cells. Biochemistry 28:9641 Carson DD, Rosenberg LI, Blaner WS, Kato M, Lennarz WJ 1984 Synthesis and secretion of a novel binding protein from retinol by a cell line derived from Sertoli cells. J Biol Chem 259:3117 Ong DE, Chytil F 1988 Presence of novel retinoic acid-binding proteins in the lumen of rat epididymis. Arch Biochem Biophys 267:474 Jegou B, Laws AO, de Kretser DM 1984 Changes in testicular function induced by short-term exposure of the rat testis to heat: further evidence for interaction of germ cells, Sertoli cells and Leydig cells. Int J Androl 7:244 Hatier R, Grignon G, Touati F 1982 Ultrastructural study of seminiferous tubules in the rat after prenatal irradiation. Anat Embryol 165:425 Pinon-Lataillade G, Velez de la Calle JF, Viguier-Martinez MC, Gamier DH, Folliot R, Maas J, Jegou B 1988 Influence of germ cells upon Sertoli cells during continuous low-dose rate gammairradiation of adult rats. Mol Cell Endocrinol 58:51 Pineau C, Velez de la Calle JF, Pinon-Lataillade G, Jegou B 1989 Assessment of testicular function after acute and chronic irradiation: further evidence for an influence of late spermatids on Sertoli cell function in the adult rat. Endocrinology 124:2720 Bartlett JM, Kerr JB, Sharpe RM 1988 The selective removal of pachytene spermatocytes usign methoxy acetic acid as an approach to the study in vivo of paracrine interactions in the testis. J Androl 9:31 Francavilla S, Bellocci M, Martini M, Bruno B, Moscardelli S, Fabbrini A, Properzi G 1982 Arrest of maturation in spermatogenesis. Boll Soc Ital Biol Sper 58:907 Morales CR, Alcivar AA, Hecht NB, Griswold MD 1989 Specific mRNAs in Sertoli and germinal cells of testes from stage synchronized rats. Mol Endocrinol 3:725 Morris ID, Bardin CW, Musto NA, Thau RB, Gunsalus GL 1987 Evidence suggesting that germ cells influence the bidirectional secretion of androgen binding protein by the seminiferous epithelium demonstrated by selective impairment of spermatogenesis with busulphan. Int J Androl 10:691 Cheng CY, Grima J, Stahler MS, Lockshin RA, Bardin CW 1989 Testins are structurally related Sertoli cell proteins whose secretion is tightly coupled to the presence of germ cells. J Biol Chem 264:21386 Hansson V, Weddington SC, McLean WS, Smith AA, Nayfeh SN, French FS, Ritzen EM 1975 Regulation of seminiferous tubular function by FSH and androgen. J Reprod Fertil 44:363 Clausen OP, Purvis K, Hansson V 1979 Endocrine correlates of meiosis in the male rat. Arch Androl 2:59 Rivarola MA, Sanchez P, Saez JM 1985 Stimulation of ribonucleic acid and deoxyribonucleic acid synthesis in spermatogenic cells by their coculture with Sertoli cells. Endocrinology 117:1796 van der Donk JA, de Ruiter-Bootsma AL, Ultee-van Gessel AM, Wauben-Penris PJ 1986 Cell-cell interaction between rat Sertoli cells and mouse germ cells in vitro. Exp Cell Res 164:191 Li LY, Seddon AP, Meister A, Risley MS 1989 Spermatogenic cell-somatic cell interactions are required for maintenance of spermatogenic cell glutathione. Biol Reprod 40:317 Vannelli BG, Barni T, Orlando C, Natali A, Serio M, Balboni GC 1988 Insulin-like growth factor-I (IGF-I) and IGF-I receptor in human testis: an immunohistochemical study. Fertil Steril 49:666 Feig LA, Bellve' AR, Erickson NH, Klagsbrun MK 1980 Sertoli cells contain a mitogenic polypeptide. Proc Natl Acad Sci USA 77:4774 Feig LA, Klagsbrun M, Bellve' AR 1983 Mitogenic polypeptide of the mammalian seminiferous epithelium: biochemical characterization and partial purification. J Cell Biol 97:1435 Bellve' AR, Feig LA 1984 Cell proliferation in the mammalian testis: biology of the seminiferous growth factor (SGF). Recent Prog Horm Res 40:531 Vol. 12, No. 1 336. Buch JP, Lamb DJ, Lipshultz LI, Smith RG 1988 Partial characterization of a unique growth factor secreted by human Sertoli cells. Fertil Steril 49:658 337. Suarez-Quian CA, Dai M, Onoda M, Kriss RM, Dym M 1989 Epidermal growth factor receptor localization in the rat and monkey testes. Biol Reprod 41:921 338. Pollanen P, Soder O, Parvinen M 1989 Interleukin-1 alpha stimulation of spermatogonial proliferation in vivo. Reprod Fertil Dev 1:85 339. O'Brien DA, Gabel CA, Rockett DL, Eddy EM 1989 Receptormediated endocytosis and differential synthesis of mannose 6phosphate receptors in isolated spermatogenic and Sertoli cells. Endocrinology 125:2973 340. Galdieri M, Zani BM, Monaco L, Ziparo E, Stefanini M 1983 Changes of Sertoli cell glycoproteins induced by removal of the associated germ cells. Exp Cell Res 145:191 341. Galdieri M, Monaco L, Stefanini M 1984 Secretion of androgen binding protein by Sertoli cells is influenced by contact with germ cells. J Androl 5:409 342. Le Magueresse B, Le Gac F, Loir M, Jegou B 1986 Stimulation of rat Sertoli cell secretory activity in vitro by germ cells and residual bodies. J Reprod Fertil 77:489 343. Le Magueresse B, Jegou B 1988 In vitro effects of germ cells on the secretory activity of Sertoli cells recovered from rats of different ages. Endocrinology 122:1672 344. Janecki A, Jakubowiak A, Steinberger A 1988 Effect of germ cells on vectorial secretion of androgen binding protein and transferrin by immature rat Sertoli cells in vitro. J Androl 9:126 345. Castellon E, Janecki A, Steinberger A 1989 Age-dependent Sertoli cell responsiveness of germ cells in vivo. Int J Androl 12:439 346. Ireland ME, Welsh MJ 1987 Germ cell stimulation of Sertoli cell protein phosphorylation. Endocrinology 120:1317 347. Schteingart HF, Rivarola MA, Cigorraga SB 1989 Hormonal and paracrine regulation of gamma-glutamyl transpeptidase in rat Sertoli cells. Mol Cell Endocrinol 67:73 348. Le Magueresse B, Jegou B 1986 Possible involvement of germ cells in the regulation of oestradiol-17/3 and ABP secretion by immature rat Sertoli cells (in vitro studies). Biochem Biophys Res Commun 141:861 349. Le Magueresse B, Jegou B 1988 Paracrine control of immature Sertoli cells by adult germ cells, in the rat (an in vitro study). Cell-cell interactions within the testis. Mol Cell Endocrinol 58:65 350. Rivarola MA, Sanchez P, Saez JM 1986 Inhibition of RNA and DNA synthesis in Sertoli cells by co-culture with spermatogenic cells. Int J Androl 9:424 351. Le Magueresse B, Pineau C, Guillou F, Jegou B 1988 Influence of germ cells upon transferrin secretion by rat Sertoli cells in vitro. J Endocrinol 118:R13 352. Djakiew D, Dym M 1988 Pachytene spermatocyte proteins influence Sertoli cell function. Biol Reprod 39:1193 353. Stallard BJ, Griswold MD 1990 Germ cell regulation of Sertoli cell transferrin mRNA levels. Mol Endocrinol 4:393 354. Persson H, Ayer-LeLievre C, Soder O, Villar MJ, Metsis M, Olson L, Ritzen M, Hokfelt T 1990 Expression of beta-nerve growth factor receptor mRNA in Sertoli cells downregulated by testosterone. Science 247:704 355. Ayer-LeLievre C, Olson L, Ebendal T, Hallbook F, Persson H 1988 Nerve growth factor mRNA and protein in the testis and epididymis of mouse and rat. Proc Natl Acad Sci USA 85:2628 356. Olson L, Ayer-LeLievre C, Ebendal T, Seiger A1987 Nerve growth factor-like immunoreactivities in rodent salivary glands and testis. Cell Tissue Res 248:275 357. Clermont Y 1958 Contractile elements in the limiting membranes of the seminiferous tubules in the rat. Exp Cell Res 15:438 358. Lacy D, Rotblat J 1960 Study of normal and irradiated boundary tissue of the seminiferous tubules of the rat. Exp Cell Res 21:49 359. Leeson CR, Leeson TS 1963 The postnatal development and differentiation of the boundary tissue of the seminiferous tubule of the rat. Anat Rec 147:243 360. Kagayama M, Irisawa S, Shirai M, Matsushita K 1965 Contractile cells in the tissue surrounding the seminiferous tubules. Jpn Excerpta Med 20:1124 February, 1991 CELL-CELL INTERACTIONS IN THE TESTIS 361. Crabo B 1963 Fine structure of the interstitial cells of the rabbit testis. Z Zellforsch Mikrosk Anat 61:587 362. Ross MH, Long IR 1966 Contractile cells in human seminiferous tubules. Science 153:1271 363. Ross MH 1967 The fine structure and development of the peritubular contractile cell component in the seminiferous tubules of the mouse. Am J Anat 121:523 364. Fawcett DW, Heidger PM, Leak LV 1960 Lymph vascular system of the interstitial tissue of the testis as revealed by electron microscopy. J Reprod Fertil 19:109 365. McCord RG 1970 Fine structural observations of the peritubular cell layer in the hamster testis. Protoplasma 69:283 366. Bressler RS, Ross MH 1972 Differentiation of peritubular myoid cells of the testis: effects of intratesticular implantation of newborn mouse testes into normal and hypophysectomized adults. Biol Reprod 6:148 367. Unsicker K, Burnstock G 1975 Myoid cells in the peritubular tissue (Lamina propria) of the reptilian testis. Cell Tissue Res 163:545 368. Kormano M, Hovatta 0 1972 Contractility and histochemistry of the myoid cell layer of the rat seminiferous tubule during postnatal development. Z Anat Entwicklungsgesch 137:239 369. Suvanto 0, Kormano M 1970 The relation between in vitro contractions of the rat seminiferous tubules and the cyclic stage of the seminiferous epithelium. J Reprod Fertil 21:227 370. van Vorstenbosch CJ, Spek E, Colenbrander B, Wensing CJ 1984 Sertoli cell development of pig testis in the fetal and neonatal period. Biol Reprod 31:565 371. Clermont Y, Perey B 1957 Quantitative study of the cell population of the seminiferous tubules in immature rats. Am J Anat 100:241 372. Flickinger CJ 1967 The postnatal development of the Sertoli cells of the mouse. Z Zellforsch Mikrosk Anat 78:92 373. Hovatta O 1972 Effect of androgens and antiandrogens on the development of the myoid cells of the rat seminiferous tubules (organ culture). Z Zellforsch Mikrosk Anat 131:299 374. De Kretser DM, Kerr JB, Paulsen CA 1975 The peritubular tissue in the normal and pathological human testis. An ultrastructural study. Biol Reprod 12:317 375. Narbaitz R, Tolnai G, Jolly EE, Barwin N, McKay DE 1978 Ultrastructural studies on testicular biopsies from eighteen cases of hypospermatogenesis. Fertil Steril 30:679 376. Millonig G, Morano E, Bollero E, Vecchietti G, Zanoio L 1978 Ultrastructural study of gonads in the complete and incomplete feminization syndrome. Gynecol Obstet Invest 9:16 377. Gravis CJ 1978 Testicular involution after optic enucleation: ultrastructure and alkaline phosphatase cytochemistry of the peritubular tissue. Am J Anat 151:213 378. Kerr JB, Rich KA, de Kretser DM 1979 Effects of experimental cryptorchidism on the ultrastructure and function of the Sertoli cell and peritubular tissue of the rat testis. Biol Reprod 21:823 379. Lacy D, Rotblat J 1960 Study of normal and irradiated boundary tissue of the seminiferous tubules of the rat. Exp Cell Res 21:49 380. Tung PS, Fritz IB 1978 Histopathological changes in testes of adult inbred rats immunized against pachytene spermatocytes or Sertoli cells. Int J Androl [Suppl] 2:459 381. Pollanen PP, Kallajoki M, Risteli L, Risteli J, Suominen JJ 1985 Laminin and type IV collagen in the human testis. Int J Androl 8:337 382. Hadley MA, Dym M 1987 Immunocytochemistry of extracellular matrix in the lamina propria of the rat testis: electron microscopic localization. Biol Reprod 37:1283 383. Tung PS, Fritz IB 1980 Interactions of Sertoli cells with myoid cells in vitro. Biol Reprod 23:207 384. Cameron DF, Markwald RR 1981 Structural response of adult rat Sertoli cells to peritubular fibroblasts in vitro. Am J Anat 160:343 385. Tung PS, Frit IB 1986 Extracellular matrix components and testicular peritubular cells influence the rate and pattern of Sertoli cell migration in vitro. Dev Biol 113:119 386. Tung PS, Fritz IB 1987 Morphogenetic restructuring and formation of basement membranes by Sertoli cells and testis peritubular cells in co-culture: inhibition of the morphogenetic ca$cade by 387. 388. 389. 390. 391. 392. 393. 394. 395. 396. 397. 398. 399. 400. 401. 402. 403. 404. 405. 406. 407. 408. 409. 71 cyclic AMP derivatives and by blocking direct cell contact. Dev Biol 120:139 Mather JP, Phillips DM 1984 Establishment of a peritubular myoid-like cell line and interactions between established testicular cell lines in culture. J Ultrastruct Res 87:263 Tung PS, Fritz IB 1984 Extracellular matrix promotes rat Sertoli cell histotype expression in vitro. Biol Reprod 30:213 Hadley MA, Byers SW, Suarez-Quian CA, Kleinman HK, Dym M 1985 Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol 101:1511 Enders GC, Henson JH, Millette CF 1986 Sertoli cell binding to isolated testicular basement membrane. J Cell Biol 103:1109 Mather JP, Wolpe SD, Gunsalus GL, Bardin CW, Phillips DM 1984 Effect of purified and cell-produced extracellular matrix components on Sertoli cell function. Ann NY Acad Sci 438:572 Anthony CT, Skinner MK 1989 Actions of extracellular matrix on Sertoli cell morphology and function. Biol Reprod 40:691 Tung PS, Fritz IB 1986 Cell-substratum and cell-cell interactions promote testicular peritubular myoid cell histotypic expression in vitro. Dev Biol 115:155 Byers SW, Hadley MA, Djakiew D, Dym M 1986 Growth and characterization of polarized monolayers of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl 7:59 Janecki A, Steinberger A 1986 Polarized Sertoli cell functions in a new two-compartment culture system. J Androl 7:69 Hadley MA, Djakiew D, Byers SW, Dym M 1987 Polarized secretion of androgen-binding protein and transferrin by Sertoli cells grown in a bicameral culture system. Endocrinology 120:1097 Janecki A, Steinberger A 1987 Bipolar secretion of androgenbinding protein and transferrin by Sertoli cells cultured in a twocompartment culture chamber. Endocrinology 120:291 Janecki A, Steinberg A 1987 Vectorial secretion of transferrin and androgen binding protein in Sertoli cell cultures: effect of extracellular matrix, peritubular myoid cells and medium composition. Mol Cell Endocrinol 52:125 Janecki A, Steinberger A 1988 Experimental pitfalls in evaluating vectorial protein secretion in vitro; Sertoli cell secretion of androgen-binding protein and transferrin in two-compartment culture chambers. In Vitro Cell Dev Biol 24:518 Ailenberg M, Fritz IB 1989 Influences of follicle-stimulating hormone, proteases, and antiproteases on permeability of the barrier generated by Sertoli cells in a two-chambered assembly. Endocrinology 124:1399 Ailenberg M, Tung PS, Pelletier M, Fritz IB 1988 Modulation of Sertoli cell functions in the two-chamber assembly by peritubular cells and by extracellular matrix. Endocrinology 122:2064 Ailenberg M, Fritz IB 1988 Control of levels of plasminogen activator activity secreted by Sertoli cells maintained in a twochambered assembly. Endocrinology 122:2613 Hutson JC 1983 Metabolic cooperation between Sertoli cells and peritubular cells in culture. Endocrinology 112:1375 Bols NC, Bowen BW, Byrne PA 1984 Rat Sertoli cells in culture undergo metabolic co-operation with each other and with fibroblasts. J Reprod Fertil 70:191 Hutson JC, Stocco DM 1981 Peritubular cell influence on the efficiency of androgen-binding protein secretion by Sertoli cells in culture. Endocrinology 108:1362 Holmes SD, Lipshultz LI, Smith RG 1984 Regulation of transferrin secretion by human Sertoli cells cultured in the presence or absence of human peritubular cells. J Clin Endocrinol Metab 59:1058 Cameron DF, Snydle E 1985 Selected enzyme histochemistry of Sertoli cells. 2. Adult rat Sertoli cells in co-culture with peritubular fibroblasts. Andrologia 17:185 Ueda H, Tres LL, Kierszenbaum AL 1988 Culture patterns and sorting of rat Sertoli cell secretory proteins. J Cell Sci 89:175 Skinner MK, Fritz IB 1986 Identification of a non-mitogenic paracrine factor involved in mesenchymal-epithelial cell interactions between testicular peritubular cells and Sertoli cells. Mol Cell Endocrinol 44:85 72 SKINNER 410. Hutson JC, Yee JB, Yee JA 1987 Peritubular cells influence Sertoli cells at the level of translation. Mol Cell Endocrinol 52:11 411. Verhoeven G, Cailleau J 1988 Testicular peritubular cells secrete a protein under androgen control that inhibits induction of aromatase activity in Sertoli cells. Endocrinology 123:2100 412. Morris PL, Vale WW, Cappel S, Bardin CW 1988 Inhibin production by primary Sertoli cell-enriched cultures: regulation by follicle-stimulating hormone, androgens, and epidermal growth factor. Endocrinology 122:717 413. Mallea LE, Machado AJ, Navaroli F, Rommerts FFG 1986 Epidermal growth factor stimulates lactate production and inhibits cromatization in cultured Sertoli cells from immature rats. Int J Androl 9:221 414. Nargolwalla C, McCabe D, Fritz IB 1990 Modulation of levels of messenger RNA for tissue-type plasminogen activator in rat Sertoli cells, and levels of messenger RNA for plasminogen activator inhibitor in testis peritubular cells. Mol Cell Endocrinol 70:73 415. Benahmed M, Esposito G, Sordoille TC, dePeretti E, Chauvin MA, Ghiglieri C, Revol A, Morera AM 1989 Transforming growth actor /3 and its related peptides in the testis: an intragonadal polypeptide control system. Perspect Androl 53:191 416. Casella SJ, Smith EP, Van Wyk JJ, Joseph DR, Hynes MA, Hoyt EC, Lund PK 1987 Isolation of rat testis cDNAs encoding an insulin-like growth factor I precursor. DNA 6:325 417. Oonk RB, Grootegoed JA 1988 Insulin-like growth factor I (IGFI) receptors on Sertoli cells from immature rats and age-dependent testicular binding of IGF-I and insulin. Mol Cell Endocrinol 55:33 418. Closset J, Gothot A, Sente B, Scippo ML, Igout A, Vandenbroeck M, Dombrowicz D, Hennen G 1989 Pituitary hormones dependent expression of insulin-like growth factors I and II in the immature hypophysectomized rat testis. Mol Endocrinol 3:1125 419. Jost A, Magre S, Agelopoulos R 1981 Early stages of testicular differentiation in the rat. Hum Genet 58:59 420. Leydig F 1850 Zur anatomie der mannlichen geschlechtsorganie und analdrusen der saugetiere. Z Wiss Zool 2:1 421. Wattenberg LW 1958 Microscopic histochemical demonstration of steroid-3/3-ol-dehydrogenase in tissue sections. J Histochem Cytochem 6:225 422. Levy H, Deane HW, Rubin BL 1959 Visualization of steroid-3(8ol-dehydrogenase activity in tissues of intact and hypophysectomized rats. Endocrinology 65:932 423. Christensen AK, Mason NR 1965 Comparative ability of seminiferous tubules and interstitial tissue of rat testes to synthesize androgens from progesterone-4-14C in vitro. Endocrinology 76:646 424. Hall PF, Irby DL, de Kretser DM 1969 Conversion of cholesterol to androgens by rat testes: comparison of interstitial cells and seminiferous tubules. Endocrinology 84:488 425. Bell JB 1972 Interconversion of testosterone and androstenedione in the human testis: a comparison between the activities displayed by the interstitium and the seminiferous tubules. Experientia 28:212 426. Cooke BA, de Jong FH, van der Molen NH, Rommerts FG 1972 Endogenous testosterone concentrations in rat testis interstitial tissue and seminiferous tubules during in vitro incubation. Nature 237:255 427. Lostroh AJ 1969 Regulation by FSH and ILSH (LH) at reproductive function in the immature male rat. Endocrinology 85:438 428. Cooke BA, Van Beurden WMO, Rommerts FFG, van der Molen NJ 1972 Effects of trophic hormones on 3',5'-cyclic AMP levels in rat testis interstitial tissue and seminiferous tubules. FEBS Lett 25:83 429. Moyle WR, Ramachandran J 1973 Effect of LH on steroidogenesis and cyclic AMP accumulation in rat Leydig cell preparations and mouse tumor Leydig cells. Endocrinology 93:127 430. Dorrington JH, Fritz IB 1974 Effects of gonadotropins on cyclic AMP production by isolated seminiferous tubule and interstitial cell preparations. Endocrinology 94:395 431. Steinberger A 1975 Studies on spermatogenesis and steroidogenesis in culture. Am Zool 15:273 432. Clermont Y, Harvey SC 1965 Duration of the cycle of the seminiferous epithelium of normal, hypophysectomized and hypophysectomized-hormone treated albino rats. Endocrinology 76:80 Vol. 12, No. 1 433. Cunningham GR, Huckins C 1979 Persistence of complete spermatogenesis in the presence of low intratesticular concentrations of testosterone. Endocrinology 105:177 434. Huang HF, Nieschlag E 1986 Suppression of the intratesticular testosterone is associated with quantitative changes in spermatogonial populations in intact adult rats. Endocrinology 118:619 435. Friend DS, Gilula NB 1972 Variations in tight and gap junctions in mammalian tissues. J Cell Biol 53:758 436. Nagano T, Suzuki F 1976 Freeze-fracture observations on the intercellular junctions of Sertoli cells and of Leydig cells in the human testis. Cell Tissue Res 166:37 437. Connell CJ, Christensen AK 1975 The ultrastructure of the canine testicular interstitial tissue. Biol Reprod 12:368 438. Christensen AK 1975 Leydig cells. In: Hamilton DW, Greep RO (eds) Handbook of Physiology, sect 7, vol 5. American Physiological Society, Washington DC, p 57 439. Connell CJ, Connell GM 1977 The interstitial tissue of the testis. In: Johnson A, Gomes W (eds) The Testis. Academic Press New York, vol 4:333 440. Adashi EY, Hsueh AJW 1981 Autoregulation of androgen production in a primary culture of rat testicular cells. Nature 293:737 441. Darney Jr KJ, Ewing L 1981 Autoregulation of testosterone secretion in perfused rat testes. Endocrinology 109:993 442. Dufau ML 1988 Endocrine regulation and communicating functions of the Leydig cell. Annu Rev Physiol 50:483 443. Hedger MP, Eddy EM 1987 The heterogeneity of isolated adult rat Leydig cells separated on Percoll density gradients: an immunological, cytochemical and functional analysis. Endocrinology 121:1824 444. Hedger MP, Eddy EM 1990 Leydig cell cooperation in vitro: evidence for communication between adult rat Leydig cells. J Androl 11:9 445. Sanborn BM, Steinberger A, Meistrich ML, Steinberger E 1975 Androgen binding sites in testis cell fractions as measured by a nuclear exchange assay. J Steroid Biochem 6:1459 446. Sanborn BM, Steinberger A, Tcholakian RK, Steinberger E 1977 Direct measurement of androgen receptors in cultured Sertoli cells. Steroids 29:493 447. Sanborn BM, Steinberger A, Tcholakian RK 1979 Effects of ionic strength on the interaction of androgens with Sertoli cell nuclei. Steroids 34:401 448. Mulder E, Peters MJ, De Vries J, Van Der Molen HJ 1975 Characterization of a nuclear receptor for testosterone in seminiferous tubules of mature rat testes. Mol Cell Endocrinol 2:171 449. Schmidt WN, Danzo BJ 1980 Androgen and progesterone binding components in cytosol prepared from cultures enriched in Sertoli cells from immature rat testes. Biol Reprod 23:495 450. Tindall DJ, Miller DA, Means AR 1977 Characterization of androgen receptor in Sertoli cell-enriched testis. Endocrinology 101:13 451. Tsai YH, Sanborn BM, Steinberger A, Steinberger E 1980 Sertoli cell chromatin acceptor sites for androgen-receptor complexes. J Steroid Biochem 13:711 452. Verhoeven G, Cailleau I 1988 Follicle-stimulating hormone and androgens increase the concentrations of the androgen receptor in Sertoli cells. Endocrinology 122:1541 453. Buzek SW, Sanborn BM 1988 Increase in testicular androgen receptor during sexual maturation in the rat. Biol Reprod 39:39 454. Sanborn BM, Wagle JR, Steinberger A 1984 Control of androgen cytosol receptor concentrations in Sertoli cells: effect of androgens. Endocrinology 114:2388 455. Buzek SW, Caston LA, Sanborn BM 1987 Evidence for agedependent changes in Sertoli cell androgen receptor concentration. J Androl 8:83 456. Nakhla AM, Mather JP, Janne OA, Bardin CW 1984 Estrogen and androgen receptors in Sertoli, Leydig, myoid, and epithelial cells: effects of time in culture and cell density. Endocrinology 115:121 457. Isomaa V, Parvinen M, Janne OA, Bardin CW 1985 Nuclear androgen receptors in different stages of the seminiferous epithelial cycle and the interstitial tissue of rat testis. Endocrinology 116:132 February, 1991 CELL-CELL INTERACTIONS IN THE TESTIS 458. Louis BG, Fritz IB 1979 Follicle stimulating hormone and testosterone independently increase the production of androgen binding protein by Sertoli cells in culture. Endocrinology 104:454 459. Cailleau J, Heyns W, Verhoeven G 1984 A filter disc assay for the measurement and characterization of androgen-binding protein in unconcentrated media from Sertoli cell-enriched cultures. J Steroid Biochem 21:691 460. Rommerts FFG, Kruger-Sewnarain BCh, Van Woerkom-Blik A, Grootegoed JA, Van Der Molen HJ 1978 Secretion of proteins by Sertoli cell enriched cultures: effects of FSH, dibutyryl cyclic AMP and testosterone and correlation with secretion of oestradiol and ABP. Mol Cell Endocrinol 10:39 461. Roberts K, Griswold MD 1989 Testosterone induction of cellular proteins in cultured Sertoli cells from hypophysectomized rats and rats of different agents. Endocrinology 125:1174 462. Ailenberg M, McCabe D, Fritz IB 1990 Androgens inhibit plasminogen activator activity secreted by Sertoli cells in culture in a two-chambered assembly. Endocrinology 126:1561 463. Skinner MK, Fritz IB 1985 Androgen stimulation of Sertoli cell function is enhanced by peritubular cells. Mol Cell Endocrinol 40:115 464. Parmentier M, Inagami T, Pochet R, Desclin JC 1983 Pituitarydependent renin-like immunoreactivity in the rat testis. Endocrinology 112:1318 465. Pandey KN, Maki M, Inagami T 1984 Detection of renin mRNA in mouse testis by hybridization with renin cDNA probe. Biochem Biophys Res Commun 125:662 466. Pandey KN, Ascoli M, Inagami T 1985 Induction of renin activity by gonadotropic hormones in cultured Leydig tumor cells. Endocrinology 117:2120 467. Pandey KN, Inagami T 1986 Regulation of renin angiotensins by gonadotropic hormones in cultured murine Leydig tumor cells. Release of angiotensin but not renin. J Biol Chem 261:3934 468. Douglass J, Cox B, Quinn B, Civelli O, Herbert E 1987 Expression of the Prodynorphin gene in male and female mammalian reproductive tissues. Endocrinology 120:707 469. Nicholson HD, Swann RW, Burford GD, Wathes DC, Porter DG, Pickering BT 1983 Identification of oxytoxin and vasopressin in the testis and in adrenal tissue. Regul Pept 8:141 470. Guldenaar SEF, Pickering BT 1985 Immunocytochemical evidence for the presence of oxytocin in rat testis. Cell Tissue Res 240:485 471. Pickering BT, Ayad VJ, Birkett SD, Gilbert CL, Guldenaar SEF, Nicholson HD, Worley RTS, Wathes DC 1990 Neurohypophysial peptides in the gonads: are they real and do they have a function? Reprod Fertil Dev 2:245 472. Chen CL, Madigan MB 1987 Regulation of testicular proopiomelanocortin gene expression. Endocrinology 121:590 473. Gizang-Ginsberg E, Wolgemuth DJ 1987 Expression of the proopiomelanocortin gene is developmentally regulated and affected by germ cells in the male mouse reproductive system. Proc Natl Acad Sci USA 84:1600 474. Bardin CW, Chen CL, Morris PL, Gerendai I, Boitani C, Liotta AS, Margioris A, Krieger DT 1987 Proopiomelanocortic-derived peptides in testis, ovary, and tissues of reproduction. Recent Prog Horm Res 43:1 475. Valenca MM, Negro-Vilar A 1986 Proopiomelanocortin-derived peptides in testicular interstitial fluid: characterization and changes in secretion after human chorionic gonadotropin or luteinizing hormone-releasing hormone analog treatment. Endocrinology 118:32 476. Fabbri A, Knox G, Buczko E, Dufau ML 1988 Beta-endorphin production by the fetal Leydig cell: regulation and implications for paracrine control of Sertoli cell function. Endocrinology 122:749 477. Fabbri A, Dufau ML 1988 Hormonal regulation of beta-endorphin in the testis. J Steroid Biochem 30:347 478. Lebouille JL, Burbach JP, De Kloet ER, Rommerts FF 1986 TEndorphin-generatingendopeptidase: distribution in body tissues and cellular localization in rat testis. Endocrinology 118:372 479. Fabbri A, Tsai-Morris CH, Luna S, Fraioli F, Dufau ML 1985 480. 481. 482. 483. 484. 485. 486. 487. 488. 489. 490. 491. 492. 493. 494. 495. 496. 497. 498. 499. 500. 501. 73 Opiate receptors are present in the rat testis. Identification and localization in Sertoli cells. Endocrinology 117:2544 Bardin CW, Morris PL, Chen CL, Boitani C, Krieger DT 1987 The action of pro-opiomelanocortin-derived peptides on testicular cells. Ann NY Acad Sci 512:308 Gerendai I, Shaha C, Gunsalus GL, Bardin CW 1986 The effects of opioid receptor antagonists suggest that testicular opiates regulate Sertoli and Leydig cell function in the neonatal rat. Endocrinology 118:2039 Morris PL, Vale WW, Bardin CW 1987 Beta-endorphin regulation of FSH-stimulated inhibin production is a component of a short loop system in testis. Biochem Biophys Res Commun 148:1513 Boitani C, Farini D, Canipari R, Bardin CW 1989 Estradiol and plasminogen activator secretion by cultured rat Sertoli cells in response to melanocyte-stimulating hormones. J Androl 10:202 Orth JM 1986 FSH-induced Sertoli cell proliferation in the developing rat is modified by /3-endorphin produced in the testis. Endocrinology 119:1876 Orth JM, Boehm R 1990 Endorphin suppresses FSH-stimulated proliferation is isolated neonatal Sertoli cells by a pertussis toxinsensitive mechanism. Anat Rec 226:320 Juniewicz PE, Keeney DS, Ewing LL 1988 Effect of adrenocorticotropin and other proopiomelanocortin-derived peptides on testosterone secretion by the in vitro perfused testis. Endocrinology 122:891 Margioris AN, Koukoulis G, Grino M, Chrousos GP 1989 In vitroperifused rat testes secrete (8-endorphin and dynorphin: their effect on testosterone secretion. Biol Reprod 40:776 Rich KA, de Kretser DM 1977 Effect of differing degrees of destruction of the rat seminiferous epithelium on levels of serum follicle stimulating hormone and androgen binding protein. Endocrinology 101:959 Aoki A, Fawcell DW 1978 Is there a local feedback from the seminiferous tubules affecting activity of the Leydig cells? Biol Reprod 19:144 Rich KA, Kerr JB, de Kretser DM 1979 Evidence for Leydig cell dysfunction in rats with seminiferous tubule damage. Mol Cell Endocrinol 13:123 Surina MN 1980 Dynamics of development of the interstitial tissue and seminiferous tubules in the testis of human fetuses. Probl Endokrinol 26:41 Kula K, Romer TE, Wlodarczyk WP 1980 Somatic and germinal cells' interrelationship in the course of seminiferous tubule maturation in man. Arch Androl 4:9 Steinberger E, Root A, Ficher M, Smith KD 1973 The role of androgens in the initiation of spermatogenesis in man. J Clin Endocrinol Metab 37:746 Neaves WB, Johnson L, Petty CS 1987 Seminiferous tubules and daily sperm production in older adult men with varied numbers of Leydig cells. Biol Reprod 36:301 Kerr JB, Rich KA, de Kretser DM 1979 Alterations of the fine structure and androgen secretion of the interstitial cells in the experimentally cryptorchid rat testis. Biol Reprod 20:409 de Kretser DM, Sharpe RM, Swanston IA 1979 Alterations in steroidogenesis and human chorionic gonadotropin binding in the cryptorchid rat testis. Endocrinology 105:135 Risbridger GP, Kerr JB, Peake R, Rich KA, de Kretser DM 1981 Temporal changes in rat Leydig cell function after the induction of bilateral cryptorchidism. J Reprod Fertil 63:415 Risbridger GP, Kerr JB, de Kretser DM 1981 Evaluation of Leydig cell function and gonadotropin binding in unilateral and bilateral cryptochidism: evidence for local control of Leydig cell function by the seminiferous tubule. Biol Reprod 24:534 Risbridger GP, Kerr JB, Peake RA, de Kretser DM 1981 An assessment of Leydig cell function after bilateral or unilateral efferent duct ligation: further evidence for local control of Leydig cell function. Endocrinology 109:1234 Bergh A, Damber JE 1984 Local regulation of Leydig cells by the seminiferous tubules. Effect of short-term cryptorchidism. Int J Androl 7:409 Jegou B, Laws AO, de Kretser DM 1983 The effect ofcryptorchi- 74 502. 503. 504. 505. 506. 507. 508. 509. 510. 511. 512. 513. 514. 515. 516. 517. 518. 519. 520. 521. 522. 523. 524. SKINNER dism and subsequent orchidopexy on testicular function in adult rats. J Reprod Fertil 69:137 Jegou B, Peake RA, Irby DC, de Kretser DM 1984 Effects of the induction of experimental cryptorchidism and subsequent orchidopexy on testicular function in immature rats. Biol Reprod 30:179 Bergh A 1982 Local differences in Leydig cell morphology in the adult rat testis: evidence for a local control of Leydig cells by adjacent seminiferous tubules. Int J Androl 5:325 Bergh A 1983 Paracrine regulation of Leydig cells by the seminiferous tubules. Int J Androl 6:57 Bergh A 1985 Development of stage-specific paracrine regulation of Leydig cells by the seminiferous tubules. Int J Androl 8:80 Reventos J, Benahmed M, Tabone E, Saez JM 1983 Modulation of Leydig cell activity by Sertoli cell: an in vitro sutdy. C R Acad Sci [III] 296:123 Tabone E, Benahmed M, Reventos, J, Saez JM 1984 Interactions between immature porcine Leydig and Sertoli cells in vitro. An ultrastructural and biochemical study. Cell Tissue Res 237:357 Benahmed M, Reventos J, Tabone E, Saez JM 1985 Cultured Sertoli cell-mediated FSH stimulatory effect on Leydig cell steroidogenesis. Am J Physiol 248:E176 Perrard-Sapori MH, Chatelain P, Vallier P, Saez JM 1986 In vitro interactions between Sertoli cells and steroidogenic cells. Biochem Biophys Res Commun 134:957 Saez JM, Tabone E, Perrard-Sapori MH, Rivarola MA 1986 Paracrine role of Sertoli cells. Med Biol 63:225 Saez JM, Sanchez P, Berthelon MC, Avallet O 1989 Regulation of pig Leydig cell aromatase activity by gonadotropins and Sertoli cells. Biol Reprod 41:813 Reventos J, Perrard-Sapori MH, Chatelain PG, Saez JM 1989 Leydig cell and extracellular matrix effects on Sertoli cell function: biochemical and morphologic studies. J Androl 10:359 Parvinen M, Nikula H, Huhtaniemi I 1984 Influence of rat seminiferous tubules on Leydig cell testosterone production in vitro. Mol Cell Endocrinol 37:331 Bartlett JM, Wu FC, Sharpe RM 1987 Enhancement of Leydig cell testosterone secretion by isolated seminiferous tubules during co-perifusion in vitro: comparison with static co-culture systems. Int J Androl 10:603 Benahmed M, Grenot C, Tabone E, Sanchez P, Morera AM 1985 FSH regulates cultured Leydig cell function via Sertoli cell proteins: an in vitro study. Biochem Biophys Res Commun 132:729 Janecki A, Jakubowiak A, Lukaszyk A 1985 Stimulatory effect of Sertoli cell secretory products on testosterone secretion by purified Leydig cells in primary culture. Mol Cell Endocrinol 42:235 Verhoeven G, Cailleau J 1985 A factor in spent media from Sertoli cell-enriched cultures that stimulates steroidogenesis in Leydig cells. Mol Cell Endocrinol 40:57 Papadopoulos V, Carreau S, Drosdowsky MA 1986 Effects of seminiferous tubule secreted factor(s) on Leydig cell cyclic AMP production in mature rat. FEBS Lett 202:74 Benahmed M, Tabone E, Grenot C, Sanchez P, Chauvin MA, Morera AM 1986 Paracrine control of Leydig cell activity by FSH dependent proteins from Sertoli cells: an in vitro study. J Steroid Biochem 24:311 Verhoeven G, Cailleau J 1986 Specificity and partial purification of a factor in spent media from Sertoli cell-enriched cultures that stimulates steroidogenesis in Leydig cells. J Steroid Biochem 25:393 Papadopoulos V, Kamtchouing P, Drosdowsky MA, Hochereau de Reviers MT, Carreau S 1987 Adult rat Sertoli cells secrete a factor or factors which modulate Leydig cell function. J Endocrinol 114:459 Verhoeven G, Cailleau J 1987 A Leydig cell stimulatory factor produced by human testicular tubules. Mol Cell Endocrinol 49:137 Perrard-Sapori MH, Chatelain PC, Rogemond N, Saez JM 1987 Modulation of Leydig cell functions by culture with Sertoli cells or with Sertoli cell-conditioned medium: effect of insulin, somatomedin-C and FSH. Mol Cell Endocrinol 50:193 Liu YX, Dahl KD 1988 A factor(s) produced by Sertoli cells 525. 526. 527. 528. 529. 530. 531. 532. 533. 534. 535. 536. 537. 538. 539. 540. 541. 542. 543. 544. 545. Vol. 12, No. 1 stimulates androgen biosynthesis by Leydig cells in neonatal rats. Sci Sin [B] 31:818 Carreau S, Papadopoulos V, Drosdowsky MA 1988 Paracrine regulation of Leydig cell aromatase in the rat: development with age. Pathol Biol 36:1002 Carreau S, Papadopoulos V, Drosdowsky MA 1988 Stimulation of adult rat Leydig cell aromatase activity by a Sertoli cell factor. Endocrinology 122:1103 Saez JM, Sanchez P, Berthelon MC, Avallet O 1989 Regulation of pig Leydig cell aromatase activity by gonadotropins and Sertoli cells. Biol Reprod 41:813 Syed V, Khan SA, Ritzen EM 1985 Stage-specific inhibition of interstitial cell testosterone secretion by rat seminiferous tubules in vitro. Mol Cell Endocrinol 40:257 Benahmed M, Morera AM, Chauvin MA 1986 Evidence for a Sertoli cell, FSH-suppressible inhibiting factor(s) of testicular steroidogenic activity. Biochem Biophys Res Commun 139:169 Syed V, Khan SA, Lindh M, Ritzen EM 1987 Ontogeny and cellular origin of a rat seminiferous tubule factor(s) that inhibits LH-dependent testosterone production by interstitial cells in vitro. Int J Androl 10:711 Papadopoulos V, Kamtchouing P, Drosdowsky MA, Carreau S 1987 Spent media from immature seminiferous tubules and Sertoli cells inhibit adult rat Leydig cell aromatase activity. Horm Metab Res 19:62 Syed V, Khan SA, Lindh M, Ritzen EM 1988 Mechanism of action of the factor(s) secreted by rat seminiferous tubules and inhibiting interstitial cell testosterone production in vitro. Acta Endocrinol (Copenh) 119:427 Takase M, Tsutsui K, Kawashima S 1988 Inhibitory effect of Sertoli cell-cultured media on LH binding to mouse Leydig cells in culture. Endocrinol Jpn 35:285 Syed V, Lindh M, Khan SA, Ritzen EM 1989 Hormonal regulation of a rat seminiferous tubule factor which inhibits LH action on interstitial cells. Int J Androl 12:464 Vihko KK, Huhtaniemi 11989 A rat seminiferous epithelial factor that inhibits Leydig cell cAMP and testosterone production: mechanism of action, stage-specific secretion, and partial characterization. Mol Cell Endocrinol 65:119 Syed V, Karpe B, Ploen L, Ritzen EM 1986 Regulation of interstitial cell function by seminiferous tubules in intact and cryptorchid rats. Int J Androl 9:271 Sharpe RM, Cooper 11984 Intratesticular secretion of a factor(s) with major stimulatory effects on Leydig cell testosterone secretion in vitro. Mol Cell Endocrinol 37:159 Sharpe RM 1985 Intratesticular regulation of testosterone secretion: comparison of the effects and interactions of hCG, an LHRH agonist and testicular interstitial fluid on Leydig cell testosterone secretion in vitro. Mol Cell Endocrinol 41:247 Risbridger GP, Jenkin G, de Kretser DM 1986 The interaction of hCG, hydroxysteroids and interstitial fluid on rat Leydig cell steroidogenesis in vitro. J Reprod Fertil 77:239 Sharpe RM, Kerr JB, Fraser HM, Bartlett JM 1986 Intratesticular factors and testosterone secretion. Effect of treatments that alter the level of testosterone within the testis. J Androl 7:180 Sharpe RM, Kerr JB, Cooper I, Bartlett JM 1986 Intratesticular factors and testosterone secretion: the effect of treatment with ethane dimethanesulphonate (EDS) and the induction of seminiferous tubule damage. Int J Androl 9:285 Bartlett JM, Sharpe RM 1987 Effect of local heating of the rat testis on the levels of interstitial fluid of a putative paracrine regulator of the Leydig cells and its relationship to changes in Sertoli cell secretory function. J Reprod Fertil 80:279 Bartlett JMS, Sharpe RM 1987 Effect of local heating of the rat testis on the levels in interstitial fluid of a putative paracrine regulator of the Leydig cells and its relationship to changes in Sertoli cell secretory function. J Reprod Fertil 80:279 Sharpe RM, Cooper 11987 Relationship between the exposure of Leydig cells to factor(s) present in testicular interstitial fluid and changes in their capacity to secrete testosterone during culture or after hCG-induced desensitization. Mol Cell Endocrinol 51:105 Ishida H, Risbridger GP, de Kretser DM 1987 Variation in the L February, 1991 546. 547. 548. 549. 550. 551. 552. 553. 554. 555. 556. 557. 558. 559. 560. 561. 562. 563. 564. 565. 566. 567. V CELL-CELL INTERACTIONS IN THE TESTIS effect of a nongonadotropic Leydig cell stimulating factor in testicular interstitial fluid after exposure of the testis to a single episode of heat treatment. J Androl 8:247 Maddocks S, Sharpe RM 1989 Interstitial fluid volume in the rat testis: androgen-dependent regulation by the seminiferous tubules? J Endocrinol 120:215 Dorrington JH, Armstrong DT 1975 Follicle-stimulating hormone stimulates estradiol-17/3 synthesis in cultured Sertoli cells. Proc Natl Acad Sci USA 72:2677 Brinkmann AO, Mulder E, Lamers-Stahlhofen GJM, Mechielsen MJ, Van der Molen HJ 1972 An oestradiol receptor in rat testis interstitial tissue. FEBS Lett 26:301 Rommerts FF, Brinkman AO 1981 Modulation of steroidogenic activities in testis Leydig cells. Mol Cell Endocrinol 21:15 Valladares LE, Payne AH 1979 Induction of testicular aromatization by luteinizing hormone in mature rats. Endocrinology 105:431 Sharpe RM, Fraser HM 1980 Leydig cell receptors for luteinizing hormone releasing hormone and its agonists and their modulation of administration or deprivation of the releasing hormone. Biochem Biophys Res Commun 95:256 Sharpe RM, Fraser HM, Cooper I, Rommerts FF 1981 SertoliLeydig cell communication via an LHRH-like factor. Nature 290:785 Sharpe RM Fraser HM, Cooper I, Rommerts FF 1982 The secretion, measurement, and function of a testicular LHRH-like factor. Ann NY Acad Sci 383:272 Sharpe RM 1984 Intratesticular factors controlling testicular function. Biol Reprod 30:29 Kerr JB, Sharpe RM 1985 Follicle-stimulating hormone induction of Leydig cell maturation. Endocrinology 116:2592 Sharpe RM, Cooper 11987 Comparison of the effects on purified Leydig cells of four hormones (oxytocin, vasopressin, opiates and LHRH) with suggested paracrine roles in the testis. J Endocrinol 113:89 Saint Pol PS, Hermand E, Tramu G 1988 Paracrine factors in adult rat testis gonadotropin control of opioids and LHRH-like peptide. Andrologia 20:173 Bourne GA, Regiani S, Payne AH, Marshall JC 1980 Testicular GnRH receptors-characterization and localization on interstitial tissue. J Clin Endocrinol Metab 51:407 Clayton RN, Katikineni M, Chan V, Dufau ML, Catt KJ 1980 Direct inhibition of testicular function by gonadotropin-releasing hormone: mediation by specific gonadotropin-releasing hormone receptors in interstitial cells. Proc Natl Acad Sci USA 77:4459 Lefebvre FA, Reeves JJ, Seguin C, Massicotte J, Labrie F 1980 Specific binding of a potent LHRH agonist in rat testis. Mol Cell Endocrinol 20:127 Hedger MP, Risbridger GP, de Kretser DM 1985 Autoradiographical localization of luteinizing hormone releasing hormone (LHRH) receptors on rat testicular intertubular cells fractionated on Percoll density gradients. Aust J Biol Sci 38:435 Sharpe RM, Doogan DG, Cooper 11983 Direct effects of a luteinizing hormone-releasing hormone agonist on intratesticular levels of testosterone and interstitial fluid formation in intact male rats. Endorinology 113:1306 Sharpe RM, Doogan DG, Cooper I 1983 Factors determining whether the direct effects of an LHRH agonist on Leydig cell function in vivo are stimulatory or inhibitory. Mol Cell Endocrinol 32:57 Hedger MP, Robertson DM, Tepe SJ, Browne CA, de Kretser DM 1986 Degradation of luteinizing hormone-releasing hormone (LHRH) and an LHRH agonist by the rat testis. Mol Cell Endocrinol 46:59 Dutlow CM, Millar RP 1981 Rat testis immunoreactive LH-RH differs structurally from hypothalamic LH-RH. Biochem Biophys Res Commun 101:486 Bhasin S, Heber D, Peterson M, Swerdloff R1983 Partial isolation and characterization of testicular GnRH-like factors. Endocrinology 112:1144 Arimura A, Turkelson CM 1984 LHRH-like substance in the rat testis. Ann NY Acad Sci 438:390 75 568. Tres LL, Smith EP, Svoboda ME, Van Wyk JJ, Kierzenbaum AL 1986 Immunoreactive sites and accumulation of somatomedin-C in rat Sertoli-spermatogenic cell co-cultures. Exp Cell Res 162:33 569. Chatelain PG, Naville D, Saez JM 1987 Somatomedin-C/insulinlike growth factor 1-like material secreted by porcine Sertoli cells in vivo: characterization and regulation. Biochem Biophys Res Commun 146:1009 570. Benahmed M, Morera AM, Chauvin MC, de Peretti E 1987 Somatomedin C/insulin-like growth factor-1 as a possible intratesticular regulator of Leydig cell activity. Mol Cell Endocrinol 50:69 571. Handelsman DJ, Spaliviero JA, Scott CD, Baxter RC 1985 Identification of insulin-growth factor-I and its receptors in the rat testis. Acta Endocrinol (Copenh) 109:543 572. Lin T, Blaisdell J, Haskell JF 1987 Type I IGF receptors of Leydig cells are upregulated by human chorionic gonadotropin. Biochem Biophys Res Commun 149:852 573. Kasson BG, Hsueh AJ 1987 Insulin-like growth factor-I augments gonadotropin-stimulated androgen biosynthesis by cultured rat testicular cells. Mol Cell Endocrinol 52:27 574. Morera AM, Chauvin MA, de Peretti E, Binoux M, Benahmed M 1987 Somatomedin C/insulin-like growth factor 1: an intratesticular differentiative factor of Leydig cells? Horm Res 28:50 575. Haugen TB, Fritzson P, Hansson V 1987 Cellular localization and developmental changes of deoxyribonucleoside-activated nucleotidase in the rat testis. Int J Biochem 19:193 576. Lin T, Haskell J, Vinson N, Terracio L 1986 Direct stimulatory effects of insulin-like growth factor-I on Leydig cell steroidogenesis in primary culture. Biochem Biophys Res Commun 137:950 577. Verhoeven G, Cailleau J 1989 Tubule-Leydig cell interactions. Andrology 53:227 578. Ascoli M 1981 Regulation of gonadotropin receptors and gonadotropin responses in a clonal strain of Leydig tumor cells by epidermal growth factor. J Biol Chem 256:179 579. Welsh Jr TH, Hsueh AJW 1982 Mechanism of the inhibitory action of epidermal growth factor on testicular androgen biosynthesis in vitro. Endocrinology 110:1498 580. Verhoeven G, Cailleau J 1986 Stimulatory effects of epidermal growth factor on steroidogenesis in Leydig cells. Mol Cell Endocrinol 47:99 581. Pignataro OP, Ascoli M 1990 Studies with insulin and insulinlike growth factor-1 show that the increased labeling of phosphatidylinositol-3,4-bisphosphate is not sufficient to elicit the diverse actions of epidermal growth factor on MA-10 Leydig tumor cells. Mol Endocrinol 4:758 582. Teerds KJ, Rommerts FF, Dorrington JH 1990 Immunohistochemical detection of transforming growth factor-alpha in Leydig cells during the development of rat testis. Mol Cell Endocrinol 69:R1 583. Avallet O, Vigier M, Perrard-Sapori MH, Saez JM 1987 Transforming growth factor /? inhibits Leydig cell functions. Biochem Biophys Res Commun 146:575 584. Lin T, Blaisdell J, Haskell JF 1987 Transforming growth factor0 inhibits Leydig cell steroidogenesis in primary culture. Biochem Biophys Res Commun 146:387 585. Morera AM, Cochet C, Keramidas M, Chauvin MA, de Peretti E, Benahmed M 1988 Direct regulating effects of transforming growth factor /3 on the Leydig cell steroidogenesis in primary culture. J Steroid Biochem 30:443 586. Fauser BC, Hsueh AJ 1988 Effect of transforming growth factor0 on human chorionic gonadotropin induced testosterone production by cultured rat testicular cells. Life Sci 43:1363 587. Benahmed M, Cochet C, Keramidas M, Chauvin MA, Morera AM 1988 Evidence for a FSH dependent secretion of a receptor reactive transforming growth factor /3-like material by immature Sertoli cells in primary culture. Biochem Biophys Res Commun 154:1222 588. McCullagh DR 1932 Dual endocrine activity of the testis. Science 76:19 589. Franchimont P, Verstraelen-Proyard J, Hazee-Hagelstein MT, Renard CH, Demoulin A, Bourguignon JP, Hustin J 1979 Inhibin: from concepts to reality. Vitam Horm 37:243 76 SKINNER 590. Ying SY 1988 Inhibins, activins and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev 9:267 591. Steinberger A, Steinberger E 1976 Secretion of an FSH inhibiting factor by cultured Sertoli cells. Endocrinology 99:918 592. Bicsak TA, Vale W, Vaughan J, Tucker EM, Cappel S, Hsueh AJ 1987 Hormonal regulation of inhibin production by cultured Sertoli cells. Mol Cell Endocrinol 49:211 593. Morris PL, Vale WW, Cappel S, Bardin CW 1988 Inhibin production by primary Sertoli cell-enriched cultures: regulation by follicle-stimulating hormone, androgens, and epidermal growth factor. Endocrinology 122:717 594. Gonzales GF, Risbridger GP, de Kretser DM 1988 In vitro synthesis and release of inhibin in response to FSH stimulation by isolated segments of seminiferous tubules from normal adult male rats. Mol Cell Endocrinol 59:179 595. Conti M, Culler MD, Negro-Vilar A 1988 Adenosine receptordependent modulation of inhibin secretion in cultured immature rat Sertoli cells. Mol Cell Endocrinol 59:255 596. Gonzales GF, Risbridger GP, de Kretser DM 1989 The effect of insulin on inhibin production in isolated seminiferous tubule segments from adult rats cultured in vitro. Mol Cell Endocrinol 61:209 597. Hsueh AJ, Dahl KD, Vaughan J, Tucker E, Rivier J, Bardin CW, Vale W 1987 Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. Proc Natl Acad Sci USA 84:5082 598. Lin T, Calkins JK, Morris PL, Vale W, Bardin CW 1989 Regulation of Leydig cell function in primary culture by inhibin and activin. Endocrinology 125:2134 599. Sharpe RM, Kerr JB, Maddocks S 1988 Evidence for a role of the Leydig cells in control of the intratesticular secretion of inhibin. Mol Cell Endocrinol 60:243 600. Drummond AE, Risbridger GP, de Kretser DM 1989 The involvement of Leydig cells in the regulation of inhibin secretion by the testis. Endocrinology 125:510 601. Roberts V, Meunier H, Sawchenko PE, Vale W 1989 Differential production and regulation of inhibin subunits in rat testicular cell types. Endocrinology 125:2350 602. Shaha C, Morris PL, Chen CL, Vale W, Bardin CW 1989 Immunostainable inhibin subunits are in multiple types of testicular cells. Endocrinology 125:1941 603. Lee W, Mason AJ, Schwall R, Szonyi E, Mather JP1988 Secretion of activin by interstitial cells in the testis. Science 243:396 604. van Dissel-Emiliani FM, Grootenhuis AJ, de Jong FH, de Rooij DG 1989 Inhibin reduces spermatogonial numbers in testes of adult mice and Chinese hamsters. Endocrinology 125:1899 605. Calkins JH, Sigel MM, Nankin HR, Lin T 1988 Interleukin-I inhibits Leydig cell steroidogenesis in primary culture. Endocrinology 123:1605 606. Calkins JH, Guo H, Sigel MM, Lin T 1990 Differential effects of recombinant interleukin-1 a and fi on Leydig cell function. Biochem Biophys Res Commun 167:548 607. Kasson BG, Hsueh AJ 1986 Arginine vasopressin as an intragonadal hormone in Brattleboro rats: presence of a testicular vasopressin-like peptide and functional vasopressin receptors. Endocrinology 118:23 608. Awoniyi CA, Santuilli R, Sprando RL, Ewing LL, Zirkin BR 1989 Restoration of advanced spermatogenic cells in the experimentally regressed rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 124:1217 609. Awoniyi CA, Santulli R, Chandrashekar V, Schanbacher BD, Zirkin BR 1989 Quantitative restoration of advanced spermatogenic cells in adult male rats made azoospermic by active immunization against luteinizing hormone or gonadotropin-releasing hormone. Endocrinology 125:1303 610. Sun Y-T, Irby DC, Robertson DM, de Kretser D 1989 The effects of exogenously administered testosterone on spermatogenesis in intact and hypophysectomized rats. Endocrinology 125:1000 611. Santulli R, Sprando RL, Awoniyi CA, Ewing LL, Zirkin BR 1990 To what extent can spermatogenesis be maintained in the hypo- 612. 613. 614. 615. 616. 617. 618. 619. 620. 621. 622. 623. 624. 625. 626. 627. 628. 629. 630. 631. 632. 633. Vol. 12, No. 1 physectomized adult rat testis with exogenously administered testosterone? Endocrinology 126:95 Lording DW, de Kretser DM 1972 Comparative ultrastructural and histochemical studies of the interstitial cells of the rat testis during fetal and postnatal development. J Reprod Fertil 29:261 Tapanainen J, Kuopio T, Pelliniemi LJ, Huhtaniemi I 1984 Rat testicular endogenous steroids and number of Leydig cells between the fetal period and sexual maturity. Biol Reprod 31:1027 Prince FP 1984 Ultrastructure of immature Leydig cells in the human prepubertal testis. Anat Rec 209:165 Teerds KJ, De Rooij DG, Rommerts FF, van der Tweel I, Wensing CJ 1989 Turnover time of Leydig cells and other interstitial cells in testes of adult rats. Arch Androl 23:105 Verhoeven G 1980 Androgen receptor in cultured interstitial cells derived from immature rat testis. J Steroid Biochem 13:469 Anthony CT, Kovacs WJ, Skinner MK 1989 Analysis of the androgen receptor in isolated testicular cell types with a microassay that uses an affinity ligand. Endocrinology 125:2628 Cunha GR 1973 The role of androgens in the epithelio-mesenchymal interactions involved in prostatic morphogenesis in embryonic mice. Anat Rec 175:87 Sugimura Y, Cunha GR, Donjacour AA 1986 Morphogenesis of ductal networks in the mouse prostate. Biol Reprod 34:961 Sugimura Y, Cunha GR, Donjacour AA 1986 Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol Reprod 34:973 Swinnen K, Cailleau J, Heyns W, Verhoeven G 1989 Stromal cells from the rat prostate secrete androgen-regulated factors which modulate Sertoli cell function. Mol Cell Endocrinol 62:147 Swinnen K, Cailleau J, Heyns W, Verhoeven G 1990 Prostatic stromal cells and testicular peritubular cells produce similar paracrine mediators of androgen action. Endocrinology 126:142 Kerr JB, Donachie K, Rommerts FF 1985 Selective destruction and regeneration of rat Leydig cells in vivo. A new method for the study of seminiferous tubular-interstitial tissue interaction. Cell Tissue Res 242:145 Molenaar R, de Rooij DG, Rommerts FF, Reuvers PJ, van der Molen HJ 1985 Specific destruction of Leydig cells in mature rats after in vivo administration of ethane dimethyl sulphonate. Biol Reprod 33:1213 Morris ID, Phillips DM, Bardin CW 1986 Ethylene dimethanesulfonate destroys Leydig cells in the rat testis. Endocrinology 118:709 Verhoeven G, Cailleau J, Morris ID 1989 Inhibitory effects of alkane sulphonates on the function of immature rat Leydig, Sertoli and peritubular cells cultured in vitro. J Mol Endocrinol 2:145 Roberts KP, Griswold MD 1990 Ethylene dimethanesulphonate inhibits the functions of Sertoli cells in culture at sublethal doses. Endocrinology 126:1618 Kerr JB, Bartlett JM, Donachie K 1986 Acute response of testicular interstitial tissue in rats to the cytotoxic drug ethane dimethanesulphonate. An ultrastructural and hormonal assay study. Cell Tissue Res 243:405 Kerr JB, Donachie K 1986 Regeneration of Leydig cells in unilaterally cryptorchid rats: evidence for stimulation by local testicular factors. Cell Tissue Res 245:649 Bartlett JM, Kerr JB, Sharpe RM 1986 The effect of selective destruction and regeneration of rat Leydig cells on the intratesticular distribution of testosterone and morphology of the seminiferous epithelium. J Androl 7:240 Kerr JB, Bartlett JM, Donachie K, Sharpe RM 1987 Origin of regenerating Leydig cells in the testis of the adult rat. An ultrastructural, morphometric and hormonal assay study. Cell Tissue Res 249:367 Risbridger GP, Kerr JB, de Kretser DM 1987 Influence of the cryptorchid testis on the regeneration of rat Leydig cells after administration of ethane dimethane sulphonate. J Endocrinol 112:197 Hardy MP, Zirkin BR, Ewing LL 1989 Kinetic studies on the February, 1991 634. 635. 636. 637. 638. 639. 640. 641. 642. 643. 644. 645. 646. 647. 648. 649. 650. 651. 652. 653. 654. 655. 656. CELL-CELL INTERACTIONS IN THE TESTIS development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology 124:762 Hardy MP, Kelce WR, Klinefelter GR, Ewing LL 1990 Differentiation of Leydig cell precursors in vitro: a role for androgen. Endocrinology 127:488 Christensen AK, Gillim SW 1969 The correlation of fine structure and function in steroid-secreting cells with emphasis on those of the gonad. In: McKerns KW (ed) The Gonads. Appleton-CenturyCrofts, New York, p 415 Wing T-Y, Lin H-S 1977 The fine structure of testicular interstitial cells in the adult golden hamster with special reference to seasonal changes. Cell Tissue Res 183:385 Miller SC, Bowman BM, Rowland HG 1983 Structure, cytochemistry, endocytic activity, and immunoglobulin (FC) receptors of rat testicular interstitial-tissue macrophages. Am J Anat 168:1 Yee JB, Hutson JC 1983 Testicular macrophages: isolation, characterization and hormonal responsiveness. Biol Reprod 29:1319 Berg A 1985 Effect of cryptorchidism on the morphology of testicular macrophages: evidence for a Leydig cell-macrophage interaction. Int J Androl 8:86 Yee JB, Hutson JC 1985 Effects of testicular macrophage-conditioned medium on Leydig cells in culture. Endocrinology 116:2682 Wei RQ, Yee JB, Straus DC, Hutson JC 1988 Bactericidal activity of testicular macrophages. Biol Reprod 38:830 Miller SC, Bowman BM, Roberts LK 1984 Identification and characterization of mononuclear phagocytes isolated from rat testicular interstitial tissues. J Leukocyte Biol 36:679 Niemi M, Sharpe RM, Brown WR 1986 Macrophages in the interstitial tissue of the rat testis. Cell Tissue Res 243:337 el-Demiry MI, Hargreave TB, Busuttil A, Elton R, James K, Chisholm GD 1987 Immunocompetent cells in human testis in health and disease. Fertil Steril 48:470 Hedger MP, Eddy EM 1987 The heterogeneity of isolated adult rat Leydig cells separated on Percoll density gradients: an immunological, cytochemical and functional analysis. Endocrinology 121:1824 Pollanen P, Niemi M 1987 Immunohistochemical identification of macrophages, lymphoid cells and HLA antigens in the human testis. Int J Androl 10:37 Pollanen P, Maddocks S 1988 Macrophages, lymphocytes and MHC II antigen in the ram and rat testis. J Reprod Fertil 82:437 Head JR, Neaves WB, Billingham RE 1983 Immune privilege in the testis. I. Basic parameters of allograft survival. Transplantation 36:423 Hedger MP 1989 The testis: an 'immunologically suppressed' tissue? Reprod Fertil Dev 1:75 Tung KSK, Teuscher C, Meng AL 1981 Autoimmunity to spermatozoa and the testis. Immunol Rev 55:217 Johnson MH 1973 Physiological mechanisms for the immunological isolation of spermatozoa. Adv Reprod Physiol 6:279 Hedger MP, Qin J-X, Robertson DM, de Kretser DM 1990 Intragonadal regulation of immune system functions. Reprod Fertil Dev 2:263 Born W, Wekerle H 1981 Leydig cells nonspecifically suppress lymphoproliferation in vitro: implications for the testis as an immunologically privileged site. Am J Reprod Immunol 2:291 Head Jr, Billingham RE 1985 Immune privilege in the testis. II. Evaluation of potential local factors. Transplantation 40:269 Pollanen P, Soder O, Uksila J 1988 Testicular immunosuppressive protein. J Reprod Immunol 14:125 Orava M, Centell K, Vihko R 1985 Human leukocyte interferon inhibits human chorionic gonadotropin stimulated testosterone 657. 658. 659. 660. 661. 662. 663. 664. 665. 666. 667. 668. 669. 670. 671. 672. 673. 674. 675. 676. 77 production by porcine Leydig cells in culture. Biochem Biophys Res Commun 127:809 Orava M, Voutilainen R, Vihko R 1989 Interferon-gamma inhibits steroidogenesis and accumulation of mRNA of the steroidogenic enzymes P450scc and P450cl7 in cultured porcine Leydig cells. Mol Endocrinol 3:887 Mealy K, Robinson B, Millette CF, Majzoub J, Wilmore DW 1990 The testicular effects of tumor necrosis factor. Ann Surg 211:470 Free MJ 1977 Blood supply to the testis and its role in local exchange and transport of hormones. In: Johnson AD, Gomes WR (eds) The Testis. Academic Press, New York, vol 4:39 Kormano M 1968 Penetration of intravenous trypan blue into the rat testis and epididymis. Acta Histochem 30:133 Kormano M 1973 Blood supply to testes and excurrent duct. Adv Biosci 10:73 Setchell BP 1970 Testicular blood supply, lymphatic drainage, and secretion of fluid. In: Johnson AD, Gomes WR, Vandemark NL (eds) The Testis. Academic Press, New York, vol 4:101 Setchell BP 1990 Local control of testicular fluids. Reprod Fertil Dev 2:291 Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G 1987 Structural characterization and biological functions of fibroblast growth factor. Endocr Rev 8:95 Ueno N, Baird A, Esch F, Ling N, Guillemin R 1987 Isolation and partial characterization of basic fibroblast growth factor form bovine testis. Mol Cell Endocrinol 49:189 Story MT, Sasse J, Kakuska D, Jacobs SC, Lawson RK 1988 A growth factor in bovine and human testes structurally related to basic fibroblast growth factor. J Urol 140:422 Fawcett DW 1973 Observations on the organization of the interstitial tissue of the testis and on the occluding cell junctions in the seminiferous epithelium. Adv Biosci 10:83 Fawcett DW, Heidger PM, Leak LV 1969 Lymph vascular system of the interstitial tissues of the testis as revealed by electron microscopy. J Reprod Fertil 19:109 Fawcett DW, Neaves WB, Flores MN 1973 Comparative observations on intertubular lymphatis and the organization of the interstitial tissue of the mammalian testis. Biol Reprod 9:500 Bellve AR, Zheng W 1989 Growth factors as autocrine and paracrine modulators of male gonadal functions. J Reprod Fertil 85:771 Fauser BC, Baird A, Hsueh AJ 1988 Fibroblast growth factor inhibits luteinizing hormone-stimulated androgen production by cultured rat testicular cells. Endocrinology 123:2935 Raeside JI, Berthelon MC, Sanchez P, Saez JM 1988 Stimulation of aromatase activity in immature porcine Leydig cells by fibroblast growth factor (FGF). Biochem Biophys Res Commun 151:163 Sordoillet C, Chauvin MA, Revol A, Morera AM, Benahmed M 1988 Fibroblast growth factor is a regulator of testosterone secretion in cultured immature Leydig cells. Mol Cell Endocrinol 58:283 Murono EP, Washburn AL 1990 Basic fibroblast growth factor inhibits delta5-3j8-hydroxysteroid dehydrogenase-isomerase activity in cultured immature Leydig cells. Biochem Biophys Res Commun 168:248 Jaillard C, Chatelain PG, Saez JM 1987 In vitro regulation of pig Leydig cell growth and function: effects of fibroblast growth factor and somatomedin-C. Biol Reprod 37:665 Skinner MK 1990 Mesenchymal (stromal)-epithelial interactions in the testis and ovary which regulate gonadal function. Reprod Fertil Dev 2:237