May/June 2010 • Volume 8 • Issue 3
EDITORIAL
Contemporary American Dermatology:
Continuing Medical Education I
Lambert and Parish
DEPARTMENTS
Historical Vignette
The Advent of a Novel Diagnosis:
Melanoma Through the Ages
Ezra and Rabie
ORIGINAL CONTRIBUTIONS
Incidence of Childhood Dermatosis in India
Patel, Vyas, Berman, and Vierra
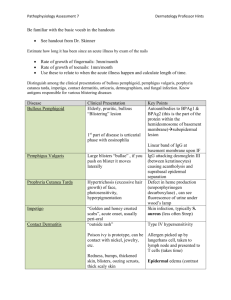
Pemphigus Vulgaris and Pregnancy
Drenovska, Darlenski, Kazandjieva, and Vassileva
Hydrocortisone Butyrate 0.1% Cream
(Proprietary Lipid Rich Cream Vehicle) Does Not
Significantly Suppress Hypothalamic-Pituitary-Adrenal Axis
and Is Effective in Pediatric Patients 3 Months and Older
With Extensive Atopic Dermatitis
Abramovits and Oquendo
REVIEWS
Segmental Neurofibromatosis and Malignancy
Dang and Cohen
Diagnosis and Treatment of Acanthosis Nigricans
Kapoor
History of Teledermatology:
A Technique of the Future in Dermatology
Şenel
New to the Clinic
Vibativ (Telavancin) for Complicated Skin and
Skin Structure Infections
Ghasri and Scheinfeld
MYTHS AND MISCONCEPTIONS
Mothers With Anogenital HPV
Should Avoid Breastfeeding: Myth or…?
Wolf, Wolf, and Davidovici
CASE STUDIES
A Rare Manifestation of Nail Changes With
Docetaxel Therapy
Halvorson, Erickson, and Gaspari
Faun Tail: A Rare Cutaneous Marker of
Spinal Dysraphism
Polat, Polat, Öztaş, Kaya, and Alli
Werner Syndrome in an Iranian Family
Hallaji, Barzegari, and Kiavash
Ulcerative Granulomatous Mycosis Fungoides
Parker, Traywick, and Arbiser
Contact Dermatitis Due to Temporary Henna Tattoos
Kazandjieva, Balabanova, Kircheva, and Tsankov
Recommend a stretch marks therapy
that lives up to its promises.
Wear your skin proudly.™
Introducing New Mederma® Stretch Marks Therapy. Dermatologist tested and clinically proven to
reduce discoloration, improve texture and enhance softness.1
ACTUAL RESULTS
80% of women in a clinical study showed visible improvement
in 12 weeks. Now, when your patients ask about stretch marks,
you can confidently answer, “There is something you can do.”
Available for in-office dispensing.
More information at Mederma.com
1 Data
on file, MEDE-200-301. April 2009.
Before
After 12 weeks
TABLE OF CONTENTS
May/June 2010 • Volume 8 • Issue 3
EDITORIAL
Contemporary American Dermatology: Continuing Medical Education I......................................................... 134
W. Clark Lambert, MD, PhD; Lawrence Charles Parish, MD, MD (Hon)
ORIGINAL CONTRIBUTIONS
Incidence of Childhood Dermatosis in India................................................................................................... 136
Jitendrakumar K. Patel, MD; Aniruddha P. Vyas, MD; Brian Berman, MD, PhD; Martha Vierra, MD
Pemphigus Vulgaris and Pregnancy.............................................................................................................. 144
Kossara Drenovska, MD, PhD; Razvigor Darlenski, MD; Jana Kazandjieva, MD, PhD; Snejina Vassileva, MD, PhD
Hydrocortisone Butyrate 0.1% Cream (Proprietary Lipid Rich Cream Vehicle)
Does Not Significantly Suppress Hypothalamic-Pituitary-Adrenal Axis and
Is Effective in Pediatric Patients 3 Months and Older With Extensive Atopic Dermatitis................................... 150
William Abramovits, MD; Marcial Oquendo, MD
REVIEWS
Segmental Neurofibromatosis and Malignancy.............................................................................................. 156
Julie D. Dang, DO; Philip R. Cohen, MD
Diagnosis and Treatment of Acanthosis Nigricans......................................................................................... 161
Shailendra Kapoor, MD
Self-Test Review Questions (p. 165)
History of Teledermatology: A Technique of the Future in Dermatology.......................................................... 167
Engin Şenel, MD
Departments
Historical Vignette
Charles Steffen, MD, Section Editor
The Advent of a Novel Diagnosis: Melanoma Through the Ages...................................................................... 172
Navid Ezra, BS; Jason Rabie, BS
New to the Clinic
Noah S. Scheinfeld, MD, JD, Section Editor
Vibativ (Telavancin) for Complicated Skin and Skin Structure Infections......................................................... 175
Pedram Ghasri, BA; Noah S. Scheinfeld, MD, JD
Myths and Misconceptions
Ronni Wolf, MD, Section Editor
Mothers With Anogenital HPV Should Avoid Breastfeeding: Myth or…?........................................................... 177
Ronni Wolf, MD; Danny Wolf, MD; Batya Davidovici, MD
129
May/June 2010
TABLE OF CONTENTS
CASE STUDIES
Vesna Petronic-Rosic, MD, MSc, Section Editor
A Rare Manifestation of Nail Changes With Docetaxel Therapy...................................................................... 179
Christian R. Halvorson, MD; Corinne L. Erickson, MD; Anthony A. Gaspari, MD
Faun Tail: A Rare Cutaneous Marker of Spinal Dysraphism............................................................................ 181
Muhterem Polat, MD; Fazli Polat, MD; Pinar Öztaş, MD; Canan Kaya, MD; Nuran Alli, MD
Werner Syndrome in an Iranian Family......................................................................................................... 184
Zahra Hallaji, MD; Massumeh Barzegari, MD; Katrin Kiavash, MD
Ulcerative Granulomatous Mycosis Fungoides............................................................................................... 188
Sareeta R.S. Parker, MD; Carmen Traywick, MD; Jack L. Arbiser, MD, PhD
Contact Dermatitis Due to Temporary Henna Tattoos..................................................................................... 191
Jana Kazandjieva, MD; Maria Balabanova, MD; Kamelia Kircheva, MD; Nikolai Tsankov, MD
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
130
For your patients with tinea pedis, tinea cruris, and tinea corporis
For the fungicidal power you trust, turn to Naftin® (naftifine HCl 1%).
Designed to suit a range of patients and preferences, Naftin® delivers
power against tinea pedis, cruris and corporis. Choose Naftin® Gel,
Naftin® Cream, or the innovative Naftin® Cream Pump and give your
patients the customized care they deserve.
Naftin® (naftifine HCl 1%) Cream and Gel are indicated for the topical treatment of tinea pedis,
tinea cruris and tinea corporis caused by Trichophyton rubrum, Trichophyton mentagrophytes,
Epidermophyton floccosum and Trichophyton tonsurans (Gel only).
Naftin® Cream and Gel are contraindicated in individuals who have shown hypersensitivity
to any of their components and are for topical use only.
During clinical trials with Naftin® Cream and Gel, the following side effects were most commonly
reported: burning/stinging, dryness, erythema, itching, local irritation, skin tenderness and rash.
Please see brief summary on the following page.
©2010 Merz Pharmaceuticals
All rights reserved.
5/10
Power made personal
May/June 2010
Volume 8 • Issue 3
ABOUT OUR JOURNAL
SKINmed: Dermatology for the Clinician®, print ISSN 1540-9740, online ISSN
1751-7125, is published bimonthly by Pulse Marketing & Communications,
LLC, located at 4 Peninsula Avenue, Sea Bright, NJ 07760.
BRIEF SUMMARY
Rx ONLY
INDICATIONS AND USAGE: Naftin® Cream, 1% is
indicated for the topical treatment of tinea pedis, tinea
cruris, and tinea corporis caused by the organisms
Trichophyton rubrum, Trichophyton mentagrophytes,
and Epidermophyton floccosum. Naftin® Gel, 1% is
indicated for the topical treatment of tinea pedis, tinea
cruris, and tinea corporis caused by the organisms
Trichophyton rubrum, Trichophyton mentagrophytes,
Trichophyton tonsurans*, Epidermophyton floccosum*.
*Efficacy for this organism in this organ system was
studied in fewer than 10 infections.
CONTRAINDICATIONS: Naftin® Cream and Gel, 1%
are contraindicated in individuals who have shown
hypersensitivity to any of their components.
WARNINGS: Naftin® Cream and Gel, 1% are for
topical use only and not for ophthalmic use.
PRECAUTIONS: General: Naftin® Cream and
Gel, 1%, are for external use only. If irritation or sensitivity develops with the use of Naftin® Cream or Gel,
1%, treatment should be discontinued and appropriate
therapy instituted. Diagnosis of the disease should be
confirmed either by direct microscopic examination of
a mounting of infected tissue in a solution of potassium hydroxide or by culture on an appropriate medium.
Information for patients: The patient should be told to:
1. Avoid the use of occlusive dressings or wrappings
unless otherwise directed by the physician.
2. Keep Naftin® Cream and Gel, 1% away from the
eyes, nose, mouth and other mucous membranes.
Carcinogenesis, mutagenesis, impairment of fertility:
Long-term studies to evaluate the carcinogenic
potential of Naftin® Cream and Gel, 1% have not been
performed. In vitro and animal studies have not
demonstrated any mutagenic effect or effect on fertility.
Printed in the USA.
Disclaimer: The Publisher, Editors, and Editorial Board cannot be held responsible for errors or any consequences arising from the use of information contained
in this journal; the views and opinions expressed herein do not necessarily reflect
those of the Publisher, Editors, and Editorial Board, neither does the publication
of advertisements constitute any endorsement by the Publisher, Editors, and Editorial Board of the products or services advertised. The Publisher, Editors, Editorial
Board, Reviewers, Authors, and Affiliated Agents shall not be held responsible or
in any way liable for the continued accuracy of the information or for any errors,
inaccuracies, or omissions of any kind in this publication, whether arising from
negligence or otherwise, or for any consequences arising thereafter.
Copyright: ©2010 Pulse Marketing & Communications, LLC. All rights reserved.
No part of this publication may be reproduced, stored, or transmitted in any form or
by any means without the prior permission in writing from the Publisher. Requests
should be addressed to the Permissions Editor at: Pulse Marketing & Communications, LLC, 4 Peninsula Avenue, Sea Bright, NJ 07760.
Abstracting & Indexing: The journal is indexed in Index Medicus/MEDLINE.
Editorial
MANAGING EDITOR
Sarah D. Staats
sstaats@skinmedjournal.com
PRODUCTION DIRECTOR
Scott C. Bouchard
Distinct Layouts, LLC
www.distinctlayouts.com
EDITORIAL DIRECTOR
Gila Berkowitz
gberkowitz@skinmedjournal.com
ASSOCIATE MANAGING EDITOR
Elizabeth Holcomb
eholcomb@skinmedjournal.com
Pregnancy: Teratogenic Effects: Pregnancy
Category B: Reproduction studies have been performed
in rats and rabbits (via oral administration) at doses
150 times or more than the topical human dose and
have revealed no evidence of impaired fertility or harm
to the fetus due to naftifine. There are, however, no
adequate and well-controlled studies in pregnant
women. Because animal reproduction studies are not
always predictive of human response, this drug should
be used during pregnancy only if clearly needed.
Nursing mothers: It is not known whether this drug
is excreted in human milk. Because many drugs are
excreted in human milk, caution should be exercised
when Naftin® Cream or Gel,1% are administered to a
nursing woman.
Pediatric use: Safety and effectiveness in pediatric
patients have not been established.
Publishing
PUBLISHER
Art Kalaka
Corporate
President
Arthur Kalaka
akalaka@skinmedjournal.com
skinmedbriefPI.indd 1
Chief Executive Officer
Jo-Ann Kalaka-Adams
jadams@skinmedjournal.com
General Counsel
Marianne Mckenzie
mmckenzie@skinmedjournal.com
ADVERSE REACTIONS: During clinical trials with
Naftin® Cream, 1%, the incidence of adverse reactions
was as follows: burning/stinging (6%), dryness (3%)
erythema (2%), itching (2%), local irritation (2%).
During clinical trials with Naftin® Gel, 1%, the
incidence of adverse reactions was as follows:
burning/stinging (5.0%), itching (1.0%), erythema
(0.5%), rash (0.5%), skin tenderness (0.5%).
Manufactured for Merz Pharmaceuticals, Greensboro, NC 27410
© 2010 Merz Pharmaceuticals Rev 3/10
Associate Publisher
James R. Adams
jadams@skinmedjournal.com
Pulse Marketing & Communications, LLC
4 Peninsula Avenue • Suite 401 • Sea Bright, NJ 07760 • Tel (732) 747-6525 • Fax (732) 747-7010
6/11/10 12:05 PM
132
May/June 2010
EDITORIAL BOARD
EDITOR IN CHIEF
Lawrence Charles Parish, MD, MD (Hon)
Philadelphia, PA
DEPUTY EDITORS
William Abramovits, MD
Dallas, TX
Larry E. Millikan, MD
Meridian, MS
Jennifer L. Parish, MD
Philadelphia, PA
Marcia Ramos-e-Silva, MD, PhD
Rio de Janeiro, Brazil
EDITORIAL BOARD
Mohamed Amer, MD
Cairo, Egypt
Anthony A. Gaspari, MD
Baltimore, MD
George M. Martin, MD
Kihei, HI
Virendra N. Sehgal, MD
Delhi, India
Robert L. Baran, MD
Cannes, France
Michael Geiges, MD
Zurich, Switzerland
David I. McLean, MD
Vancouver, British Columbia
J. Graham Smith Jr, MD
Mobile, AL
Anthony V. Benedetto, DO
Philadelphia, PA
Michael H. Gold, MD
Nashville, TN
Marc S. Micozzi, MD, PhD
Bethesda, MD
Charles Steffen, MD
Oceanside, CA
Brian Berman, MD, PhD
Miami, FL
Orin M. Goldblum, MD
Abbott Park, IL
George F. Murphy, MD
Boston, MA
Alexander J. Stratigos, MD
Athens, Greece
Jack M. Bernstein, MD
Dayton, OH
Lowell A. Goldsmith, MD, MPH
Chapel Hill, NC
Oumeish Youssef Oumeish, MD, FRCP
Amman, Jordan
James S. Studdiford III, MD
Philadelphia, PA
Sarah Brenner, MD
Tel Aviv, Israel
Aditya K. Gupta, MD, PhD, FRCP(C)
London, Ontario
Joseph L. Pace, MD, FRCP
Naxxar, Malta
Robert J. Thomsen, MD
Los Alamos, NM
Joaquin Calap Calatayud, MD
Cadiz, Spain
Seung-Kyung Hann, MD, PhD
Seoul, Korea
Art Papier, MD
Rochester, NY
Julian Trevino, MD
Dayton, OH
Vesna Petronic-Rosic, MD, MSc
Chicago, IL
Sandy Sharon Tsao, MD
Boston, MA
Henry H.L. Chan, MB, MD, PhD, FRCP Roderick J. Hay, BCh, DM, FRCP, FRCPath
Hong Kong, China
London, UK
Ncoza C. Dlova, MBChB, FCDerm
Durban, South Africa
Tanya R. Humphreys, MD
Philadelphia, PA
Johannes Ring, MD, DPhil
Munich, Germany
Snejina Vassileva, MD, PhD
Sofia, Bulgaria
Richard L. Dobson, MD
Mt Pleasant, SC
Camila K. Janniger, MD
Englewood, NJ
Roy S. Rogers III, MD
Rochester, MN
Daniel Wallach, MD
Paris, France
William H. Eaglstein, MD
Palo Alto, CA
Abdul-Ghani Kibbi, MD
Beirut, Lebanon
Donald Rudikoff, MD
New York, NY
Michael A. Waugh, MB, FRCP
Leeds, UK
Boni E. Elewski, MD
Birmingham, AL
W. Clark Lambert, MD, PhD
Newark, NJ
Robert I. Rudolph, MD
Wyomissing, PA
Wm. Philip Werschler, MD
Spokane, WA
Charles N. Ellis, MD
Ann Arbor, MI
Andrew P. Lazar, MD
Highland Park, IL
Vincenzo Ruocco, MD
Naples, Italy
Joseph A. Witkowski, MD
Philadelphia, PA
Howard A. Epstein, PhD
Gibbstown, NJ
Jasna Lipozencic, MD, PhD
Zagreb, Croatia
Noah S. Scheinfeld, MD, JD
New York, NY
Ronni Wolf, MD
Rechovot, Israel
Ibrahim Hassan Galadari, MD, PhD, FRCP
Dubai, United Arab Emirates
133
May/June 2010
Volume 8 • Issue 3
EDITORIAL
Contemporary American Dermatology:
Continuing Medical Education I
W. Clark Lambert, MD, PhD;1 Lawrence Charles Parish, MD, MD (Hon)2
“‘The time has come,’ the Walrus said, ‘to talk of many things: of [CME, and MOC, and recertification,] and kings.’”*
Lewis Carroll, “The Walrus and the Carpenter,” Through the Looking-Glass, and What Alice Found There
C
ontinuing medical education (CME) continues to be
among the buzzwords of the 21st century. Physicians are
subject to penalties for not completing a specified number
of hours of CME in a calendar year. Medical societies continue to
thrive on providing CME programs, while medical schools have
deans whose sole responsibilities are CME programs.
Definitions
The American Medical Association defines CME in the following terms: “A physician’s continuing professional development
is critical to keeping up with advances in medicine and with
changes in the delivery of care.”
Harvard Medical School’s CME Web site states: “Our continuing
education mission is to optimize patient care. To this end, our
programs are designed to provide the most up-to-date information and strategies for physicians and allied health professionals.”
On their Web site, the Johns Hopkins School of Medicine offers
a slightly different approach: “We strive to provide exemplary
educational activities, which teach evidence-based practices and
identify new and emerging health care needs and opportunities
from research through delivery of care so that, through education, we can significantly improve health.”
If the definition were stated more succinctly, it might read: Physicians have an obligation to stay continually abreast of advancements in their respective fields.
Purpose and Means
CME “credits” can be obtained in any number of ways, from attending a meeting to reading a journal. There have been annual
meetings, international congresses, and specific courses offered
by societies, schools, hospitals, and independent organizations.
There are journals, books, and even independent supplements
purporting to provide educational information.
Why then an editorial on the subject? CME is not something
new but something that has been an integral part of medicine.
Even when American medical schools consisted of a 3-year ungraded curriculum, there were spring and fall courses to supplement the curriculum and to permit practitioners to learn about
recent advancements. The postgraduate schools and polyclinics
developed out of this need.
As medicine evolved in the 20th century in the United States,
organized postgraduate training in residencies and then examinations to measure competency emerged. A physician became
“board certified” in one and/or another specialty, with the certification generally awarded for life, following the passing of a thorough examination and, in some specialties, a designated number
of years of practice beyond residency. More recently, more has
been required. Initially, CME was sufficient, but not so long ago,
“maintenance of certification (MOC)” and now “recertification”
have become the norm, with initial board certification awarded
on a time-limited basis. Recertification has itself been expanded
to include not just periodically taking a rigorous, proctored examination, but also maintaining a list of other activities, such as
the monitoring of office records.
The Downside
With this progress came rules and regulations and new accrediting and regulatory bodies. State boards of medicine began to
demand a certain number of CME hours to maintain licensure. With each political wind, they added new requirements,
*With apologies to Lewis Carroll.
From the Department of Pathology, Divisions of Dermatopathology and Anatomic Pathology; the Department of Dermatology, University
of Medicine and Dentistry of New Jersey–New Jersey Medical School, Newark, NJ;1 and the Department of Dermatology and Cutaneous
Biology and the Jefferson Center for International Dermatology, Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA2
Address for Correspondence: W. Clark Lambert, MD, PhD, Room C520, Medical Science Building, University of Medicine and Dentistry of
New Jersey, New Jersey Medical School, 185 South Orange Avenue, Newark, NJ 07103 • E-mail: lamberwc@umdnj.edu
134
May/June 2010
EDITORIAL
such as safety and quality (whatever these should mean). Forget
that medicine is a broad specialty, and many physicians do not
provide hospital care or practice general medicine. We wonder
whether these legislators take recurring examination themselves,
before they are permitted to write laws or pass them.
Each of the activities described above has itself become formalized,
with presenters of talks at CME-approved events, for example, required to present “goals and objectives,” often requiring extensive
information on why and how each topic was presented and to
have a disinterested party present to evaluate the event. Then, the
presenter is required to document how the success or failure of
each aspect of the presentation was measured, and these results
have to be presented to an accrediting agency. All of these regulatory activities have laudable goals; they are ostensibly designed to
help the physician and protect the public, and each one, individually, may arguably do so. This, in turn, fuels and justifies further
calls for increased regulation1; however, we believe that, collectively, these regulatory measures have become counterproductive. For
the physician, keeping up and helping other physicians keep up
have become ways of life. The tail is now wagging the dog!
Stricter and more stringent rules have permitted offices of CME
to develop. Because they are now able to exert more authority,
the bureaucrats running these departments have become more
powerful. With this, new regulations and charges have emerged,
and the entire process is expanding and redoubling on itself, creating an absurd atmosphere. We are leaving Lewis Carroll behind and are progressing to the world of George Orwell.
to ensure that the program was appropriate. What happens if the
speaker diverges from his or her abstract or the presentation is
a somnolent affair? Should the audience be punished even more
by the unnecessary charges?
Consider a journal contribution that disseminates questionable
information. The author is an alleged authority, and the peer
reviewers agreed with his or her ideas. The participant must swallow the dribble completely, for fear of not passing the recertification examination thrust upon him or her. Alternatively, the
information may be correct but irrelevant to the physician, who
has subspecialized within his or her specialty; he or she must
learn it anyway, taking time from learning what is actually relevant and which may help his or her patients to satisfy certification/recertification requirements.
The Last Straw
All of this is occurring in an environment in which the United
States is educating and training far too few physicians to meet
our needs, not just in primary care specialties, but in other ones
as well.2 These regulatory burdens are stressing an already overburdened system. We are also draining other, less developed
countries of physicians sorely needed at home.3 Surely, these absurd physician shortages should be addressed before additional
regulatise is added, even to the extent that it is justified.
References
The Downside Magnified
Imagine organizing an international meeting and being directed
to pay for an associate dean and a secretary to attend the congress
1 Herman JB. Increasing the value of the state medical license. N
Engl J Med. 2010;362:1459–1461.
2 Pardes H. Health insurance doesn’t mean much without doctors.
[Letter] The Wall Street Journal. April 27, 2010:A16.
3 Wiley E. [Letter to the editor.] The Wall Street Journal. April 27,
2010:A16.
BODY ODOR (BROMHIDROSIS)
Medications known to cause body odor
Felbatol
Omega-3 acids
Selenium
Levocarnitine
Provera
Synarel
Lupron
Salagen
Topamax
Adapted from Litt JZ. Curious, Odd, Rare, and Abnormal Reactions to Medications. Fort Lee, NJ: Barricade Books; 2009:150–155.
135
May/June 2010
Volume 8 • Issue 3
Original Contribution
Incidence of Childhood Dermatosis in India
Jitendrakumar K. Patel, MD;1* Aniruddha P. Vyas, MD;2 Brian Berman, MD, PhD;1 Martha Vierra, MD1
Abstract
The incidence of dermatologic conditions in the pediatric age group presents a pattern that often differs from that in adults; this is important
for epidemiologic studies and population-based analysis. This clinical study was carried out in children up to age 14 in the western part of
India. Dermatologic conditions were tabulated based on the etiology, incidence, age, and sex distribution, as well as seasonal variations,
and the results were analyzed. There were a total of 390 boys and 310 girls. The majority of skin conditions in newborns are transient. The
most common dermatoses found were of infectious etiology (38.43%) in which impetigo (11.13%) and pyoderma (8.9%) were the most
common. In infectious etiology, incidence of scabies was 5.32%. Viral warts were the most common viral infections followed by molluscum
contagiosum. Incidence of eczema, atopic dermatitis, and sweat gland disorders were 6.64%, 0.83%, and 8.86%, respectively. The study
shows various unique features of tropical pediatric dermatology in a developing country, such as high frequency of infections and infectious,
nutritional, and environmentally associated disorders. Many of these dermatoses can be controlled by proper environmental sanitation,
improving nutrition, awareness among parents and children, and preventing overcrowding. (SKINmed. 2010;8:136–142)
T
he incidence of dermatologic conditions in the pediatric
age group presents a pattern that often differs from that
in adults; this is important for epidemiologic studies
and population-based analysis. Also, the disease pattern differs
in a given population by different ecological factors.1 Dermatologic conditions constitute at least 30% of all outpatient visits
to pediatricians, and 30% of all visits to dermatologists involve
children.2,3 The majority of dermatoses in newborns are physiological and transient, but they cause anxiety and concern for
the parents.4 The incidence of various dermatologic conditions
varies according to age, race, geographic locations, climate, nutrition, hygiene, socioeconomic conditions, and heredity.5–8 In a
developing country such as India, with the second largest population in the world, there are several problems including lack of
education, social backwardness, lack of health care facilities in
the rural area, lack of sanitation, excess pollution, overcrowding, and long wait times in health centers and hospitals. All of
these and other unknown factors cause patients to avoid going to
the hospital. Consequently, patients usually arrive at the hospital
in an advanced, complicated stage of diseases. The incidence of
skin diseases among children in various parts of India has ranged
from 8.7% to 38.8% in different studies, usually school-based
surveys.9,10 The principal aim of this study was to study incidence, age distribution, seasonal variations, and different clinical
manifestations of various dermatologic conditions in children
(up to age 14) in the western part of India (Gujarat).
PARTICIPANTS and Methods
This study was conducted during the period of April 2000 to
March 2002 in the dermatology and venereology department of
Sheth L.G. General Hospital (LGGH) and Sheth Vadilal Sarabhai General Hospital (VSGH), which are part of the Sheth K.M.
School of P.G. Medicine Research and Smt NHL Municipal
Medical College, Ahmedabad, India. Ahmedabad is a megacity
with a population of more than 4.5 million people. The climate
is typically hot and humid (up to 48°C) throughout the year, except during monsoon season (June to September), when it is predominantly rainy. Seven hundred patients younger than 14 years
were examined in the outpatient department of dermatology. Of
these, 550 patients were examined in LGGH and 150 patients
were examined in VSGH. All patients were divided into two different age groups, <1 month old (neonate) and >1 month to 14
years old. The diagnosis of the dermatologic condition was made
based on a detailed review of history, clinical features, complete
From the Department of Dermatology and Cutaneous Surgery, University of Miami, Miller School of Medicine, Miami, FL;1 and the Department of Dermatology and Venereology, Sheth L.G. General Hospital and Vadilal Sarabhai Hospital, NHL Municipal Medical College, Sheth
K.M. School of Post-Graduate Medicine and Research, Ahmedabad, Gujarat, India2
*Jitendrakumar K. Patel, MD, was a resident in the Department of Dermatology, Sheth L.G. General Hospital, Gujarat, India, from February
2000 to February 2003.
Address for Correspondence: Jitendrakumar K. Patel, MD, Department of Dermatology and Cutaneous Surgery, University of Miami, Miller
School of Medicine, 1600 NW 10th Avenue, RMSB, Room 2023A (R250), Miami, FL 33136 • E-mail: drjitupatel@yahoo.com
136
May/June 2010
ORIGINAL CONTRIBUTION
Table I. Age and Sex Distribution of Children
Age Group
Boys, No. (%)
Girls, No. (%)
Total, No. (%)
<1 Month (neonates)
56 (8.00)
42 (6.01)
98 (14.01)
>1 Month to 14 years
334 (47.71)
268 (38.28)
602 (85.99)
Total
390 (55.71)
310 (44.29)
700 (100)
Table II. Comparative Analysis of Etiological Distribution of Various Dermatoses in Different Age Groups
Percentage (n=98)
<1 Month Age Group
Percentage (n=602)
>1 Month Age Group
Percentage (n=700)
Sweat gland disorders
36.74 (36)
4.31 (26)
8.86 (62)
Pigmentary disorders
30.61 (30)
11.46 (69)
14.14 (99)
Dermatitis
17.35 (17)
21.42 (129)
20.86 (146)
Infectious disorders
9.18 (9)
43.19 (260)
38.43 (269)
Allergic disorders
3.06 (3)
1.33 (8)
1.57 (11)
Miscellaneous
3.06 (3)
19.61 (110)
16.14 (113)
Etiology
physical examination including skin and necessary tests such as
Gram’s stain, potassium hydroxide examination, Tzanck test, hematology and biochemistry analysis, urine analysis, Wood’s lamp
examination, diascopy, purified protein derivative (tuberculin),
chest x-ray, skin biopsy, and other investigations as needed. The
diseases were tabulated based on the etiology, incidence, age distribution, sex distribution, and seasonal variations and results
were analyzed. We did not include patients who had more than
one dermatological condition.
Results
In all age groups, there were a total of 390 boys (55.71%) and
310 girls (44.29%) in the study, with a boy to girl ratio of 1.2:0.8.
There were 56 (8.00%) boys and 42 (6.01%) girls in the neonate
group and 334 (47.71%) boys and 268 (38.28%) girls in the >1
month to 14 years old group (Table I). The dermatologic conditions vary according to age, climate, nutrition, hygiene, socioeconomic class, and heredity (Table II–Table VII). The majority of
the skin conditions in the newborn group were transient (physiological) and constituted 73.47%. The most common dermatoses
found were of infectious etiology (Table VI), which was 38.43%
of the study population. Impetigo and pyoderma were the most
common infectious diseases and comprised 11.13% and 8.97%,
respectively. The incidence of scabies and pediculosis capitis were
5.32% and 0.5%, respectively. Viral warts are the most common
of all viral infections followed by molluscum contagiosum, and
viral infection was most common in the 11- to 14-year age group
(Table VII). In the hot and humid climate of Ahmedabad, sweat
137
Table III. Pattern of Various Dermatoses in Neonates
No. of
Cases
Percentage
(n=98)
Milia
20
20.41
Miliaria
13
13.27
3
3.06
25
25.51
Hemangioma
3
3.06
Congenital melanocytic nevus
1
1.02
Sebaceous nevus
1
1.02
10
10.20
Intertrigo
5
5.10
Atopic dermatitis
1
1.02
Pityriasis alba
1
1.02
Bacterial
8
8.16
Parasitic
1
1.02
3
3.06
Dermatoses
Sweat gland disorders
Erythema toxicum
Pigmentary disorders
Mongolian spots
Dermatitis
Seborrheic dermatitis
Infectious disorders
Allergic disorders
Drug eruption
May/June 2010
ORIGINAL CONTRIBUTION
Papular urticaria (insect bite reaction) was found in 6.27%,
while nutritional disorders were found in about 6.3%. Among
the papulosquamous disorders, lichen planus was observed in
2.16% and psoriasis in 0.99%. In the present study, herpes zoster
(0.33%), tinea capitis (0.17%), and generalized pruritus (0.17%)
were found in children infected with human immunodeficiency
virus. Other miscellaneous disorders found were alopecia areata
(1.50%), allergic drug reactions (1.33%), vesicobullous disorders
(0.99%), collagen disorders (0.99%), nail disorders (0.66%), pruritus (0.66%), geographic tongue (0.17%%), syringoma (0.17%),
acneform eruption (0.17%), and erythroderma verrucous variable
(0.17%). Bacterial infections and sweat gland disorders (miliaria)
peak in summer months (April to June) while parasitic infections
and eczema were observed more in fall months (October through
December) (Table VI). Pityriasis alba was also more common in
the summer and fall seasons.
Table IV. Pattern of Various Dermatoses in the >1-Month
Age Group
No. of
Cases
Percentage
(n=602)
26
4.31
Congenital melanocytic nevus
27
4.49
Vitiligo
19
3.16
Hemangioma
14
2.33
Post-inflammatory
hyperpigmentation and
hypopigmentation
5
0.83
Xeroderma pigmentosum
2
0.33
Mongolian spots
1
0.17
Lentigines
1
0.17
Eczema
40
6.64
Pityriasis alba
30
4.98
Papular urticaria
26
4.31
Seborrheic dermatitis
11
1.82
Contact dermatitis
6
0.99
Lichenoid dermatitis
6
0.99
Atopic dermatitis
4
0.66
Perianal dermatitis
4
0.66
Pompholyx
2
0.33
Bacterial
145
24.90
Parasitic
35
5.81
Fungal
47
7.81
Viral
33
5.48
Dermatoses
Sweat gland disorders
Miliaria
Pigmentary disorders
Dermatitis
Infectious disorders
gland disorders (milia, miliaria, and erythema toxicum) were common and we observed 8.86% in our study. Also, sweat gland disorders are more often observed in neonates (36.74%) than in older
children (4.31%) (Table III). The percentage of congenital pigmentary disorders in neonates was 10.57% (Table II). Mongolian
spots was the most common pigmentary disorder in the neonate
group, followed by congenital melanocytic nevus. In the present
study, the incidence of eczema was 6.64% and atopic dermatitis was 0.83%. We also observed that eczema was more common
in the 6- to 14-year age group (school age group) (Table VII).
Discussion
The various dermatologic conditions vary according to age, geographic location, climate, nutrition, hygiene, socioeconomic
class, and heredity8,11–15 (Table II–Table VII). The majority of
skin conditions in newborns are transient (physiological) and
constituted 73.47%, which is similar to studies done by Nobbay
and Chakrabarty (69%),16 Baruah and colleagues (93%),17 and
Kulkarni and Singh (72%).18 In our study, older children had
more incidence of skin conditions than younger children, which
is similar to a study done in Turkey (P<.001).19 The most common
dermatoses found were infectious disorders, which were found in
38.43% of the study population (Table II). In their study, Dogra
and Kumar10 found only 11.4% of disorders of infectious etiology;
however, various other authors in India have reported that disorders of infectious and infestations etiology contributed to 35.6%
to 85.2%.20–24 We found 5.81% of infestations etiology, similar to
the 5% found by Dogra and Kumar.10 In all these studies, whether
institution-based or community-based, infection was the main
etiological agent for pediatric dermatoses. Impetigo and pyoderma
were the most common infections in our study and comprised
11.13% and 8.97%, respectively. In most of the studies, including one conducted in rural Pakistan, researchers found that pyoderma and impetigo were the most common infections.20–25 Poor
hygiene, lack of awareness, overcrowding, poverty, and high prevalence of biting flies appeared to be underlying causes for the largest
number of cases of pyoderma and impetigo.
We found the incidence of scabies to be 5.32%, compared
with the incidence rate found in other reports that range from
5.1% to 22.4%.21–24,26 In this study, however, the incidence of
infection of pediculosis capitis was 0.5%, which is similar to a
study by Rao and colleagues (0.5%).26 Other studies in India
found more incidence of pediculosis, about 54%.23,27,28 Diverse
138
May/June 2010
ORIGINAL CONTRIBUTION
overseas studies have different infestation rates, such as 19% in
Israel, 33.7% in Australia, 50% in Brazil, and 81.5% in Argentina.29–32 The low incidence of pediculosis capitis in our study
may be due to more awareness of hair care and easy availability
of over-the-counter products for pediculosis capitis in the urban area. Viral warts are common in children and prevalence
increases in childhood, with its peak in adolescence, and declines in the second decade of life.6,33 In this study, viral warts
were the most common of all viral infections, followed by molluscum contagiosum; the 11- to 14-year age group had more
incidence than the rest of the study group (Table VII), which is
similar to findings observed in other studies.6,33 These observations are also supported by studies in Turkey and Switzerland
and recently in Taiwan and Nigeria, where the higher incidence
of warts in children was also found.19,34–36 Surprisingly, the incidence of fungal infection was 7.81% in our study, which was
mainly observed in the older age group. Low incidence may be
related to newspaper and television advertisements of antifungal
products, maturation of sweat glands, and easy availability of
over-the-counter products in the urban area. A recently published study also mentioned that dermatophyte infections are
declining among new-patient outpatient visits.36
In the hot and humid climate of Ahmedabad, incidence of sweat
gland disorders (milia, miliaria, and erythema toxicum) was
more common and observed as 8.86%. Another study, however,
found the incidence of sweat gland disorders to be between 30%
and 40%.37 Incidence of congenital pigmentary disorders (Mongolian spots, hemangioma, melanocytic nevus, sebaceous nevus,
and xeroderma pigmentosum) in all age groups in our study was
10.57%, which is much lower than that found by Cordova38
(80%) and Baruah and colleagues (78.40%).17 Their studies
mainly related to newborn inpatients, however, while our study
mainly refers to outpatients. This may be a reason for the low incidence of pigmentary disorders in our study. Congenital melanocytic nevus was found in 5.51%, while a study done in Hong
Kong found 3.6%.39 Incidence of vitiligo in the >1-month age
group was 3.16%, similar to the 2.9% observation of Negi and
colleagues,21 but higher than that observed in Taiwan (0.09%),35
and lower than that observed in China (20%).40
In the present study, the incidence of eczema was 6.64%, which is
similar to Dogra and Kumar (5.2%)10 and Johnson and colleagues
(4.66%).41 Other western studies, however, ranged their incidence
of eczema from 18% to 34%.10,41–44 We also observed that eczema
occurred more in the 6- to 14- year age group (Table VII), similar
to a study done in central Taiwan in 2007.45 These findings may
be related to more contact with the allergen while playing, while in
school, or by some unknown factors. The incidence of atopic dermatitis (0.83%) was very low when compared with other studies
Table V. Distribution of Patients (>1-Month Age Group) With
Infectious Disorders According to Their Individual Etiology
No. of
Patients
Percentage
(n=602)
Impetigo
67
11.13
Pyoderma
54
8.97
Furuncle
21
3.49
Mycobacterium
2
0.33
Cellulitis
1
0.17
32
5.32
3
0.50
Tinea
33
5.48
Candidiasis
14
2.33
Warts
9
1.50
Molluscum contagiosum
8
1.33
Herpes zoster
8
1.33
Measles
4
0.66
Chicken pox
2
0.33
Herpes virus
2
0.33
Etiology
Bacterial
Parasitic
Scabies
Pediculosis
Fungal
Viral
performed in developed countries, where they found rates ranging from 3% to 28%.35,46–49 Low frequency of atopic dermatitis
and eczema may be related to climate, dietary habits, genetics, or
other unknown factors. The incidence of papular urticaria in our
study was 6.27%, which is similar to Karthikeyan and colleagues
(5.27%)50 and Sharma and colleagues9 (7.5%). Other overseas
studies, however, found lower incidences such as 3.3% to 3.6%
in Nigeria and 2.3% in Thailand.27,36,50–52 Higher incidence can be
explained by the higher prevalence of flea bites and wearing scanty
clothes in the hot climatic conditions of Ahmedabad.
Nutritional disorders were found in about 6.3% of children, which
was less than the 17.5% found in a study by Negi and associates.21
This difference may exist because the study by Negi and associates21 was done in a rural area while our study center was in an
urban area. Pityriasis alba was observed more in the 1- to 10-year
old age group. The higher incidence may be because of the growth
period in children, irregular and inadequate food habit, worm infestation, and in some cases socioeconomic factors. Among the
139
May/June 2010
ORIGINAL CONTRIBUTION
Table VI. Seasonal Distributions of Various Dermatoses in the >1-Month Age Group
Month
Bacterial, No. (%)
Parasitic, No. (%)
Miliaria, No. (%)
Eczema, No. (%)
Pityriasis Alba, No. (%)
January to March
12 (8.27)
2 (5.71)
-
4 (10.00)
5 (16.66)
April to June
68 (46.89)
9 (25.71)
23 (88.46)
7 (17.50)
9 (30.00)
July to September
25 (17.24)
5 (14.28)
-
5 (12.50)
4 (13.33)
October to December
40 (27.58)
19 (54.28)
3 (11.53)
24 (60.00)
12 (40.00)
Total
145 (100)
35 (100)
26 (100)
40 (100)
30 (100)
found that bacterial infections and sweat gland disorders (miliaria) peaked in the summer months of the hot and humid climate
(April to June), while parasitic infections and eczema were more
often present in the fall months (October to December). Higher
incidence of bacterial infections can be explained by higher prevalence of insect bites in the summer. We did not find the incidence
of any specific dermatoses to occur more during January to March
or July to September.
Table VII. Common Dermatoses Among Different Age Groups
Percentage as per
Age Group (No.)a
Age Group
Dermatosis
<1 Month
(n=98)
Mongolian spots
25.51 (25)
Milia
20.41 (20)
1 Month to 1 year
(n=114)
Intertrigo
12.28 (14)
Hemangioma
11.40 (13)
Melanocytic nevus
8.77 (10)
Bacterial infections
37.21 (83)
Miliaria
8.07 (18)
Papular urticaria
6.27 (14)
Pityriasis alba
5.82 (13)
Bacterial infections
18.33 (33)
Fungal infections
10.00 (18)
Pityriasis alba
6.11 (11)
Eczema
6.11 (11)
Viral infections
10.58 (9)
Bacterial infections
9.41 (8)
Vitiligo
9.41 (8)
Eczema
8.23 (7)
1 Year to 5 years
(n=223)
6 Years to 10 years
(n=180)
11 Years to 14 years
(n=85)
Conclusions
Total number of common dermatoses for that age group. Total
number of cases in that age group may be different.
a
papulosquamous disorders, lichen planus was observed in 2.16%,
which is similar to Samman (2%)53 and Handa and Sahoo (2%),54
while Kumar and colleagues55 and Luis-Montoya and colleagues56
found higher incidences of about 11.2% and 10.2%, respectively.
Psoriasis (0.99%) was the second most common papulosquamous
disorder after lichen planus in our study which is similar to the
findings of Rao and associates (2.4%).26 Seasonal variations in
dermatologic disorders in our study were comparable with those
noticed by other investigators.11–13 In our study (Table VI) we
Seven hundred pediatric patients were examined, 55.71% were
boys and 44.29% were girls. Patients in the neonate group constituted 14%, while patients >1 month of age constituted 86%
of the total number of patients examined. Physiological skin
conditions were more common in the neonate group, while infections and infestations were more common in the older age
group. Sweat gland disorders were more common after the infectious etiology, which was also more common in neonates, along
with pigmentary disorders. Infection and dermatitis were more
common in the older age group. In the category of infectious
disease, pyoderma and impetigo were more common. Bacterial
infections and sweat gland disorders (miliaria) peak during the
summer months, while parasitic infections and eczema occurred
more often in the fall months. Pityriasis alba was more common
in the summer and fall seasons. Our study shows various unique
features of tropical pediatric dermatology such as high frequency of infections and infectious disorders, nutritional disorders,
and environmentally associated disorders such as miliaria and
papular urticaria. We would like to highlight the fact that many
of these dermatoses can be controlled by proper environmental
sanitation; improving nutrition; educating parents, children, and
society; improvements in living standards; and personal hygiene.
REFERENCES
140
1 Dayal SG, Gupta GD. A cross section of skin diseases in
Bundelkhand Region, up. Indian J Dermatol Venereol Leprol.
1977;43:258–261.
2 Thappa DM. Common skin problems. Indian J Pediatr.
2002;69:701–706.
May/June 2010
ORIGINAL CONTRIBUTION
3 Federman DG, Reid M, Feldman SR, et al. The primary care
provider and the care of skin disease: the patient’s perspective.
Arch Dermatol. 2001;137:25–29.
4 Illingworth RS. The Normal Child: Some Problems of Early
Years and Their Treatment. Vol 450. 10th ed. London, England:
Churchill Livingstone; 1987.
5 Roger M, Barnetson RSC. Disease of skin. In: Campbell AGM,
McIntosh N, eds. Forfar and Arneil’s Textbook of Pediatrics. 5th
ed. New York, NY: Churchill Livingstone; 1998:1633–1635.
6 Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2003;(3):CD001781.
24 Ghosh SK, Saha DK, Roy A. A clinico aetiological study of dermatosis in pediatric age group. Indian J Dermatol. 1996;41:29–31.
25 Porter MJ, Mack RW, Chaudhary MA. Pediatric skin disease
in Pakistan. A study of three Punjab villages. Int J Dermatol.
1984;23:613–616.
26 Rao GS, Kumar SS, Sandhya. Pattern of skin diseases in an
Indian village. Indian J Med Sci. 2003;57:108–110.
27 Sharma NL, Sharma RC. Prevalence of dermatological diseases
in school children of a high altitude tribunal area of Himachal
Pradesh. Indian J Dermatol Venereol Leprol. 1990;56:375–376.
7 Bhattacharya M, Kaur I, Kumar B. Lichen planus: a clinical and
epidemiological study. J Dermatol. 2000;27:576–582.
28 Kumar V, Garg BR, Baruah MC. Prevalence of dermatological
diseases in school children in a semi urban area in Pondicherry.
Indian J Dermatol Venereol Leprol. 1998;54:300–302.
8 Ersoy-Evans S, Greco MF, Mancini AJ, et al. Pityriasis lichenoides in childhood: a retrospective review of 124 patients. J Am
Acad Dermatol. 2007;56:205–210.
29 Speare R, Buettner PG. Head lice in pupils of a primary
school in Australia and implications for control. Int J Dermatol.
1999;38:285–290.
9 Sharma N, Garg Bk, Goel M. Pattern of skin diseases in urban school children. Indian J Dermatol Venereol Leprol.
1986;52:330–331.
30 Chouela E, Abeldano A, Cirigliano M, et al. Head louse infestations: epidemiologic survey and treatment evaluation in Argentinian schoolchildren. Int J Dermatol. 1997;36:819–825.
10 Dogra S, Kumar B. Epidemiology of skin diseases in school
children: a study from northern India. Pediatr Dermatol.
2003;20:470–473.
31 Mumcuoglu KY, Klaus S, Kafka D, et al. Clinical observations related
to head lice infestation. J Am Acad Dermatol. 1991;25:248–251.
11 Hancox JG, Sheridan SC, Feldman SR, et al. Seasonal variation
of dermatologic disease in the USA: a study of office visits from
1990 to 1998. Int J Dermatol. 2004;43:6–11.
32 Bechelli LM, Haddad N, Pimenta WP, et al. Epidemiological survey
of skin diseases in schoolchildren living in the Purus Valley (Acre
State, Amazonia, Brazil). Dermatologica. 1981;163:78–93.
12 Kaur I, Handa S, Kumar B. Natural history of psoriasis: a study
from the Indian subcontinent. J Dermatol. 1997;24:230–234.
33 Sterling JC, Kurtz JB. Viral infections. In: Champion RH, Burton JL,
Burns DA, Breathnach SM, eds. Rook Textbook of Dermatology. Vol 4.
6th ed. New York, NY: Oxford:Blackwell Scientific; 1998:995–1095.
13 Borelli D, Jacobs PH, Nall L. Tinea versicolor: epidemiologic, clinical, and therapeutic aspects. J Am Acad Dermatol.
1991;25:300–305.
34 Wenk C, Itin PH. Epidemiology of pediatric dermatology and allergology in the region of Aargau, Switzerland. Pediatr Dermatol. 2003;20:482–487.
14 Habif TP. Atopic dermatitis. In: Hodgson S, Cook L, eds. Clinical
Dermatology. 4th ed. New York, NY: Mosby; 2004:105.
35 Chen GY, Cheng YW, Wang CY, et al. Prevalence of skin diseases
among schoolchildren in Magong, Penghu, Taiwan: a communitybased clinical survey. J Formos Med Assoc. 2008;107:21–29.
15 James WD, Berger TG, Elston DM. Pityriasis rosea. In: Hodgson S, Bowler K, eds. Andrews’ Diseases of the Skin: Clinical
Dermatology. 10th ed. Toronto, Canada: Saunders Elsevier;
2000:208–209.
16 Nobbay B, Chakrabarty N. Cutaneous manifestation in the newborn. Indian J Dermatol Venereol Leprol. 1992;58:69–72.
17 Baruah C, Bhat V, Bhargava R, et al. Prevalence of dermatoses
in the neonates in Pondicherry. Indian J Dermatol Venereol Leprol. 1991;57:25–28.
18 Kulkarni ML, Singh R. Normal variants of skin in neonates. Indian
J Dermatol Venereol Leprol. 1996;62:83–86.
19 Serarslan G, Savas N. Prevalence of skin diseases among children and adolescents living in an orphanage in Antakya, Turkey.
Pediatr Dermatol. 2005;22:490–492.
20 Anand IS, Gupta S. A profile of skin disorders in children in Saurashtra. J Indian Med Assoc. 1998;96:245–246.
21 Negi KS, Kandpal SD, Parsad D. Pattern of skin diseases in
children in Garhwal region of Uttar Pradesh. Indian Pediatr.
2001;38:77–80.
36 Yahya H. Change in pattern of skin disease in Kaduna, northcentral Nigeria. Int J Dermatol. 2007;46:936–943.
37 Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218–222.
38 Cordova A. The Mongolian spot: a study of ethnic differences
and a literature review. Clin Pediatr (Phila). 1981;20:714–719.
39 Fung WK, Lo KK. Prevalence of skin disease among school children and adolescents in a student health service center in Hong
Kong. Pediatr Dermatol. 2000;17:440–446.
40 Hu Z, Liu JB, Ma SS, et al. Profile of childhood vitiligo in China: an
analysis of 541 patients. Pediatr Dermatol. 2006;23:114–116.
41 Prevalence, morbidity, and cost of dermatological diseases. J
Invest Dermatol. 1979;73:395–401.
42 Bowker NC, Cross KW, Fairburn EA, et al. Sociological implications of an epidemiological study of eczema in the city of
Birmingham. Br J Dermatol. 1976;95:137–144.
43 Horn R. The pattern of skin diseases in general practice. Dermatol Pract. 1986;2:14–19.
22 Bhatia V. Extent and pattern of in paediatric dermatoses in central India. Indian J Dermatol Venereol Leprol. 1997;63:22–25.
44 Katelaris CH, Peake JE. 5. Allergy and the skin: eczema and
chronic urticaria. Med J Aust. 2006;185:517–522.
23 Sharma RC, Mendiratta V. Clinical profile of cutaneous infections
and infestations in the pediatric age group. Indian J Dermatol.
1999;44:174–178.
45 Chiang LC, Chen YH, Hsueh KC, et al. Prevalence and severity
of symptoms of asthma, allergic rhinitis, and eczema in 10- to
15-year-old schoolchildren in central Taiwan. Asian Pac J Allergy
141
May/June 2010
ORIGINAL CONTRIBUTION
Immunol. 2007;25:1–5.
2004;41:373–377.
46 Foley P, Zuo Y, Plunkett A, et al. The frequency of common skin
conditions in preschool-age children in Australia: atopic dermatitis. Arch Dermatol. 2001;137:293–300.
51 Ogunbiyi AO, Owoaje E, Ndahi A. Prevalence of skin disorders in school children in Ibadan, Nigeria. Pediatr Dermatol.
2005;22:6–10.
47 Sladden MJ, Dure-Smith B, Berth-Jones J, et al. Ethnic differences in the pattern of skin disease seen in a dermatology department--atopic dermatitis is more common among Asian referrals
in Leicestershire. Clin Exp Dermatol. 1991;16:348–349.
52 Wisuthsarewong W, Viravan S. Analysis of skin diseases in a
referral pediatric dermatology clinic in Thailand. J Med Assoc
Thai. 2000;83:999–1004.
48 Larsen FS, Holm NV, Henningsen K. Atopic dermatitis. A genetic-epidemiologic study in a population-based twin sample. J Am
Acad Dermatol. 1986;15:487–494.
49 Peroni DG, Piacentini GL, Bodini A, et al. Prevalence and risk factors for atopic dermatitis in preschool children. Br J Dermatol.
2008;158:539–543.
50 Karthikeyan K, Thappa DM, Jeevankumar B. Pattern of pediatric
dermatoses in a referral center in south India. Indian Pediatr.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
142
53 Samman PD. Lichen planus: an analysis of 200 cases. Trans St
Johns Hosp Dermatol Soc. 1961;46:36–38.
54 Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423–427.
55 Kumar V, Garg BR, Baruah MC, et al. Childhood lichen planus
(LP). J Dermatol. 1993;20:175–177.
56 Luis-Montoya P, Dominguez-Soto L, Vega-Memije E. Lichen planus in 24 children with review of the literature. Pediatr Dermatol.
2005;22:295–298.
Available soon...
%
A New Tretinoin Therapy
From Triax Pharmaceuticals
©2010 Triax Pharmaceuticals, LLC.
All Rights Reserved.
Printed in USA
TX-0610-02
May/June 2010
Volume 8 • Issue 3
Original Contribution
Pemphigus Vulgaris and Pregnancy
Kossara Drenovska, MD, PhD; Razvigor Darlenski, MD; Jana Kazandjieva, MD, PhD; Snejina Vassileva, MD, PhD
Abstract
The management and counseling of patients with pemphigus vulgaris during pregnancy is a challenge. The frequency of the association is
very low and the current knowledge is based only on case reports or small series. The authors report 2 cases of pemphigus vulgaris and pregnancy that differed from each other in the time of occurrence and clinical course but had similar favorable outcomes. Based on a literature
review and their personal observations, the authors discuss the characteristics of this association, the therapeutic behavior, patients’ followup, and fetal prognosis. (SKINmed. 2010;8:144–149)
P
emphigus vulgaris (PV) is a severe, potentially life-threatening autoimmune blistering disease of the skin and mucous membranes associated with autoantibodies against the
cadherin-type adhesion molecules desmoglein (Dsg)3 and Dsg1.
The binding of immunoglobulin (Ig)G antibodies to Dsg on epidermal keratinocytes leads to intraepithelial blister formation. It
is a well recognized fact that PV more frequently affects middleaged women, including those in their childbearing years. The
disease may occur for the first time or flare during pregnancy or
immediately after delivery.1 The association of PV with pregnancy,
although very rare, raises numerous questions with regard to its
clinical manifestation, management, and prognosis for both the
mother and child. Maternal pemphigus autoantibodies are able to
cross the placenta, thereby causing neonatal pemphigus in 30% to
45% of the cases, the latter being a transient condition with good
prognosis after antibodies are metabolized.2,3
The influence of pregnancy on the evolution of PV has been
a matter of debate for decades. Since the description of “acantholytic bullous eruption in the newborn infant of a pemphigus mother” in 1975,4 almost 50 well documented cases of PV
associated with pregnancy have been reported in the literature,
adding to earlier existing data.5–8 In contrast to a common former belief that women with PV should avoid pregnancy due to
high risk of detrimental outcomes to both the mother and the
fetus, newer data demonstrate that advances in the treatment of
pemphigus and the management of pregnant women with this
disease have improved the prognosis for mother and child.
We report 2 more cases of PV and pregnancy, which, while differing from each other in time of occurrence and clinical course,
had similar favorable outcomes.
CASE REPORTS
Case 1
A 27-year-old Caucasian primiparous woman was admitted
to the Department of Dermatology, Sofia Faculty of Medicine
for a severe vesiculobullous eruption affecting the face, neck,
trunk, and extremities (Figure 1) that had occurred 1 week after
delivery of a healthy, term-appropriate-for-gestational-age boy.
She described an episode of similar but milder eruption, the
onset of which coincided with a delayed menstrual cycle and a
positive pregnancy test. Subsequent gynecological consultation
confirmed an intrauterine pregnancy with a probable period of
conception about 2 weeks before the onset of the first blisters.
The patient was given a diagnosis of PV at a different institution
based on the histology results that revealed suprabasal bulla
with acantholysis, spongiosis, and sparse mixed perivascular inflammatory infiltrate in the upper dermis. At that time, oral
methylprednisolone 12 mg/d had been administered and was
sufficient to control the disease at a stable dose throughout the
whole pregnancy. At admission to our department, the diagnosis of PV was confirmed based on histological assessment and
positive direct immunofluorescence (DIF) on perilesional skin
(Figure 2). High titers (1:640) of circulating IgG antiepithelial
cell surface antibodies were detected by indirect immunofluorescence (IIF) on human esophagus substrate. The disease was
successfully controlled with 80 mg/d methylprednisolone, subsequently tapered to a maintenance oral dose of 8 mg/d. Oral
azathioprine 100 mg/d was initially added but was discontinued
in 5 days due to nausea and vomiting. During the next 3-year
follow-up the mother’s disease remained stable and her child
was in good health.
From the Department of Dermatology and Venereology, Sofia Faculty of Medicine, Sofia, Bulgaria
Address for Correspondence: Snejina Vassileva, MD, PhD, Department of Dermatology and Venereology, Sofia Faculty of Medicine, 1 G.
Sofiiski str, 1431 Sofia, Bulgaria • E-mail: snejina.vassileva@gmail.com
144
May/June 2010
ORIGINAL CONTRIBUTION
Case 2
A 24-year-old Caucasian woman presented to our department
due to vesiculobullous eruption initially spread next to a free
autologous skin graft following a thermal burn in the area of the
right breast. Later lesions disseminated to the abdomen, back,
and extremities (Figure 3). The diagnosis of PV was confirmed
through histological examination, positive DIF on perilesional
skin, and positive IgG antiepithelial cell surface antibodies at a
titer of 1:320 by IIF on human esophagus. Methylprednisolone
60 mg/d and azathioprine 100 mg/d led to clinical remission
followed by gradual tapering of methylprednisolone to a maintenance dose of 4 mg/d. Since the onset of her disease the patient
was regularly assessed with regard to clinical and laboratory data
and therapeutic monitoring. During the whole observation period she did not experience severe flare ups and demonstrated
negative IIF or low titers of pemphigus antibodies (up to 1:20).
Three years after the beginning of PV and nearly 1 year prior
to a planned pregnancy, azathioprine was discontinued and the
patient remained disease free. She conceived at the age of 28.
The dose of methylprednisolone was raised to 16 mg/d at the
first month of gestation and was kept unchanged throughout
the pregnancy in order to prevent possible exacerbations. After
an uneventful pregnancy during which the patient remained
clinically healthy, she gave birth through Cesarean section to a
healthy, appropriate-for-gestational-age boy. Forty days after delivery, methylprednisolone was tapered again to 8 mg/d. During
the next 2 years of follow-up, both the mother and her child
remained disease-free.
Figure 1. Clinical manifestation of the pemphigus vulgaris
exacerbation postpartum in patient 1: multiple erosions with
crusts and single flaccid blisters over the trunk and upper
extremities.
DISCUSSION
The prevalence of PV in women and its possible occurrence in
childbearing age oblige the specialist to take into account disease
and therapy effects on conception, pregnancy, and the period of
after delivery.1,9
Various autoimmune disorders, such as systemic sclerosis, rheumatoid arthritis, Crohn’s disease, insulin-dependant diabetes
mellitus, chronic active hepatitis, etc., are associated with impairment of fertility. In a retrospective study, 8 of 9 patients
suffering from PV failed to conceive. Four patients had luteal
phase defects, 4 had follicle stimulating hormone defects, and
antisperm antibodies were detected in 2 patients. None of the
8 patients conceived even after discontinuation of their therapy
(corticosteroids, azathioprine, or cyclophosphamide). Only one
became pregnant, but during full remission.10 In contrast to
these data, a recent report described a pemphigus patient who
conceived during the active phase of severe PV, which required
high doses of prednisone, thus implying that active disease is not
necessarily associated with infertility.11 Nevertheless, it is advised
Figure 2. Direct immunofluorescence on perilesional skin:
intercellular deposits of immunoglobulin G in the lower two
thirds of the epidermis.
that conception coincide with a period of clinical remission and
low titers of pemphigus antibodies, as was the case with our patient 2.12 In addition, literature data analysis showed that PV was
diagnosed before conception in more than half of the reported
cases of PV in pregnancy.
This raises the question, how should the dermatosis be treated
safely and efficiently whenever a pregnancy is planned? No prospective, controlled studies exist to evaluate the efficacy and safety of PV management before conception. The conclusions are
mainly drawn from case reports. It is proposed that every attempt
should be made to taper or discontinue any immunosuppressive
145
May/June 2010
ORIGINAL CONTRIBUTION
such as mycophenolate mofetil, cyclophosphamide, methotrexate, cyclosporine A, dapsone, intravenous immunoglobulins,
rituximab, or plasmapheresis, remain to be proven safe and efficient in the preconception period in PV patients.
Figure 3. Extensive erosion of the nipple and areola with
small, ruptured blisters in the surrounding skin in patient 2.
agent, including prednisone which should be reduced to the
lowest effective dose.3 On the other hand, a sufficient control
of the disease is required before conception as it is expected that
pregnancy can aggravate preexisting PV as it does in other autoimmune diseases (eg, myasthenia gravis, lupus erythematosus).
Most authors believe that adverse pregnancy outcome is more
closely related to poor control of maternal disease and high titers of pemphigus antibodies than to particular medication.13–15)
Corticosteroids remain the first-choice treatment for PV if low
doses are sufficient to control the disease, but when high doses
are required steroid-sparing immunosuppressive agents may be
added to therapy. Azathioprine is the most common adjuvant
therapy in PV although its use during conception and early
pregnancy has not been evaluated systematically within the field
of dermatology.15 It is widely accepted that azathioprine (US
Food and Drug Administration [FDA] pregnancy category D)
increases human fetal risk, and therefore, should be avoided in
pregnancy. Having this in mind, in our second patient it was discontinued a year before the planned conception which did not
result in exacerbation of the disease. In 2 formerly reported cases,
on the contrary, azathioprine was used in the preconception period in a dose regimen of 15 mg/d14 and 75 mg/d,15 respectively.
Both pregnancies were generally uneventful without signs of fetal teratogenicity. The lower dosage of azathioprine was related
to transient neonatal pemphigus lesions that resolved within 3
weeks postpartum,14 while the 75 mg/d regimen resulted in the
delivery of a healthy newborn.15 Other therapeutic modalities,
It is of interest to the clinician to investigate what is the effect of
pregnancy on the course and manifestations of PV. Current information on the association of pemphigus and pregnancy is based
on 38 reports and 49 pregnancies.3 Age at onset of PV in mothers
ranged from 18 to 42 years (mean, 30 years) and there was no
correlation with the number of pregnancies. PV appeared de novo
during pregnancy in 18 patients and our first case adds to this
number. In the rest of cases, the disease preceded the pregnancy,
this period ranging from 2 months to 8 years (mean, 4 years). Clinical records available for 40 patients demonstrated the following
distribution: 25% of the patients presented with mucosal lesions,
45% showed both mucosal and cutaneous involvement, while in
30% only skin was affected.3 Gingival erosions were reported as
the first and/or only manifestation of PV during pregnancy.16,17
Seven patients had no active disease during pregnancy.3,12,18 Our
second case should be included in that group. Exacerbation of PV
was observed in 11 of 49 patients, occurring during the second
trimester in 2 cases, and postpartum in 5 patients.3
Similarly to other autoimmune diseases, such as systemic lupus erythematosus, myasthenia gravis, etc., aggravation of PV
should be expected during pregnancy.1,19,20 The first and second
trimester, as well as the postpartum period, are the critical time
points.12 Difficulty in controlling disease flare-up has been reported during the period of conception and early pregnancy,11 as
was observed in our first clinical case. Improvement or remission
of PV during the third trimester of pregnancy was related to the
elevated endogenous corticosteroid production by the chorion,
and consequent immunosuppression.21 Postpartum flare-up of
PV is also likely to appear, as in our case 1, so attention and close
follow-up of patients at that period is recommended.12
Two of the most important questions that arise with regard to
the association of pemphigus and pregnancy are the possible
influence of the disease on the intrauterine fetal development,
and that of pregnancy prognosis. PV during pregnancy showed
various outcomes ranging from stillbirth, retardation of the intrauterine growth, premature delivery, transient neonatal pemphigus, or delivery of a healthy neonate completely free of skin
disease, as in our clinical observations.2,22
The reported perinatal mortality rate was 12% (6 of 49 cases) although there were not enough cases for representative statistics.
Of these, there were 5 stillbirths and 1 newborn who died 2 days
postpartum due to meconium aspiration syndrome.3,12 Of the 5 intrauterine deaths, one was related to umbilical cord prolapse, one
146
May/June 2010
ORIGINAL CONTRIBUTION
to placental dysfunction, and one to cytomegalovirus pneumonitis.
In the other 2 cases the reason remained unknown. All 5 stillbirths
occurred in mothers with severe active disease, and azathioprine was
administered in 2 of the cases. Despite these observations and the
impression that poor pregnancy prognosis is related to severe PV,
there is no clear evidence in the literature that PV plays a causative
role in fetal death, as in several reports mothers with severe active
disease gave birth to completely healthy neonates.3,20 The exact mortality causes could not be strictly identified, although intrauterine
growth retardation, placental insufficiency, immune suppression,
infections, and adverse drug reactions have been suspected.12
Premature deliveries were mainly related to high doses of systemic
corticosteroid treatment.11,23 Other authors do not find consistent association between maternal treatment regimen and fetal outcome.15
Twenty newborns of 44 reported live births presented with transient pemphigus lesions, which resolved within 4 weeks postpartum with or without treatment.3 Clinical manifestation of PV in
the neonate is a result of transplacental transmission of maternally
derived IgG pemphigus antibodies. A correlation between the
maternal disease severity, antibody titers, and the development of
neonatal pemphigus seemed probable.13,14 However, mothers with
severe disease gave birth to healthy newborns, and infants with
neonatal pemphigus were born by asymptomatic mothers.18,24–26
On the other hand, mothers without active disease most commonly deliver healthy children, although some reports contradict
this assumption. A probable explanation is the different sensitivity
of fetal skin to the maternally derived antibodies.12,20 These data
lead to the conclusion that clinical presentation or antibody titers
in the mother cannot be predictive for the development of pemphigus in the neonate, but parents and pediatricians should be
aware of the possible occurrence of neonatal lesions.22,27
More often neonatal pemphigus presented with skin lesions (12
out of 20 cases) and this did not always correlate to the clinical
manifestation in the mother. Isolated cutaneous involvement in
the child was observed even in mucosal dominant PV in the
mother.27,28 Only rarely, mucocutaneous lesions in the newborn
were reported.29 This was explained by the different tissue distribution of Dsg1 and Dsg3 in the adult and neonatal skin.3,30
Healthy, full-term, and completely free of skin lesions neonates were
reported in 4 women with milder PV,22 but the same pregnancy
outcome has been observed in mothers with severe, active disease.3,20
There is no consensus on the choice of delivery type in PV mothers. Vaginal birth can result in worsening and spreading of the
pemphigus lesions in the mother.20 On the other hand, systemic
steroid therapy delays and complicates the healing of the Cesarean section.31 Despite that caveat, in an analysis of 4 cases
of PV, all the patients underwent Cesarean section at their own
request, and none demonstrated impaired wound healing.22 The
method of choice should be individualized in each patient in
accordance with the medical indications and the patient’s preferences. Whenever there are PV lesions in the genital mucosa,
Cesarean section is advisable.
The management of PV during pregnancy is similar to that in
the nonpregnant woman, however there is no doubt that the
control of the disease during pregnancy is a challenge for both
dermatologists and obstetricians.11,12
Systemic steroids are widely accepted as first-choice treatment of
PV with or without pregnancy. Prednisone and/or its equivalents
are usually administered in doses varying from 5 to 300 mg/d
for several weeks with subsequent tapering to a maintenance
dose.3,28 Although corticosteroids, mainly prednisone, have been
established as safe during pregnancy with no increase of congenital malformations, high dosage may increase fetal risk for low
birth weight, prematurity, infection, and adrenal insufficiency.15
Preterm premature rupture of membranes and preterm delivery
may be the result of aggressive steroid therapy during pregnancy.11,23 Some authors recommend introducing systemic steroid
therapy after the twelfth week of gestation to avoid possible teratogenic effects of high doses administered in early pregnancy.17
Azathioprine was coadministered with the steroid treatment
during pregnancy in 5 cases, with doses ranging from 15 to 150
mg/d.3 In one case the child was healthy,15 in 2 cases the infants
presented with self-limiting pemphigus lesions,12,14 and 2 stillbirths were reported.32,33 In each of these cases, the mother’s condition was severe, which was considered a possible independent
risk factor for neonatal PV or fetal death. In most reports, the
use of azathioprine during pregnancy is not recommended.17 Recent data suggest that azathioprine could be a reasonable treatment in pregnant patients who require steroid-sparing agents for
serious medical conditions.34
Dapsone (200 mg/d) was used in one case in combination with
prednisone.35 Fetal death at 33 weeks of gestation was attributed
to placental insufficiency due to the steroid use. Dapsone (US
FDA pregnancy category C) can be used for its antiinflammatory effect in PV and pregnancy and it was even suggested that
dapsone 100 mg/d should be preferred to prednisone.3,36
Regarding treatment of PV during breastfeeding, only prednisone and azathioprine were approved for use in nursing mothers
by the American Academy of Pediatrics.3,37 Dapsone is also considered compatible with breastfeeding due to its minimal excretion in breast milk.38
Cyclosporine A (US FDA pregnancy category C) was reported to be
the safest corticosteroid-sparing agent in pregnancy, but was considered
147
May/June 2010
ORIGINAL CONTRIBUTION
less effective in the treatment of PV than other therapies.15,39 On the
contrary, in another review of the literature it was characterized as
strongly contraindicated in pregnancy in pemphigus patients.3
Mycophenolate mofetil, cyclophosphamide, and methotrexate
are strongly discouraged and contraindicated in pregnancy because of their immunosuppressive and teratogenic effect.15,17
There are still no reports on the use of intravenous immunoglobulins for PV during pregnancy but they may be necessary in
some patients to achieve clinical control of the disease.3
Plasmapheresis was reported to be a successful alternative to immunosuppressive therapy in pregnant PV patients.40,41 It was
regarded as a useful way to decrease fetal risk that may be associated with high-dose maternal medication, but it is still considered an experimental procedure in PV and should be restricted
to severe corticosteroid-resistant cases.11,12
Other therapeutic modalities include topical corticosteroid therapy,27
intralesional triamcinolone acetonide application,3 or no treatment.18
CONCLUSIONS
Despite the lack of systemic controlled studies there is growing
evidence on the association between pemphigus and pregnancy.
Still, many practical approaches with regard to clinical and therapeutic monitoring of PV during pregnancy remain controversial. Whenever this association occurs, every attempt should be
made by clinicians, dermatologists, obstetricians, pediatricians,
and parents to lead such a pregnancy to a favorable outcome.
Possible aggravation of PV postpartum requires special attention
during the period after delivery as well. We believe that a careful,
individualized approach based on personal experience, published
data, and patients’ choices, is the most appropriate strategy.
REFERENCES
1 Maharshak N, Brenner S. Gender differences in vesiculobullous
autoimmune skin diseases. Skinmed. 2002;1:25–30.
2 Meurer M. Pemphigus diseases in children and adolescents.
Hautarz. 2009;60:208–216.
3 Kardos M, Levine D, Gurcan HM, et al. Pemphigus vulgaris in
pregnancy: analysis of current data on the management and
outcomes. Obstet Gynecol Surv. 2009;64:739–749.
4 Ruocco V, Rossi A, Astarita C, et al. A congenital acantholytic
bullous eruption in the newborn infant of a pemphigus mother.
Ital Gen Rev Dermatol. 1975;12:169–174.
5 Kolonja S. Pemphigus and pregnancy. Zentralbl Gynakol.
1952;74:1831–1836.
6 Samitz MH, Greenberg MS, Coletti JM. Pemphigus in association with pregnancy; observations of the effect of corticotropin
(ACTH) and cortisone on the pemphigus, the pregnancy, and the
infant. AMA Arch Derm Syphilol. 1953;67:10–17.
7 Bolgert M, Le Sourd M. Pemphigus vegetans treated since
148
1952 with combined quinacrine & aureomycin (5th case report);
twin pregnancy with live birth of one infant; current status [in
French]. Bull Soc Fr Dermatol Syphiligr. 1958;65:8–12.
8 Lapiere S. Pemphigus, cortisone and pregnancy [in French].
Arch Belg Dermatol Syphiligr. 1960;16:164–174.
9 Brenner S, Wohl Y. A survey of sex differences in 249 pemphigus
patients and possible explanations. Skinmed. 2007;6:163–165.
10 Ouahes N, Tabarak AQ, Razzaque A. Infertility in women with
pemphigus vulgaris and other autoimmune disease. J Am Acad
Dermatol. 1997;36:383–387.
11 Fainaru O, Mashiach R, Kupferminc M, et al. Pemphigus vulgaris
in pregnancy: a case report and review of literature. Hum Reprod. 2000;15:1195–1197.
12 Ruach M, Ohel G, Rahav D, et al. Pemphigus vulgaris and pregnancy. Obstet Gynecol Surv. 1995;50:755–760.
13 Metzker A, Merlob P. Pemphigus in pregnancy: a reevaluation of
fetal risk. Am J Obstet Gynecol. 1987;157(4 pt 1):1012–1013.
14 Hup JM, Bruinsma RA, Boersma ER, et al. Neonatal pemphigus
vulgaris: transplacental transmission of antibodies. Pediatr Dermatol. 1986;3:468–472.
15 Lehman JS, Mueller KK, Schraith DF. Do safe and effective
treatment options exist for patients with active pemphigus
vulgaris who plan conception and pregnancy? Arch Dermatol.
2008;144:783–785.
16 Wells GC, Ballard RM. Management of pemphigus vulgaris during pregnancy. Proc R Soc Med. 1969;62:666–667.
17 Lopez-Jornet P, Bermejo-Fenoll A. Gingival lesions as a first
symptom of pemphigus vulgaris in pregnancy. Br Dent J.
2005;199:91–92.
18 Bonifazi E, Milioto M, Trashlieva V, et al. Neonatal pemphigus
vulgaris passively transmitted from a clinically asymptomatic
mother. J Am Acad Dermatol. 2006;55(5 suppl):S113–S114.
19 Kaufman AJ, Ahmed AR, Kaplan RP. Pemphigus, myasthenia gravis,
and pregnancy. J Am Acad Dermatol. 1988;19(2 pt 2):414–418.
20 Goldberg NS, DeFeo C, Kirshenbaum N. Pemphigus vulgaris and
pregnancy: risk factors and recommendations. J Am Acad Dermatol. 1993;28(5 pt 2):877–879.
21 Weinberg ED. Pregnancy-associated depression of cell-mediated immunity. Rev Infect Dis. 1984;6:814–831.
22 Kalayciyan A, Engin B, Serdaroglu S, et al. A retrospective analysis of patients with pemphigus vulgaris associated with pregnancy. Br J Dermatol. 2002;147:396–397.
23 Laskin CA, Bombardier C, Hannah ME, et al. Prednisone and
aspirin in women with autoantibodies and unexpected recurrent
fetal loss. N Engl J Med. 1997;337:148–153.
24 Okano M, Takijiri C, Aoki T, et al. Pemphigus vulgaris associated
with pregnancy. A case report from Japan. Acta Derm Venereol.
1990;70:517–519.
25 Tope WD, Kamino H, Briggaman RA, et al. Neonatal pemphigus
vulgaris in a child born to a woman in remission. J Am Acad
Dermatol. 1993;29:480–485.
26 Sasidharan CK, Anoop P, Gokul E. Transient neonatal autoimmune blistering in pemphigus complicating pregnancy. Kuwait
Med J. 2005;37:197–199.
27 Chowdhury MM, Natarajan S. Neonatal pemphigus vulgaris associated with mild oral pemphigus vulgaris in the mother during
May/June 2010
ORIGINAL CONTRIBUTION
pregnancy. Br J Dermatol. 1998;139:500–503.
28 Hern S, Vaughan Jones SA, Setterfield J, et al. Pemphigus vulgaris in pregnancy with favourable prognosis. Clin Exp Dermatol. 1998;23:260–263.
29 Campo-Voegeli A, Muñiz F, Mascaró JM, et al. Neonatal pemphigus
vulgaris with extensive mucocutaneous lesions from a mother with
oral pemphigus vulgaris. Br J Dermatol. 2002;147:801–805.
30 Anhalt GJ. Making sense of antigens and antibodies in pemphigus. J Am Acad Dermatol. 1999;40:763–766.
31 Daniel Y, Shenhav M, Botchan A, et al. Pregnancy associated
with pemphigus. Br J Obstet Gynaecol. 1995;102(8):667–669.
32 Ross MG, Kane B, Frieder R, et al. Pemphigus in pregnancy: a
reevaluation of fetal risk. Am J Obstet Gynecol. 1986;155:30–33.
33 Wasserstrum N, Laros RK Jr. Transplacental transmission of
pemphigus. JAMA. 1983;249:1480–1482.
34 EBPG Expert Group on Renal Transplantation. European best
practice guidelines for renal transplantation, section IV: longterm management of the transplant recipients. Nephrol Dial
Transplant. 2002;17(suppl 4):50–55.
35 Terpstra H, de Jong MC, Klokke AH. In vivo bound pemphigus
antibodies in a stillborn infant. Passive intrauterine transfer of
pemphigus vulgaris? Arch Dermatol. 1979;115:316–319.
36 Armenti VT, Moritz MJ, Davison JM. Drug safety issue in pregnancy following transplantation and immunosuppression: effects and outcomes. Drug Saf. 1998;19:219–232.
37 Christensen LA, Dahlerup JF, Nielsen MJ, et al. Azathioprine treatment
during lactation. Aliment Pharmacol Ther. 2008;28:1209–1213.
38 Edstein MD, Veenendaal JR, Newman K, et al. Excretion of chloroquine, dapsone and pyrimethamine in human milk. Br J Clin
Pharmacol. 1986;22:733–735.
39 Mimouni D, Nousari CH, Cummins DL, et al. Differences and similarities among expert opinions on the diagnosis and treatment of
pemphigus vulgaris. J Am Acad Dermatol. 2003;49:1059–1062.
40 Piontek JO, Borberg H, Sollberg S, et al. Severe exacerbation
of pemphigus vulgaris in pregnancy: successful treatment with
plasma exchange. Br J Dermatol. 2000;143:455–456.
41 Shieh S, Fang YV, Becker JL, et al. Pemphigus, pregnancy, and
plasmapheresis. Cutis. 2004;73:327–329.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
149
May/June 2010
Volume 8 • Issue 3
ORIGINAL CONTRIBUTION
Hydrocortisone Butyrate 0.1% Cream
(Proprietary Lipid Rich Cream Vehicle) Does Not
Significantly Suppress Hypothalamic-Pituitary-Adrenal
Axis and Is Effective in Pediatric Patients 3 Months
and Older With Extensive Atopic Dermatitis
William Abramovits, MD;1 Marcial Oquendo, MD2
Abstract
Only a few corticosteroids for topical use have been proven safe and effective in pediatric populations down to 3 months of age. The authors
examined the systemic safety (adrenal suppression potential) of topically applied hydrocortisone butyrate 0.1% cream (proprietary lipid
rich cream vehicle) in the treatment of moderate to severe atopic dermatitis in pediatric patients aged 3 months to 6 years and 12 years to
18 years. An open-label hypothalamic-pituitary-adrenal axis suppression study was conducted wherein the sole treatment was 0.1% proprietary lipid rich cream vehicle. A total of 65 patients with moderate to severe atopic dermatitis and body surface area involvement of at
least 25% were included in the treatment phase of the study based on the requirement that these patients had normal baseline cortisol and
hypothalamic-pituitary-adrenal system function. All signs and symptoms of atopic dermatitis showed progressive improvement beginning
with day 8 through the day 29 evaluation. Pruritus showed the greatest improvement, with a decrease in grade of 1.3 at day 8, and continued
to show improvement at day 29, with a decrease of 1.8 from baseline. The percent body surface area affected at baseline averaged 40.5%, and
it decreased significantly to a mean of 6.5% at day 29. Only 5 (8%) of the 63 patients showed laboratory evidence of adrenal suppression
at the end of the treatment evaluation. None of these ever demonstrated any clinical signs or symptoms of adrenal suppression. This study
adds hydrocortisone butyrate 0.1% cream, to the short list of corticosteroids that have been proven effective and safe by the cosyntropin
suppression test in children 3 months and older with widespread atopic dermatitis. (SKINmed. 2010;8:150–154)
A
topic dermatitis (AD) is a pruritic disease characterized by
chronic or relapsing dermatitis in a pattern of distribution
that is linked to the age of the patient as essential features,
with xerosis, atopy (immunoglobulin E reactivity), and early age of
onset being important features, and an array of associated features.1,2
AD is the most common chronic inflammatory skin condition
of the pediatric population, affecting 2% to 20% of infants and
children in the United States, drastically impacting quality of life.3
Hydrocortisone butyrate (HCB), the active molecule in the
study drug, is a synthetic nonfluorinated glucocorticosteroid
approved for topical use for the relief of inflammatory and
pruritic manifestations of corticosteroid-responsive dermatoses. The formulation used in this study is the same approved
and marketed for adult use since 1997. It is ranked, in a I to
VII potency scale that utilizes a vasoconstriction assay, as lower
mid-strength class V.4,5
Corticosteroids for topical use are absorbed and act locally, inducing phospholipase A2 inhibitory proteins that control the
biosynthesis of inflammation mediators such as prostaglandins
and leukotrienes. Absorption can be enhanced by occlusion, application over extensive areas, or where the skin barrier function
is compromised. With increased absorption, the risk of systemic
side effects rises.6–9
Cosyntropin is a synthetic subunit of adrenocorticotropic hormone (ACTH). It exhibits the full corticosteroidogenic activity
and similar pharmacologic profile of purified natural ACTH. It
From Baylor University Medical Center and the Department of Dermatology, The University of Texas Southwestern Medical School at Dallas,
Dallas, TX;1 and the Dermatology Treatment & Research Center, Dallas, TX2
Address for Correspondence: William Abramovits, MD, Dermatology Treatment and Research Center, 5310 Harvest Hill Road, Suite 160,
Dallas TX 75230 • E-mail: dra@dermcenter.us
150
May/June 2010
ORIGINAL CONTRIBUTION
Table I. Physician’s Global Assessment Score and Description
Score
Category
0
Clear
1
Almost clear
2
Mild
3
Moderate
4
Severe
Definition
No inflammatory signs of atopic dermatitis.
Faint, barely detectable erythema and/or trace residual induration/papulation in limited areas; neither excoriation
nor oozing/crusting are present.
Light pink erythema and slightly perceptible induration/papulation; excoriation, if present, is mild.
Dull red, clearly distinguishable erythema and clearly perceptible induration/papulation but not extensive;
excoriation or oozing/crusting, if present, are mild to moderate.
Deep/dark red erythema and marked and extensive induration/papulation; excoriation and oozing/crusting are present.
has been established that 0.25 mg of cosyntropin will maximally
stimulate the adrenal cortex and to the same extent as 25 units
of natural ACTH, producing a maximal secretion of 17-OH
corticosteroids, 17-ketosteroids, and/or 17-ketogenic steroids.
Because of its rapid effect on the adrenal cortex, it may be used
to perform a 30-minute test of adrenal function (plasma cortisol
response) as an office or outpatient procedure.10
Trial Design
To examine the potential for systemic toxicity for HCB, an
open-label hypothalamic-pituitary-adrenal (HPA) axis suppression study was conducted. There was no comparator-control
group for this study; the sole treatment was HCB 0.1% cream.
The study was designed to maximally challenge the response of
the adrenal gland to cosyntropin stimulation after 4 weeks of
treatment 3 times daily with HCB 0.1% cream in a pediatric
population aged 3 months to older than 6 years and 12 years to
older than 18 years with moderate to severe AD affecting at least
25% of the body surface area (BSA). Thrice-daily dosing was included in this study design to maximally challenge these patients
with respect to adrenal axis function.
Study Population
Sixty-nine patients were enrolled into the study, 65 of whom
were included in the treatment phase of the study based on the
requirement that these patients had normal baseline cortisol and
HPA system function and did not have any clinically significant
out-of-range baseline laboratory test results.
Demographic and Other
Baseline Characteristics
The average age of the 65 treated patients was 6.31 years. Thirtysix (55%) were boys. The average BSA affected was 40.53%. Forty-eight patients (74%) had moderate disease (Physician’s Global
Assessment score [PGA]=3) and 17 patients (26%) had severe
disease (PGA=4). The majority of patients had moderate to severe erythema (55 patients, 84%), induration/papulation (57 pa-
tients, 87%), excoriations (36 patients, 55%), lichenification (45
patients, 69%), scaling (35 patients, 54%), pruritus (53 patients,
82%), and no or mild oozing/crusting (44 patients, 68%).
Clinical Diagnostic Criteria
The 3 major diagnostic criteria for entry into the study included
the following: patients had to have a clinical diagnosis of stable,
moderate to severe AD defined by criteria per Hanifin & Rajka2,11–13; the severity of AD according to PGA scale had to be 3
or 4 (Table I); and there had to be a minimum percent surface
area involvement of at least 25% BSA.
Treatment
The intent-to-treat population for safety evaluation consisted of
23 patients 3 months to older than 2 years, 22 patients 2 to older
than 6 years, and 20 patients 12 to older than 18 years, diagnosed
with moderate to severe AD affecting a minimum of 25% BSA.
Eligible patients were treated with HCB 0.1% cream 3 times daily
for up to 4 weeks, depending on improvement of the condition.
A patient who completed 4 weeks of treatment was expected to
apply 84 applications of study medication. If a patient was clear
at the day 8, 15, or 22 evaluation, they completed the study
at that point and were expected to have applied 21, 42, or 63
applications of study medication, respectively. If a patient prematurely discontinued from the study, the number of expected
applications was based on the number of dosing days the patient
participated in the study.
Although the protocol did not identify a minimum number of
study medication applications for a patient to be considered
compliant with the dosing regimen, the data were reviewed
to ensure that patients used study medication appropriately.
A standard of 75% compliance was used to determine dosing
compliance. One patient applied study medication <75% of the
expected doses, which was 3 times daily for up to 4 weeks. This
patient was 73.8% compliant and applied 62 applications of
study medication while completing the study.
151
May/June 2010
ORIGINAL CONTRIBUTION
Efficacy
At baseline, patients were required to have an overall disease severity of moderate or severe. Forty-eight of 65 patients (74%)
were enrolled into the study with moderate severity and 17 of 65
patients (26%) were enrolled with severe disease. Administration
of the study drug resulted in immediate efficacy, as noted by the
day 8 evaluations in which 12 of 64 patients (19%) were almost clear, 31 of 64 patients (48%) were mild, 19 of 64 patients
(30%) were moderate, and 2 of 64 patients (3%) were severe. By
the day 29 evaluation, 24 of 64 patients (38%) were clear, 28 of
64 patients (44%) were almost clear, 11 of 64 patients (17%)
were mild, and 1 of 64 patients (2%) was moderate (Table II).
All signs and symptoms showed progressive improvement beginning with day 8 through the day 29 evaluation. At day 8, the mean
erythema score decreased by 0.8 and continued to decrease to 1.4
grades less than baseline at day 29. Induration/papulation showed
similar efficacy with a mean decrease of 0.8 at day 8 down to 1.5
grades less than baseline at day 29. The mean excoriation score decreased by a grade of 0.9 at day 8 and continued to decrease to 1.5
grades less than baseline at day 29. The mean lichenification score
decreased by 0.6 at day 8 and continued to decrease to 1.1 grades
less than baseline at day 29. The mean oozing/crusting score decreased by 0.7 at day 8 and continued to decrease to 1.0 grade less
than baseline at day 29. The mean scaling score decreased by 0.6 at
day 8 and continued to decrease to 1.2 grades less than baseline at
day 29. Pruritus showed the greatest improvement with a decrease
in grade of 1.3 at day 8, which continued to show improvement
at day 29 with a decrease of 1.8 from baseline. The percent BSA
affected at baseline averaged 40.5%, which significantly decreased
to a mean of 6.5% at day 29.
for suppressed response in this study was a poststimulation plasma cortisol level £18 mg/dL on day 29.
The cosyntropin stimulation test (CST) was scheduled at the screening and final visit (day 29). This evaluation was performed between 7
am and 9 am, with the poststimulation blood draw being completed
by 9 am. Cosyntropin was intravenously administered following
blood sampling for baseline serum cortisol evaluation and followed
by a second blood draw for serum cortisol evaluation exactly 30
minutes later. A patient was considered to have evidence of adrenal
suppression if the serum cortisol level 30 minutes after cosyntropin
administration was £18 mg/dL.
If there was evidence of adrenal suppression from the CST at the posttreatment assessment, this was to be considered an adverse event. For
suppressed patients, the posttreatment CST testing was performed every 4 weeks until axis function was documented as returning to normal.
At the end of the study, 5 of the 63 patients (8%) showed laboratory
evidence of adrenal suppression at the end of treatment evaluation.
None of these patients ever demonstrated any clinical signs or symptoms of adrenal suppression. The average prestimulation cortisol level at baseline was 14.32 mg/dL compared with 13.89 mg/dL at end
of treatment, for an average difference of 0.45 mg/dL. The average
poststimulation cortisol level at baseline was 26.90 mg/dL compared
with 24.90 mg/dL at end of treatment, for an average difference of
2.03 mg/dL (Table III).
The CST retesting for 4 of the 5 patients with suppressed responses, with follow-up testing 28 to 65 days after end of treatment, was
>18 mg/dL (ie, nonsuppressed). The other patient was lost to follow-up. None of the patients with abnormal serum cortisol values
showed any clinical signs and symptoms of adrenal suppression.
Safety
Assessment of Adverse Effects
Cosyntropin stimulation testing is an established surrogate measure for system toxicity of topical corticosteroids. The definition
Adverse events were reported in 19 of the 65 patients (29%)
entered into the treatment phase. The most common adverse
Table II. Summary of Efficacy: Physician’s Global Assessment Score
Baseline
Day 8
Day 15
Day 22
Day 29
No.
65
64
65
64
64
Clear
0 (0%)
0 (0%)
2 (3%)
9 (14%)
24 (38%)
Almost clear
0 (0%)
12 (19%)
24 (37%)
32 (50%)
28 (44%)
Mild
0 (0%)
31 (48%)
31 (48%)
19 (30%)
11 (17%)
Moderate
48 (74%)
19 (30%)
7 (11%)
4 (6%)
1 (2%)
Severe
17 (26%)
2 (3%)
1 (2%)
0 (0%)
0 (0%)
Completely clear
0
2
7
Not reported
1
0
1
152
1
May/June 2010
ORIGINAL CONTRIBUTION
Table III. Adrenal Response to Cosyntropin
Screening
End of Treatment
Change From Screeninga
65
64
64
Mean
14.32
13.89
–0.45
Standard deviation
5.34
6.03
5.75
65
63
63
Mean
26.90
24.90
–2.03
Standard deviation
5.79
5.81
6.63
65
63
–12.58
–10.89
6.78
5.83
Prestimulation cortisol concentration, mg/dL
No.
Poststimulation cortisol concentration, mg/dL
No.
Change from prestimulation to poststimulation cortisol
concentration, mg/dL
No.
Mean
Standard deviation
Patients with adrenal suppression, No. (%)b
5 (8%)
Change from baseline was calculated as end of treatment cortisol concentration minus baseline cortisol concentration. bAdrenal suppression is
defined as a serum cortisol level £18 mg/dL 30 minutes after cosyntropin administration.
a
events reported during the treatment phase included upper respiratory infection (4 of 65 patients, 6%) and pyrexia (3 of 65
patients, 5%), which were all mild in severity and considered not
related to study medication. All other adverse events reported
during the treatment phase were reported by 3% of patients or
less, were mild to moderate in severity, and the majority was
considered not related to study medication. There were 6 events
considered by the investigator to be related to study medication.
The majority of these events were related to the 5 patients that
had laboratory evidence of adrenal suppression (day 29) that, per
protocol, were to be documented as adverse events (Table IV).
There were no serious adverse events reported during the study
and no patients prematurely discontinued due to adverse events.
Discussion
The efficacy of topical corticosteroids in AD is unquestionable.
Their systemic safety in pediatric populations by means of cosyntropin stimulation testing to assess for adrenal suppression has been
evaluated for only a few; particularly down to 3 months of age.
A PubMed search allowed us to find studies where fluocinolone
acetonide 0.01% in peanut oil showed no adrenal suppression in
24 infants as young as 3 months of age treated with twice-daily
applications for 4 weeks.13 Among 34 children aged 6 months
to 6 years who applied a desonide hydrogel 0.05% twice daily,
only 1 (3%) patient developed adrenal suppression at the 4-week
evaluation.14 A fluticasone propionate lotion 0.05% did not
cause adrenal suppression in 44 patients aged 3 to 71 months
who applied the preparation twice daily for 4 weeks.15 The same
corticosteroid at the same concentration of 0.05% in a cream
formulation caused adrenal suppression in 2 (4.7%) of 43 children 3 months of age and older who applied it twice daily for 3
to 4 weeks.16
In the daily practice of dermatology, the need for comfort in prescribing topical corticosteroids to pediatric patients often arises.
Studies that support the safety of a particular corticosteroid preparation allow mitigating corticosteroid phobia by parents, concerns
by physicians, and medicolegal risks related to their use.
Conclusions
This study adds another topical corticosteroid preparation, HCB
0.1%, a proprietary lipid rich cream vehicle, to the short list of
corticosteroids that have been proven safe by the gold standard to
evaluate for systemic toxicity, the CST for adrenal suppression, in
children 3 months and older.
153
May/June 2010
ORIGINAL CONTRIBUTION
References
Table IV. Summary of Adverse Events (N=65)
No. of events reported
No. of patients reporting ≥1 event
1 Williams HC, Grindlay DJ. What’s new in atopic eczema? An analysis of systematic reviews published in 2007 and 2008. Part
1. Definitions, causes and consequences of eczema. Clin Exp
Dermatol. 2010;35:12–15.
35
19 (29%)
Serious
Yes
0 (0%)
No
35 (100%)
2 Abramovits W. Atopic dermatitis. J Am Acad Dermatol.
2005;53(1 suppl 1):S86–S93.
3 Williams H, Stewart A, von Mutius E, et al. Is eczema really on the
increase worldwide? J Allergy Clin Immunol. 2008;121:947–954.
Severity of event
Mild
4 Locoid Lipocream [package insert]. Ferndale, MI: Ferndale Laboratories, Inc.
32 (91%)
Moderate
3 (9%)
Severe
0 (0%)
5 Fowler JF Jr, Fransway AF, Jackson JM, Rohowsky N. Hydrocortisone butyrate 0.1% cream in the treatment of chronic dermatitis. Cutis. 2005;75:125–131.
6 Aksoy MO, Li X, Borenstein M, et al. Effects of topical corticosteroids on inflammatory mediator-induced eicosanoid release by human airway epithelial cells. J Allergy Clin Immunol.
1999;103:1081–1091.
Relationship to study drug
Unassessable
0 (0%)
Not related
29 (83%)
Possible
2 (6%)
Probable
3 (9%)
Certain
1 (3%)
7 Drake LA, Dinehart SM, Farmer ER. Guidelines of care for
the use of topical glucocorticosteroids. J Am Acad Dermatol.
1996;35:615–619.
8 Cook D. FDA experience: topical corticosteroids and HPA axis
suppression. Pediatric subcommittee of the AIDAC. October
29–30, 2003.
System organ class/preferred term
Infections and infestations
Upper respiratory tract infection
4 (6%)
Rhinitis
2 (3%)
Folliculitis
1 (2%)
Otitis media
1 (2%)
Pneumonia
1 (2%)
Pustular eruption
1 (2%)
Viral infection
1 (2%)
Endocrine disorders
5 (8%)
Adrenal suppression
5 (8%)
General disorders and administration site conditions
10 Cortrosyn (cosyntropin) for Injection [package insert]. Rancho
Cucamonga, CA: Amphastar Pharmaceuticals Inc; 2005.
11 Hanifin JM, Thurston M, Omoto M, et al. The eczema area and
severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11–18.
12 Ricci G, Dondi A, Patrizi A. Useful tools for the management of
atopic dermatitis. Am J Clin Dermatol. 2009;10:287–300.
13 Dohil MA, Alvarez-Connelly E, Eichenfield LF. Fluocinolone acetonide 0.01% in peanut oil: safety and efficacy data in the treatment of childhood atopic dermatitis in infants as young as 3
months of age. Pediatr Dermatol. 2009;26:262–268.
14 Eichenfield LF, Basu S, Calvarese B, et al. Effect of desonide
hydrogel 0.05% on the hypothalamic-pituitary-adrenal axis in
pediatric subjects with moderate to severe atopic dermatitis.
Pediatr Dermatol. 2007;24:289–295.
4 (6%)
Application site irritation
1 (2%)
Pyrexia
3 (5%)
Gastrointestinal disorders
9 Hengge U, Ruzicka T, Schwartz R, Cork M. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54:1–15.
10 (5%)
15 Hebert AA, Friedlander SF, Allen DB. Topical fluticasone propionate lotion does not cause HPA axis suppression. J Pediatr.
2006;149:378–382.
16 Friedlander SF, Hebert AA, Allen DB. Safety of fluticasone propionate cream 0.05% for the treatment of severe and extensive
atopic dermatitis in children as young as 3 months. J Am Acad
Dermatol. 2002;46:387–393.
2 (3%)
Diarrhea
1 (2%)
Nausea
1 (2%)
Vomiting
1 (2%)
154
May/June 2010
Volume 8 • Issue 3
REVIEW
Segmental Neurofibromatosis and Malignancy
Julie D. Dang, DO;1 Philip R. Cohen, MD2,3,4
Abstract
Segmental neurofibromatosis is an uncommon variant of neurofibromatosis type I characterized by neurofibromas and/or café-au-lait
macules localized to one sector of the body. Although patients with neurofibromatosis type I have an associated increased risk of certain malignancies, malignancy has only occasionally been reported in patients with segmental neurofibromatosis. The published reports of patients
with segmental neurofibromatosis who developed malignancy were reviewed and the characteristics of these patients and their cancers were
summarized. Ten individuals (6 women and 4 men) with segmental neurofibromatosis and malignancy have been reported. The malignancies include malignant peripheral nerve sheath tumor (3), malignant melanoma (2), breast cancer (1), colon cancer (1), gastric cancer (1),
lung cancer (1), and Hodgkin lymphoma (1). The most common malignancies in patients with segmental neurofibromatosis are derived
from neural crest cells: malignant peripheral nerve sheath tumor and malignant melanoma. The incidence of malignancy in patients with
segmental neurofibromatosis may approach that of patients with neurofibromatosis type I. SKINmed. 2010;8:156–159)
I
n 1982, Riccardi1 described 8 subtypes of neurofibromatoses.
Classic neurofibromatosis (also referred to as both von Recklinghausen neurofibromatosis and neurofibromatosis type I) is
the most common subtype, comprising 90% of the cases of neurofibromatosis, with an estimated incidence of 1 in 3000 people.1–3
Segmental neurofibromatosis, previously referred to as neurofibromatosis type V and now designated as segmental neurofibromatosis type I, is an uncommon subtype with an estimated incidence
of 1 in 38,000 people.4 In contrast, however, to neurofibromatosis
type I, in which syndrome-associated internal malignancy may develop, cancer has not usually been considered to be prominent in
patients with segmental neurofibromatosis.1,5–9
History
Segmental neurofibromatosis was originally described by Gammel in 1931.10 Later, Crowe and colleagues11,12 in 1956, observed
4 more patients with café-au-lait macules and neurofibromas
limited to one sector of the body. These patients had no systemic
involvement and no family history. They called this syndrome
sectorial neurofibromatosis, and proposed that it may be due to
a later development of a somatic mutation of the neurofibromatosis type I gene, resulting in a localized form of disease.
Miller and Sparkes,13 in 1977, renamed this disease as segmental
neurofibromatosis. In 1982, Riccardi1 included this variant as
neurofibromatosis type V in his classification of the neurofibromatoses. He defined segmental neurofibromatosis as the pres-
ence of café-au-lait macules and neurofibromas limited to one
region of the body, without crossing of the midline, and not
associated with a family history of neurofibromatosis (Figure).
In 1987, Roth and colleagues5 further expanded the description of segmental neurofibromatosis into 4 subtypes to include
individuals that did not meet Riccardi’s strict criteria, such as
patients with bilateral segmental neurofibromatosis, and patients
with a positive family history. Most recently, in 2000, Tinschert
and associates10 confirmed what Crowe and colleagues11,12 proposed half a century earlier: that segmental neurofibromatosis is
a variant of neurofibromatosis type I. This has led to the general
acceptance of this syndrome being referred to as segmental neurofibromatosis type I.
CANCER IN NEUROFIBROMATOSIS TYPE I PATIENTS
Neurofibromatosis type I is associated with malignancy.6,8,14
According to the American Society of Clinical Oncology, the
overall lifetime risk of developing cancer in patients with neurofibromatosis type I is 7%, about twice the risk of that seen in
the general population.15 Children with neurofibromatosis type
I also have a 200-fold relative risk of developing juvenile chronic
myelogenous leukemia.16
The most common associated malignancy in neurofibromatosis
type I patients is malignant peripheral nerve sheath tumor which
has a lifetime risk of 5% in these patients, which is nearly 4000
From the Departments of Internal Medicine-Pediatrics,1 and Dermatology,2 University of Texas-Houston Medical School, Houston, Texas; the
University of Houston Health Center, University of Houston, Houston, Texas;3 and the Department of Dermatology, The University of Texas
MD Anderson Cancer Center, Houston, Texas4
Address for Correspondence: Philip R. Cohen, MD, 805 Anderson Street, Bellaire, Texas 77401-2806 • E-mail: mitehead@aol.com
156
May/June 2010
REVIEW
times the rate seen in the general population.8,17 Other neurofibromatosis type I-associated malignancies include carcinoid tumors,
lymphomas, medullary carcinoma of the thyroid, meningiomas,
optic gliomas, pheochromocytoma, rhabdomyosarcoma, and
Wilms tumor.8,18–20 Lymphomas associated with neurofibromatosis type I are uncommon and have mostly been non-Hodgkin.
To the best of our knowledge, there has only been one report of a
patient with neurofibromatosis type I and Hodgkin lymphoma.18
CANCER IN SEGMENTAL
NEUROFIBROMATOSIS PATIENTS
Ten individuals with segmental neurofibromatosis and cancer
have been reported (Table).17,21–28 The most common malignancy
associated with segmental neurofibromatosis was malignant peripheral nerve sheath tumor. This is the same tumor that is most
frequently seen in patients with neurofibromatosis type I. Painful, recurrent, and/or rapidly growing neurofibromas prompted
the detection of malignant peripheral nerve sheath tumors in
several of the neurofibromatosis type I patients with this cancer.
In contrast, only 1 of 3 patients with segmental neurofibromatosis had painful and recurrent tumors (case 1).
Malignant melanoma was the second most common cancer in
patients with segmental neurofibromatosis. With the exception
of malignant peripheral nerve sheath tumor and malignant melanoma, tumors of neural crest origin that have been previously
associated with neurofibromatosis type I such as astrocytomas,
carcinoid tumors, glioblastomas, meningiomas, neuroblastomas,
and optic nerve gliomas, were not observed in patients with segmental neurofibromatosis. The other reported malignancies were
of non-neural crest origin, including breast cancer, colon cancer,
gastric cancer, lung cancer, and lymphoma.
Malignancy was found to be more common in women (6 women as compared to 4 men). Eight patients had segmental neurofibromatosis localized to the left side (cases 2–8 and 10). One
patient had right-sided involvement (case 1) and 1 patient had
bilateral involvement (case 9). The most common associated cutaneous findings in patients with segmental neurofibromatosis
and malignancy were painless neurofibromas (cases 2–4, 9, and
10) and café-au-lait macules (cases 5, 6, and 8–10) which were
each observed in 50% of the patients.
The diagnosis of segmental neurofibromatosis preceded the discovery of cancer in at least 5 patients. Three patients were reported to have segmental neurofibromatosis develop after their
malignancy was detected (cases 3–5). Some authors have proposed that the occurrence of segmental neurofibromatosis following the onset of malignancy was related to the tumor treatment-associated lymphedema,21 whereas others have suggested
Figure. Neurofibromas that are localized to one sector of the
body—the C6 dermatome of the distal left upper extremity—
in a man with segmental neurofibromatosis who developed
Hodgkin lymphoma.
that the finding of segmental neurofibromatosis should prompt
the clinician to look for an underlying concurrent malignancy.27
Whether the development of these malignancies is related to underlying segmental neurofibromatosis or is coincidental remains
to be determined.
Previously, segmental neurofibromatosis has usually not been
considered to be associated with malignancy. However, based on
the previously reported frequency of patients with upper extremity segmental neurofibromatosis (18.2%),7 and the recent review
of 34 of these individuals in the literature,28 it would imply that
there are approximately 187 patients with segmental neurofibromatosis.9 The incidence of cancer in patients with segmental
neurofibromatosis can then be calculated to be 5.3% (10/187).
This incidence for developing malignancy in segmental neurofibromatosis patients approaches the observed 7% lifetime risk for
cancer in neurofibromatosis type I patients.15
CONCLUSIONS
Ten patients with segmental neurofibromatosis were found to
have a systemic malignancy. Malignancy was more common
in women and more frequently observed in patients with segmental neurofibromatosis involving the left side. The most
common tumor in patients with segmental neurofibromatosis
was the same as that observed in neurofibromatosis type I: malignant peripheral nerve sheath tumor. With the exception of
malignant peripheral nerve sheath tumor and malignant melanoma, all of the other cancers were of non-neural crest origin.
The incidence of malignancy in segmental neurofibromatosis
patients approaches the observed risk for cancer in that of patients with classical neurofibromatosis type I. A conservative
approach, therefore, for the management of individuals with
segmental neurofibromatosis is to monitor these patients for
the possible development of malignancy.
157
May/June 2010
REVIEW
Table. Segmental Neurofibromatosis and Malignancya
Age of Onset (y)
of SN/Malignancy
Gender
Site of SN
1
40/43
F
Right lower
extremity
Neurofibroma
2
41/48
F
Left
buttock
3
47/44
F
4
66/64
5
Other
Systemic
Family
History
Reference
Malignant
peripheral
nerve sheath
tumor
–
17
–
Malignant
peripheral
nerve sheath
tumor
–
17
Neurofibromad
–
Breast cancer
–
21
Left pubis
Neurofibromad
–
Malignant
peripheral
nerve sheath
tumor
–
22
F
Left trunk,
lower
extremity
Café au lait macule
–
Lung cancer
–
23
NS/71
F
Left lower
extremity
Café au lait macule
–
Malignant
melanoma
–
24
7
16/48
M
Left neck
Large congenital
nevus,
neurofibromae
plexiform,
neurofibroma,e
schwannoma
+h
Malignant
melanoma
–
25
8
21/61
M
Left trunk
Axillary freckling,
café au lait macule,
neurofibromae
–
Colon cancer
–
26
9
60/61
M
Bilateral
trunk
Café au lait macule,
neurofibromad
–
Gastric
carcinoma
–
27
10
NS/32
M
Left forearm,
hand
Café au lait macule,
neurofibromad
–
Hodgkin
lymphoma
–
28
Case
Cutaneous
Involvementb
Involvement
Malignancy
–
Neurofibromad
Left arm
F
72g/72
6
c,f
Race of the patient was defined in 1 patient: case 8 = Korean. bThe neurofibromas were cpainful, dpainless, or ewithout description of symptoms.
Some of the neurofibromas recurred in the same area after excision. gThe diagnosis of SN occurred 3 months after the patient’s diagnosis of lung
cancer. hOther systemic involvement was reported in 1 patient: case 7 = multiple osseous abnormalities including congenital fusion of the cervical
spine, marked kyphosis, and scoliosis. Abbreviations: +, present; –, absent; F, female; M, male; NS, not stated; SN, segmental neurofibromatosis.
a
f
REFERENCES
1 Riccardi VM. Neurofibromatosis: clinical heterogeneity. Curr
Probl Cancer. 1982;7:1–34.
5 Roth R, Martines R, James W. Segmental neurofibromatosis.
Arch Dermatol. 1987;123:917–920.
2 Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis
1. Am J Epidemiol. 2000;151(1):33–40.
6 Ruggieri M. The different forms of neurofibromatosis. Childs
Nerv Syst. 1999;15:295–308.
3 Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J
Med. 1981;305:1617–1627.
7 Hager CM, Cohen PR, Tschen JA. Segmental neurofibromatosis:
case reports and review. J Am Acad Dermatol. 1997;37:864–869.
4 Ruggieri M. Mosaic (segmental) neurofibromatosis type 1 (NF1)
and type 2 (NF2): no longer neurofibromatosis type 5 (NF5). Am
J Med Genet. 2001;101:178–180.
8 Pastar Z, Lipozencic J, Budimcic D, et al. Neurofibromatosis--review of literature and case report. Acta Dermatovenerol Croat.
2006;14:167–171.
158
May/June 2010
REVIEW
9 Ilyas AM, Nourissat G, Jupiter JJ. Segmental neurofibromatosis
of the hand and upper extremity: a case report. J Hand Surg.
2007;32:1538–1542.
20 Hayflick SJ, Hofman KJ, Tunnessen WW, et al. Neurofibromatosis 1: recognition and management of associated neuroblastoma. Pediatric Dermatol. 1989;7:293–295.
10 Tinschert S, Naumann I, Stegmann E, et al. Segmental neurofibromatosis type 1 (NF1) gene. Eur J Hum Genet. 2000;8:455–459.
21 Bhargava AK, Bryan N, Nash, AG. Localized neurofibromatosis
associated with chronic post-mastectomy lymphoedema—a
case report. Eur J Surg Oncol. 1996;22:114–120.
11 Crowe FW, Schull WJ, Neel JV. Description of clinical material.
In: A Clinical, Pathological, and Genetic Study of Multiple Neurofibromatosis. Springfield, IL: Charles C. Thomas; 1956:57–141.
12 Crowe FW, Schull WJ, Neel JV. The genetics of neurofibromatosis.
In: A Clinical, Pathological, and Genetic Study of Multiple Neurofibromatosis. Springfield, IL: Charles C. Thomas; 1956:147–168.
13 Miller RM, Sparkes RS. Segmental neurofibromatosis. Arch Dermatol. 1977;113:837–838.
14 Cohen PR. Neurofibromatosis type 1. N Engl J Med. 1993;329:1549.
15 Cancer.net guide to neurofibromatosis type I. http://www.cancer.net/patient/Cancer+Types/Neurofibromatosis+Type+1. Accessed February 26, 2010.
16 Stiller CA, Chessells, JM, Fitchett M. Neurofibromatosis and
childhood leukaemia/lymphoma: a population-based UKCCSG
study. Br J Cancer. 1994;70:969–972.
17 Schwarz J, Belzberg AJ. Malignant peripheral nerve sheath tumors in the setting of segmental neurofibromatosis. J Neurosurg. 2000;92:342–346.
18 Natori H, Tanaka K, Shiraishi K, et al. Hodgkin’s disease in a
patient with neurofibromatosis. Am J Hematol. 1994;4:349.
19 Pantoja E, Llobet R, Taveras J. Neoplastic complications of neurofibromatosis. Cutis. 1978;22:677–680.
22 Kapsok L, Chong HW, Moon SE. A superficial form of malignant
peripheral nerve sheath tumor associated with segmental neurofibromatosis. Acta Derm Venereol. 2005;85:540–541.
23 Yalcin B, Toy GG, Tamer E, et al. Increased expression of segmental neurofibromatosis with bronchoalveolar lung carcinoma.
Dermatology. 2004;209:342.
24 Selvaag E, Thune P, Larsen TE. Segmental neurofibromatosis presenting as a giant naevus spilus. Acta Derm Venereol.
1994;74:327.
25 Doherty SD, George S, Prieto VG, et al. Segmental neurofibromatosis in association with a large congenital nevus and malignant melanoma. Dermatol Online J. 2006;12(7):22.
26 Kim SE, Heo EP, Yoon TJ, et al. Segmentally distributed neurofibromatosis associated with adenocarcinoma of the colon. J
Dermatol. 2002;29:350–353.
27 Kajimoto A, Oiso N, Fukai K, et al. Bilateral segmental neurofibromatosis with gastric carcinoma. Clin Exp Dermatol.
2006;32:43–44.
28 Dang JD, Cohen PR. Segmental neurofibromatosis of the distal
arm in a man who developed Hodgkin lymphoma. Int J Dermatol.
2009;48:1105–1109.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
159
SAVE THE DATE FOR THE
7th Annual Maui Derm
A Pan-Pacific Thought Leaders Meeting
FEBRUARY 23-27, 2011 GRAND WAILEA, MAUI
Save the Date & Register Early! $395 before June 30th!
Plan to attend the 7th Annual Maui Derm Conference! “Cutting edge, a great blend of science and clinical medicine and
a world class faculty” describe Maui Derm 2011. Our faculty will share with you the most important developments in
medical and cosmetic dermatology vital to your practice. Our live patient demonstration workshops in lasers, Botulinum
Toxin A and fillers have set the standard for “hands on” workshops. Our format has been specifically designed to allow
optimal time for discussion with our faculty. We are certain that you will find this to be an outstanding and memorable
educational event that you will not want to miss. Put Maui Derm on your calendar for 2011!
FEATURED LECTURES
MAUI DERM 2011 FACULTY
Skin Cancer/Chemoprevention • Acne/Rosacea • Pediatric Dermatology •
Infectious Diseases • Psoriasis • Pigmentary Disorders • Contact Dermatitis • Cosmeceuticals • Collagen Vascular Diseases • Office Surgery Tips
Practice Management • Skin of Color
Dr. Rox Anderson
Dr. Andrew Blauvelt
Dr. Joel Cohen
Dr. Lawrence Eichenfield
Dr. Ilona Frieden
Dr. Sheila Fallon-Friedlander
Dr. Michael Gold
Dr. Mitchel Goldman
Dr. Pearl Grimes
Dr. Arthur Kavanaugh
Dr. Suzanne Kilmer
Dr. David Laub
Dr. Craig Leonardi
Medical Dermatology
Cosmetic Dermatology
Botulinum Toxin A and Fillers • Lasers • Skin of Color
“Lunch with the Faculty” –
Controversial Topics, Case-based Discussions
Interactive Workshops
Small Group, Live Patient, Hands-On Demonstrations:
Botulinum Toxins/Fillers
Chairman: Dr. George Martin
Dr. Stuart Maddin
Dr. Sam Moschella
Dr. Stuart Nelson
Dr. Kevin Pinski
Dr. Ted Rosen
Dr. Vic Ross
Dr. Alan Shalita
Dr. Bruce Strober
Dr. Hensin Tsao
Dr. Sandy Tsao
Dr. Guy Webster
Dr. Philip Werscheler
* Subject to Change
Official Meeting Publication Sponsor
Maui Derm 2011 has special/reduced rates at the
Grand Wailea and the Wailea Marriott
For more details, visit our website at
www.acmd-derm-hawaii.com
May/June 2010
Volume 8 • Issue 3
REVIEW
Diagnosis and Treatment of Acanthosis Nigricans
Shailendra Kapoor, MD
Abstract
Acanthosis nigricans is a dermatologic condition commonly seen in diabetics and obese individuals. Histologically, it is characterized by the
proliferation of epidermal keratinocytes and fibroblasts. Clinically, the lesions appear as dark-brown thickened plaques. The neck and the
axillas are the most commonly affected sites. Treatment involves management of the underlying disorder. Topical tretinoin and calcipotriol
have also been used with some limited success. Other treatment options include laser and surgical excision. (SKINmed. 2010;8:161–165)
A
canthosis nigricans (AN) is a common dermatologic
abnormality seen in numerous endocrine disorders as
well as malignancies. It was first described by Pollitzer
and Janovsky in 1890. AN is more common in dark-skinned
persons. Higher rates of incidence have also been reported in
certain groups such as Native Americans.
AN is mostly seen in individuals with underlying diabetes mellitus.
In a recent study, nearly 36% of patients with newly diagnosed diabetes had AN.1 Obese individuals are also at an increased risk for
developing AN. In a recent study, researchers2 reported that 39%
of their study population of obese children had AN. AN may also
appear as a paraneoplastic syndrome (malignant AN).3,4 Malignant
AN is usually seen in gastrointestinal adenocarcinomas such as primary malignant lesions of the gallbladder and gastric lymphomas.5,6
The lesions may also appear in individuals with no apparent identifiable underlying cause. In fact, most cases of AN are idiopathic.7
Genetic causes of AN have also been identified.8 These include
Crouzon syndrome and Berardinelli Seip syndrome (congenital
generalized lipodystrophy).9,10 In Crouzon syndrome, the transmission is autosomal dominant (caused by mutation of the fibroblast
growth factor receptor 3 gene),11,12 while in Berardinelli Seip syndrome, the transmission is autosomal recessive (caused by mutation
of the 1-acylglycerol-3-phosphate O-acyltransferase 2 gene).13
Iatrogenic AN usually occurs secondary to the use of hormonal
therapies such as birth control pills for contraception or growth
hormones and human chorionic gonadotropins.14 In addition,
AN has also been reported secondary to the use of highly active antiretroviral therapy medications such as amprenavir and
even vitamins such as nicotinic acid. AN may also be seen in
other endocrine disorders such as Cushing syndrome and acro-
megaly.15 Another rare but important cause of AN is nonalcoholic steatohepatitis.16 Researchers17 have also reported AN in
a pregnant woman without any underlying glucose intolerance
or gestational diabetes. Pathologically, the lesions of AN occur
secondary to elevated levels of serum insulin, resulting in the
activation of insulin-like growth factor-1 receptors of epidermal
keratinocytes and fibroblasts.18 Interestingly, investigators reported an increased expression of fibroblast growth factor 3 in
certain individuals with malignancy associated AN.19
DIAGNOSIS
AN tends to affect the skin folds, flexural areas, and the intertriginous areas. The neck and axillary regions are most commonly affected, although lesions may appear almost anywhere
on the body (Figure 1).18 Lesions have even been described on
the nipples, perineum, groin, and extensor surfaces of the legs.
Researchers20 have also described AN of the conjunctiva in a patient with underlying bronchial squamous cell carcinoma.
In general, the lesions appear as dark-brown, thickened plaques (Figure 2). These hyperpigmented plaques generally tend to be velvety
on touch. Lesions of AN may occur alone or may develop in other
skin lesions such as hyperkeratosis of the nipple and areola (HNA
type 2). Researchers21 have also described the case of a patient with
coexisting AN and confluent reticulated papillomatosis. Verrucous
AN may also occur. These lesions tend to affect the eyelids, lips, and
buccal mucosa. Rarely, the lesions may have a nevoid appearance.
These nevoid lesions usually appear at birth or puberty and may appear as unilateral lesions or midline umbilical lesions.22
The dermatologist should always inquire about any familial history of diabetes or AN. In fact, in a recent study, a prevalence
From the Department of Family Medicine, University of Illinois at Chicago, Chicago, IL 60612.
Dr. Kapoor is now in Private Practice in Knoxville, TN.
Address for Correspondence: Shailendra Kapoor, MD, Knoxville, TN 37923 • E-mail: shailendrakapoor@yahoo.com
161
May/June 2010
REVIEW
sary. For instance, investigators28 recently described the case of a
patient with AN and hepatic metastasis secondary to an occult
underlying primary malignant lesion. Patients with gastrointestinal neoplasms may also present with other paraneoplastic
dermatoses such as tylosis and cutaneous papillomatosis. Rarely,
multiple paraneoplastic dermatoses may be present in the same
patient. Researchers29 recently reported the case of a patient with
malignant AN who had the sign of Leser-Trelat as well as tripe
palms. AN has also been reported with other adenocarcinomas,
including those of the testes and prostate in men and the breast
and uterine parametrium in women. A detailed medication history should also be taken to rule out any iatrogenic cause such as
steroids, palifermin, or triazinate. Acanthosis nigricans can be associated with other features such as hypodontia and cutis gyrate
as in patients with Beare-Stevenson syndrome.30
Figure 1. An elderly woman with uncontrolled diabetes and
acanthosis nigricans of the neck.
Biopsy of the lesions may be performed to confirm the diagnosis. Histology reveals hypertrophy and hyperplasia of the epidermis and papillary dermis accompanied by hyperkeratosis and
acanthosis. Typically, there is an increase in extracellular matrix,
resulting in papillary extensions into the dermis. Typically, no
melanocytic hyperplasia or hyperpigmentation is seen.
Figure 2. The typical hyperpigmented lesions of acanthosis
nigricans in a woman with diabetes.
of 17% in African individuals with type II diabetes mellitus was
resported.23 Presence of AN in obese individuals usually points toward hyperinsulinemia and peripheral insulin resistance.24,25 Investigators26 have reported that children with AN are almost 4 times
more likely to have hyperinsulinemia compared with children
without AN. Presence of AN alone may also indicate increased
risk of type 2 diabetes mellitus. In a recent study, Kong and coauthors27 reported that the prevalence ratio for type 2 diabetes in
patients with AN was 1.97. In another recent study, researchers1
showed that patients with AN usually require a higher dosage of
insulin compared with diabetic patients without AN (82.3 units/d
vs 50.2 units/d). Clearly, AN has significant diagnostic and prognostic value.
Any healthy patient diagnosed with AN without an underlying
explanation should also be investigated for any occult malignancy. The gastrointestinal system should be particularly focused
on and appropriate tests should be performed if deemed neces-
Further evaluation should include fasting blood glucose and
thyroid function tests. In addition, a complete blood cell count
and serum chemistries should be performed. Patients with AN
are more likely to have the metabolic syndrome.31,32 In a recent
study, 75% of patients with AN had the metabolic syndrome16;
hence, lipid panels should also be routinely performed in all
patients with AN.33 In a recent study in Asian women, investigators34 confirmed a strong association between AN and
polycystic ovarian disease34; therefore, obese women with AN
should also have quantitative follicle-stimulating hormone and
lutenizing hormone assays performed to rule out polycystic
ovarian disease.35 Incidentally, estrogen and its corresponding
receptors have recently been shown to play a role in the evolution of skin tags.36
TREATMENT
There is no specific treatment for AN. The treatment plan focuses on management of the underlying disorder. This usually
results in partial or complete regression of the AN. Researchers37 recently showed that regression of AN correlates with the
disappearance of anti–insulin receptor antibodies in patients
with diabetes. They demonstrated that the achievement of euglycemia in diabetic patients is accompanied by marked regression of AN. Similarly, malignant AN usually improves after the
underlying malignancy is treated. The prognostic response to
treatment is, however, not significant in certain cases such as individuals with truncation mutations of Tre-2, BUB2, CDC16,
162
May/June 2010
REVIEW
1 domain family members 4 (TBC1D4).38 Researchers39 have
also shown that patients presenting with AN secondary to
underlying congenital adrenal hyperplasia show symptomatic
resolution of the skin lesions after effective treatment of the
adrenal hyperplasia. Similarly, investigators40 have reported
dramatic improvements of AN lesions in patients with congenital generalized lipodystrophy following leptin replacement
therapy. This clearly demonstrates the need to address and treat
the underlying etiology in all patients with AN.
References
The management of obesity-associated AN primarily involves behavioral modifications, particularly increased exercise and rigorous
dietary control.41 In addition, oral metformin may be potentially
used as a potent treatment agent with considerable significant response.42 It acts by increasing the sensitivity of the peripheral insulin receptors. Investigators43 have reported that patients taking
metformin therapy show significant improvements of their AN
lesions compared with those taking placebo alone; however, some
other researchers44 have reported no significant changes with metformin therapy. Oral octreotide has also been used with moderate
success. It acts by decreasing insulin secretion.
Topical application of 0.1% tretinoin cream can be tried to
lighten the lesion. In addition, combinations of tretinoin cream
with 12% ammonium lactate cream have also been successfully
used.45 Topical calcipotriol ointments have also been effective in
the management of these lesions. Investigators46 recently reported the successful treatment with topical calcipotriol ointment
of a 26-year-old obese woman with bilateral AN of the nipples.
Calcipotriol binds to vitamin D3 receptors in the keratinocytes,
thus decreasing their proliferation.
Systemic therapy that primarily relies on isotretinoin or acitretin
may be tried if the patient fails to respond to topical therapy.47
Ozdemir and researchers48 recently reported remarkable success
with the use of oral acitretin. An initial dose of 50 mg/d followed
by a maintenance dose of 25 mg/d was used.
Photothermolysis may also be attempted by using Q-switched lasers. This has produced limited success. Long-pulsed lasers may also
be tried. Rosenbach and Ram7 recently used the long-pulsed alexandrite laser to successfully treat AN of the axilla. Dermabrasion is an
alternative to laser therapy. Surgical therapy of the lesions, although
rarely used, may also be attempted if conservative therapy fails.49
CONCLUSIONS
AN is a skin disorder that is difficult to treat. It should be explained
to patients that complete resolution of the AN may never occur. As
new developments in dermatology emerge, we may one day have
a better treatment, but, for now, the management of AN should
clearly focus on the management of the underlying disorder.
163
1 Litonjua P, Pinero-Pilona A, Aviles-Santa L, et al. Prevalence of
acanthosis nigricans in newly-diagnosed type 2 diabetes. Endocr Pract. 2004;10:101–106.
2 Miura N, Ikezaki A, Iwama S, et al. Genetic factors and clinical
significance of acanthosis nigricans in obese Japanese children
and adolescents. Acta Paediatr. 2006;95:170–175.
3 Yoshino N, Yamagishi S, Kubokura H, et al. Mediastinal lymph node
metastasis of lung cancer with an unknown primary lesion having
concurrent endocrine abnormality and acanthosis nigricans: report of a case. Ann Thorac Cardiovasc Surg. 2009;15:397–400.
4 Brantsch KD, Moehrle M. Acanthosis nigricans in a patient with sarcoma of unknown origin. J Am Acad Dermatol. 2010;62:527–528.
5 Ziadi T, Alahyane A, El Fahssi M, et al. Adenocarcinoma of gallbladder revealed by acanthosis nigricans. Gastroenterol Clin
Biol. 2009;33:986–988.
6 Mignogna MD, Fortuna G, Falleti J, et al. Gastric diffuse large Bcell lymphoma (DLBCL) exhibiting oral acanthosis nigricans and
tripe palms. Dig Liver Dis. 2009;41:766–768.
7 Rosenbach A, Ram R. Treatment of acanthosis nigricans of the
axillae using a long-pulsed (5-msec) alexandrite laser. Dermatol
Surg. 2004;30:1158–1160.
8 Marshall JD, Bronson RT, Collin GB, et al. New Alstrom syndrome phenotypes based on the evaluation of 182 cases. Arch
Intern Med. 2005;165:675–683.
9 Prasad AN. Berardinelli seip syndrome. Indian Pediatr.
2005;42:1245.
10 Rego AG, Mesquita ET, Faria CA, et al. Cardiometabolic abnormalities in patients with berardinelli-Seip syndrome [in Portuguese]. Arq Bras Cardiol. 2010;94:109–118.
11 Sharda S, Panigrahi I, Gupta K, et al. A newborn with acanthosis
nigricans: can it be Crouzon syndrome with acanthosis nigricans? Pediatr Dermatol. 2010;27:43–47.
12 Gupta AK, Koley S, Choudhary S, et al. A rare association of
acanthosis nigricans with Crouzon syndrome. Indian J Dermatol
Venereol Leprol. 2010;76:65–67.
13 Miranda DM, Wajchenberg BL, Calsolari MR, et al. Novel mutations of the BSCL2 and AGPAT2 genes in 10 families with Berardinelli-Seip congenital generalized lipodystrophy syndrome. Clin
Endocrinol (Oxf). 2009;71:512–517.
14 Downs AM, Kennedy CT. Somatotrophin-induced acanthosis nigricans. Br J Dermatol. 1999;141:390–391.
15 Ben-Shlomo A, Melmed S. Skin manifestations in acromegaly.
Clin Dermatol. 2006;24:256–259.
16 Uwaifo GI, Tjahjana M, Freedman RJ, et al. Acanthosis nigricans
in patients with nonalcoholic steatohepatitis: an uncommon finding. Endocr Pract. 2006;12:371–379.
17 Kroumpouzos G, Avgerinou G, Granter SR. Acanthosis nigricans without diabetes during pregnancy. Br J Dermatol.
2002;146:925–928.
18 Hermanns-Le T, Scheen A, Pierard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management.
Am J Clin Dermatol. 2004;5:199–203.
19 Hida Y, Kubo Y, Nishio Y, et al. Malignant acanthosis nigricans
with enhanced expression of fibroblast growth factor receptor
3. Acta Derm Venereol. 2009;89:435–437.
May/June 2010
REVIEW
20 Tabandeh H, Gopal S, Teimory M, et al. Conjunctival involvement
in malignancy-associated acanthosis nigricans. Eye. 1993;7(pt
5):648–651.
21 Cannavo SP, Guarneri C, Borgia F, et al. Confluent and reticulated papillomatosis and acanthosis nigricans in an obese girl:
two distinct pathologies with a common pathogenetic pathway
or a unique entity dependent on insulin resistance? J Eur Acad
Dermatol Venereol. 2006;20:478–480.
22 Kim SK, Kim YC. Nevoid acanthosis nigricans localized to the
umbilicus. J Dermatol. 2006;33:433–434.
23 Ogbera AO, Akinlade A, Ajose O, et al. Prevalence of acanthosis
nigricans and its correlates in a cross-section of Nigerians with
type 2 diabetes mellitus. Trop Doct. 2009;39:235–236.
24 Takahashi I, Yamada Y, Kadowaki H, et al. Phenotypical variety of
insulin resistance in a family with a novel mutation of the insulin
receptor gene. Endocr J. 2010 Mar 25. [Epub ahead of print]
25 Scott AT, Metzig AM, Hames RK, et al. Acanthosis nigricans and
oral glucose tolerance in obese children. Clin Pediatr (Phila).
2010;49:69–71.
26 Mukhtar Q, Cleverley G, Voorhees RE, et al. Prevalence of acanthosis nigricans and its association with hyperinsulinemia in New
Mexico adolescents. J Adolesc Health. 2001;28:372–376.
27 Kong AS, Williams RL, Smith M, et al. Acanthosis nigricans
and diabetes risk factors: prevalence in young persons seen
in southwestern US primary care practices. Ann Fam Med.
2007;5:202–208.
28 Nair PS, Moorthy PK, Suprakasan S, et al. Malignant acanthosis
nigricans with liver secondaries from an occult primary adenocarcinoma of gastrointestinal tract. Indian J Dermatol Venereol
Leprol. 2005;71:197–198.
29 Pentenero M, Carrozzo M, Pagano M, et al. Oral acanthosis nigricans, tripe palms and sign of leser-trélat in a patient with
gastric adenocarcinoma. Int J Dermatol. 2004;43:530–532.
30 Tao YC, Slavotinek A, Vargervik K, et al. Hypodontia in BeareStevenson syndrome: an example of dental anomalies in FGFRrelated craniosynostosis syndromes. Cleft Palate Craniofac J.
2010;47:253–258.
31 Galli-Tsinopoulou A, Grammatikopoulou M, Stylianou C, et al.
Diabese youngsters have 3.7 more chances in developing metabolic syndrome compared with the obese. J Endocrinol Invest.
2010 Feb 24. [Epub ahead of print]
32 Brickman WJ, Huang J, Silverman BL, et al. Acanthosis nigricans
identifies youth at high risk for metabolic abnormalities. J Pediatr. 2010;156:87–92.
33 Felszeghy E, Kaposzta R, Juhasz E, et al. Alterations of carbohydrate and lipoprotein metabolism in childhood obesity--impact of
insulin resistance and acanthosis nigricans. J Pediatr Endocrinol
Metab. 2009;22:1117–1126.
34 Charnvises K, Weerakiet S, Tingthanatikul Y, et al. Acanthosis nigricans: clinical predictor of abnormal glucose tolerance in Asian
women with polycystic ovary syndrome. Gynecol Endocrinol.
164
2005;21:161–164.
35 Alemzadeh R, Kichler J, Calhoun M. Spectrum of metabolic dysfunction in relationship with hyperandrogenemia in obese adolescent girls with polycystic ovary syndrome. Eur J Endocrinol.
2010 Apr 6. [Epub ahead of print]
36 El Safoury O, Rashid L, Ibrahim M. A study of androgen and
estrogen receptors alpha, beta in skin tags. Indian J Dermatol.
2010;55:20–24.
37 Fareau GG, Maldonado M, Oral E, et al. Regression of acanthosis nigricans correlates with disappearance of anti-insulin
receptor autoantibodies and achievement of euglycemia in type
B insulin resistance syndrome. Metabolism. 2007;56:670–675.
38 Dash S, Sano H, Rochford JJ, et al. A truncation mutation in
TBC1D4 in a family with acanthosis nigricans and postprandial hyperinsulinemia. Proc Natl Acad Sci U S A. 2009;106:9350–9355.
39 Kurtoglu S, Atabek ME, Keskin M, et al. Acanthosis nigricans in
association with congenital adrenal hyperplasia: resolution after
treatment. case report. Turk J Pediatr. 2005;47:183–187.
40 Eberting CL, Javor E, Gorden P, et al. Insulin resistance, acanthosis nigricans, and hypertriglyceridemia. J Am Acad Dermatol. 2005;52:341–344.
41 Otto DE, Wang X, Tijerina SL, et al. A comparison of blood pressure, body mass index, and acanthosis nigricans in school-age
children. J Sch Nurs. 2010 Mar 24. [Epub ahead of print]
42 Wasniewska M, Arrigo T, Crisafulli G, et al. Recovery of acanthosis nigricans under prolonged metformin treatment
in an adolescent with normal weight. J Endocrinol Invest.
2009;32:939–940.
43 Srinivasan S, Ambler GR, Baur LA, et al. Randomized, controlled
trial of metformin for obesity and insulin resistance in children
and adolescents: improvement in body composition and fasting
insulin. J Clin Endocrinol Metab. 2006;91:2074–2080.
44 Bellot-Rojas P, Posadas-Sanchez R, Caracas-Portilla N, et al.
Comparison of metformin versus rosiglitazone in patients
with acanthosis nigricans: a pilot study. J Drugs Dermatol.
2006;5:884–889.
45 Blobstein SH. Topical therapy with tretinoin and ammonium
lactate for acanthosis nigricans associated with obesity. Cutis.
2003;71:33–34.
46 Lee HW, Chang SE, Lee MW, et al. Hyperkeratosis of the nipple
associated with acanthosis nigricans: treatment with topical calcipotriol. J Am Acad Dermatol. 2005;52:529–530.
47 Walling HW, Messingham M, Myers LM, et al. Improvement of
acanthosis nigricans on isotretinoin and metformin. J Drugs Dermatol. 2003;2:677–681.
48 Ozdemir M, Toy H, Mevlitoglu I, et al. Generalized idiopathic acanthosis nigricans treated with acitretin. J Dermatolog Treat.
2006;17:54–56.
49 Schmolke B, Grotmann P, Kreusch J, et al. Surgical therapy
of lip involvement in acanthosis nigricans maligna. Hautarzt.
1994;45:875–877.
May/June 2010
Volume 8 • Issue 3
SELF-TEST REVIEW QUESTIONS
W. Clark Lambert, MD, PhD, Editor
Instructions: For each of the following numbered questions, choose the single most appropriate lettered response.
1) Acanthosis nigricans is mostly seen in individuals with:
4) The most common malignancies associated with acanthosis
a. Addison’s disease
b. Cushing’s syndrome
a. adrenal cancers
c. diabetes mellitus
b. gastrointestinal carcinomas
d. hypothyroidism
c. metastatic hepatic cancers
e. thyrotoxicosis
d. metastatic renal cancers
e. thyroid cancers
nigricans are:
2) Lesions of acanthosis nigricans have been described in (on) the:
a. conjunctiva
b. extensor surfaces of the legs
c. groin
d. nipple
e. perineum
f. All of these are correct
g. None of these is correct
5) Which of the following statements regarding acanthosis nigricans
is correct?
a. In patients with diabetes mellitus, regression of acanthosis
nigricans correlates with appearance of anti-insulin receptor
antibodies.
b. Patients with acanthosis nigricans are more likely to have the
metabolic syndrome.
c. Oral metformin counteracts acanthosis nigricans by decreasing insulin secretion.
d. Oral octreotide counteracts acanthosis nigricans by increasing the sensitivity of peripheral insulin receptors.
3) Verrucous acanthosis nigricans tends to occur on the:
a. buccal mucosa
b. eyelids
c. lips
d. All of the above are correct
e. None of the above is correct
e. All of the above are correct.
f. None of the above is correct.
ANSWERS TO SELF-TEST REVIEW QUESTIONS:
1) c, 2) f, 3) d, 4) b, 5) b
From the Departments of Pathology and Dermatology, UMDNJ-New Jersey Medical School, Newark, NJ
Address for Correspondence: W. Clark Lambert, MD, PhD, Room C520, Medical Science Building UMDNJ-New Jersey Medical School,
Newark, NJ 07101 • E-mail: lamberwc@umdnj.edu
165
May/June 2010
Volume 8 • Issue 3
REVIEW
History of Teledermatology:
A Technique of the Future in Dermatology
Engin Şenel, MD
Abstract
Teledermatology is a developing application of telemedicine. Studies and reviews regarding diagnostic accuracy, efficacy, and feasibility of teledermatology applications have been published in the literature. There are two main methods of teledermatology technology to use: store-andforward and real-time videoconferencing. Teledermatology is a proven, effective diagnostic tool for skin lesions. (SKINmed. 2010;8:167–170)
W
orldwide economic, social, and medical standards
for teledermatology have not been established.
There are almost 8 million doctors in the world, and
the ratio of physicians to population by country vary from 1
to 440 per 100,000 people.1 For instance, the United Kingdom
has a ratio of 164 physicians for every 100,000 people, but the
doctor-to-population ratio for Rwanda is only 1.1 physicians
per 100,000 people.2 Rural/urban physician distribution is a
crucial issue in many undeveloped and developing countries. If
physicians were distributed according to population around the
world, there would be 124 physicians to every 100,000 people.
There are currently 57 countries with critical shortage, equivalent to a global deficit of 2.4 million doctors.2 Africa has 25% of
the world’s disease burden, 13.8% of the world’s population, but
only 1.3% of the world’s health workforce.3
The ideal ratio of dermatologist to population has been estimated
to be 1:50,000.4 In the aspect of dermatology, The International
Foundation for Dermatology estimates that 3 billion people living in more than 100 countries where skin diseases and sexually
transmitted diseases are common and on the increase lack basic
care for their skin diseases.5
Telemedicine is a technique in medicine that uses modern communication technologies to transfer medical information and
services.6 The World Health Organization (WHO) defines the
term telemedicine as:
The delivery of healthcare services, where distance is a
critical factor, by all healthcare professionals using information and communication technologies for the exchange
of valid information for diagnosis, treatment and preven-
tion of disease and injuries, research and evaluation, and
for the continuing education of healthcare providers, all
in the interests of advancing the health of individuals and
their communities.7
Telemedicine could reduce waiting time, treatment costs, and the
number of follow-up visits and it is a particularly promising technology for undeveloped and developing countries.8,9 Still, there are no
standard international guidelines for all telemedicine applications.
Teledermatology is an application of telemedicine that evaluates patients via the latest digital and informative technology.6,10 It is the
most exciting and fast-developing part of telemedicine by means
of the advances in digital technology.9,11 Teledermatology basically
uses telecommunication technologies to transfer images of lesions
with the demographic and clinical data of the patient. A specialist or
consultant evaluates the patient and makes the diagnosis.
Teledermatology Methods
Teledermatology can be performed in two methods: store-andforward teledermatology (SAFT) and real-time videoconferencing. Each method has its advantages and disadvantages (Table).
Store-and-Forward Teledermatology
SAFT is a relatively inexpensive and easy teledermatology application. In SAFT, demographic and clinical data of the patient
are recorded. Clinical pictures are then captured and digitally
stored.7,12 All digital materials are transferred by the clinician to
the consultant. Since prerecorded materials are evaluated by the
consultant in a later time, simultaneous presence of the clinician is
not required. SAFT is an effective diagnostic tool in dermatology.
From the Clinic of Dermatology, Merzifon Military Hospital, Merzifon, Amasya, Turkey
Address for Correspondence: Engin Şenel, MD, Merzifon Military Hospital, Clinic of Dermatology, Merzifon, Amasya, Turkey •
E-mail: enginsenel@enginsenel.com
167
May/June 2010
REVIEW
Table. Comparison of the Methods of Teledermatology9,18,22
Store-and-Forward Teledermatology
Videoconferencing System
Synchronized evaluation is not necessary
Interactive consultation
Inexpensive but effective way for diagnosis and treatment
Expensive
Often unable to accept information directly from the patient
Enables 3-way discussion between general practitioner, patient, and
the consultant dermatologist
High picture quality
Poorer picture quality but streaming
Simple computer systems and dial-up connection are sufficient
Requires faster (broadband) Internet connection
Good method for the patient triage
Effective method for general practitioner treatment
Diagnostic agreement between SAFT and conventional face-toface examination ranges from 65% to 93% in various studies.13–15
Real-Time Videoconferencing System
The real-time videoconferencing system needs a broadband connection and synchronous video and audio streaming services to
allow a live, interactive consultation. Simultaneous evaluation
can minimize information gaps, but may increase the cost of the
teledermatology application.7,16 Diagnostic accuracy agreement
ranges from 59% to 80% between real-time teledermatology
and face-to-face evaluation.17–21
History of Telemedicine
and Teledermatology
Telemedicine was first used in 1959 in Nebraska. The Nebraska
Psychiatric Institute began using a closed-circuit television system between the institute itself and Norfolk State Hospital,
about 112 miles away, in 1964.22 The name of this study was the
Nebraska Project.23 The aim of the project was the transmission
of motion visuals for psychiatric and neurologic evaluation of
patients.24 The system was used for 6 years to provide consultation and education.25
The Space Technology Applied to Rural Papago Advanced
Health Care (STARPAHC) Project26 demonstrates another significant early endeavor of telemedicine. The project was started
in the late 1950s by the National Aeronautics and Space Administration (NASA) for telemedical help for people living in
remote locations with little or no medical services, such as those
in Arizona’s Papago Indian Reservation.25 The other main purpose of the project was to develop a system to provide medical
service to astronauts in space by using audio and visual telecommunication.23,27 The project successfully provided medical care
to remote sites on the Papago Indian Reservation for 20 years.28
In 1967, an interactive television link was established between a
medical station at Boston’s Logan International Airport and Mas-
sachusetts General Hospital, miles away within the city of Boston.
Physicians at the hospital provided telemedical care to the patients
at the airport 24 hours a day by using the telemedicine link. After
the success of this program, telepsychiatric services provided by
the hospital were expanded to schools and the courts.29
There were 15 telemedicine sites receiving federal funding in
the United States in the 1970s.30 NASA started to use satellite
video consultations to improve the quality of rural health care
in Alaska in 1971. Five installed ground stations of the trial had
two-way audio but only one-way video.31 Simultaneous video
transmission and evaluation was not available. At the end of
this trial it was determined that the unique capabilities of the
video transmission may play a critical role in 5% to 10% of the
cases selected for video presentation.32 In 1972, The Health Care
Technology Division of the US Department of Health, Education and Welfare (HEW) funded 7 telemedicine research and
demonstration projects such as Dartmouth Medical School’s
Impact Project and Massachusetts’ Cambridge Hospital Project.
The North-West Telemedicine Project was started in 1984 in
Australia to pilot-test a government satellite communications
network (the Q-Network). The aim of the successful project was
to provide health care to people in 5 remote regions of the Gulf
of Carpentaria.22 In 1989, after a catastrophic earthquake that
hit the Soviet Republic of Armenia, NASA conducted the first
international telemedicine project, Space Bridge. One-way video
and audio transmission systems were used between a medical
center in Yerevan, Armenia, and 4 medical centers in the United
States. This program was later extended to Ufa, the capital of the
Republic of Bashkortostan in Russia.11
Since telemedicine applications were first started, teledermatology has become a crucial component of many telemedicine
networks. The term teledermatology was first used by Perednia
and Brown in 1995. They reported the results of the teledermatology project in the remote rural areas of Oregon where
dermatologic care was supplied by only 2 dermatologists.10 The
168
May/June 2010
REVIEW
authors reported that the installation of a teledermatology system dramatically increases the number of patients referred for
specialist evaluation. Even while the number of in-person visits
to specialists fell and although there was no statistical diagnostic
difference between dermatologist and primary care providers, a
significant difference in treatment modalities was found.
In 1997, three independent teledermatology programs to provide
dermatologic care to 3 traditionally underserved populations of
the United States, Pacific Islanders, migrant farm workers, and
prison inmates were described in one report.33 Teledermatology
was found to be a useful technology to provide dermatologic
support to remote or undeserved communities.33 In the same
year, researchers34 reported that teledermatology was an effective way of dermatology consultation in new patients referred
from rural communities. In addition, experts13 reported a high
diagnostic concordance rate (95%) between a simple videoconferencing method using a digital camera and face-to-face evaluation, which correctly diagnosed 95 of 100 patients.
no dermatologist. It may reduce waiting time for patients with
limited access to medical care and improve survival in patients
with malignant skin tumors by means of providing swift diagnosis and early excision of the lesion. Physicians should be aware
of the techniques of teledermatology and encouraged to perform
new studies to develop this technology.
References
Investigators20 reported high diagnostic concordance (77.2%)
between face-to-face examination and teledermatologic evaluation in 1998. In January 1998, the first telemedicine link for the
British Defense Medical Services was set up between the British
military hospital in Sipovo, Bosnia, and the Royal Hospital Haslar, the main tri-service hospital in the United Kingdom.35 In the
same year, telemedicine technology was used at sea in Sweden,36
from an aircraft in the United Kingdom,37 and from a moving
ambulance in Greece.38
In 1999, researchers reported a diagnostic concordance of 91%
between face-to-face examination and teledermatologic evaluation of 66 pigmented skin lesions. One year later, in 2000, the
same authors found an average of 85% correct concordance of the
evaluation in a subset of 43 cutaneous pigmented skin lesions sent
by e-mail to 11 colleagues with different degrees of experience in
dermoscopy.40 In 2003, dermatoscopic images of 108 melanocytic
and nonmelanocytic lesions were evaluated via the Internet by 40
experienced dermatoscopists for the consensus meeting performed
to describe diagnostic criteria and pattern analyses for benign and
malignant skin tumors.41 In 2006, investigators42 evaluated teledermatoscopy as a triage system for pigmented lesions in a pilot
study that included 219 pigmented skin lesions. One year later, researchers43 stated that mobile teledermatology with cellular phones
is an easy, applicable, and efficient way for melanoma screening.
39
Teledermatology is a developing field and a relatively new branch
of telemedicine. Advances in telecommunication and visual digital systems make teledermatology an area with potential to apply telemedicine resources. Teledermatology is a proven-useful
technology to provide dermatologic care in places where there is
169
1 Wharrad H, Robinson J. The global distribution of physicians and
nurses. J Adv Nurs. 1999;30:109–120.
2 World Health Organization. The World Health Report 2006:
Working Together for Health. Geneva, Switzerland: World Health
Organization; 2006.
3 King RC, Fomundam HN. Remodeling pharmaceutical care
in Sub-Saharan Africa (SSA) amidst human resources challenges and the HIV/AIDS pandemic. Inter J Health Plan Manag.
2009;25:30–48.
4 Krasner M, Ramsay DL. National dermatology manpower requirements: the experience of prepaid group practices. Arch
Dermatol. 1977;113:903–905.
5 International Foundation for Dermatology Web site. http://www.
ifd.org/. Accessed February 22, 2010.
6 Perednia DA, Allen A. Telemedicine technology and clinical applications. JAMA. 1995;273:483–488.
7 Feroze K. Teledermatology in India: practical implications. Indian
J Med Sci. 2008;62:208–214.
8 Kanthraj GR, Srinivas CR. Store and forward teledermatology.
Indian J Dermatol Venereol Leprol. 2007;73:5–12.
9 Eedy DJ, Wootton R. Teledermatology: a review. Br J Dermatol.
2001;144:696–707.
10 Perednia DA, Brown NA. Teledermatology: one application of
telemedicine. Bull Med Libr Assoc. 1995;83:42–47.
11 Massone C, Wurm EM, Hofmann-Wellenhof R, et al. Teledermatology: an update. Semin Cutan Med Surg. 2008;27:101–105.
12 Kvedar JC, Menn ER, Baradagunta S, et al. Teledermatology in a
capitated delivery system using distributed information architecture: design and development. Telemed J. 1999;5:357–366.
13 Lyon CC, Harrison PV. Digital imaging and teledermatology: educational and diagnostic applications of a portable digital imaging system for the trainee dermatologist. Clin Exp Dermatol.
1997;22:163–165.
14 Zelickson BD, Homan L. Teledermatology in the nursing home.
Arch Dermatol. 1997;133:171–174.
15 Kvedar JC, Edwards RA, Menn ER, et al. The substitution of
digital images for dermatologic physical examination. Arch Dermatol. 1997;133:161–167.
16 Kaliyadan F, Venkitakrishnan S. Teledermatology: clinical case
profiles and practical issues. Indian J Dermatol Venereol Leprol.
2009;75:32–35.
17 Loane MA, Corbett R, Bloomer SE, et al. Diagnostic accuracy
and clinical management by realtime teledermatology. Results
from the Northern Ireland arms of the UK Multicentre Teledermatology Trial. J Telemed Telecare. 1998;4:95–100.
18 Gilmour E, Campbell SM, Loane MA, et al. Comparison of teleconsultations and face-to-face consultations: preliminary results
May/June 2010
REVIEW
of a United Kingdom multicentre teledermatology study. Br J
Dermatol. 1998;139:81–87.
19 Oakley AM, Astwood DR, Loane M, et al. Diagnostic accuracy of
teledermatology: results of a preliminary study in New Zealand.
N Z Med J. 1997;110:51–53.
20 Phillips CM, Burke WA, Allen MH, et al. Reliability of telemedicine
in evaluating skin tumors. Telemed J. 1998;4:5–9.
21 Lowitt MH, Kessler II, Kauffman CL, et al. Teledermatology
and in-person examinations: a comparison of patient and physician perceptions and diagnostic agreement. Arch Dermatol.
1998;134:471–476.
22 Ceyhan A, Baysal V. Teledermatoloji. TÜRKDERM. 2003;37:58–62.
23 Lim AC, Egerton IB, Shumack SP. Australian teledermatology:
the patient, the doctor and their government. Australas J Dermatol. 2000;41:8–13.
24 Burg G, Hasse U, Cipolat C, et al. Teledermatology: just cool or
a real tool? Dermatology. 2005;210:169–173.
25 Wittson CL, Benschoter R. Two-way television: helping the Medical Center reach out. Am J Psychiatry. 1972;129:624–627.
26 Fuchs M. Provider attitudes toward STARPAHC: a telemedicine
project on the Papago reservation. Med Care. 1979;17:59–68.
27 Brown JH. Telecommunications—a system for total health care.
Crit Rev Bioeng. 1980;4:271–309.
28 Preston J, Brown FW, Hartley B. Using telemedicine to improve
health care in distant areas. Hosp Community Psychiatry.
1992;43:25–32.
29 Dranov P. Telemedicine. Sci Dig. 1981;89:112–113, 120.
30 Park B, Bashshur R. Some implications of telemedicine. J Commun. 1975;25:161–166.
31 Foote DR. Satellite communication for rural health care in Alas-
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
170
ka. J Commun. 1977;27:173–182.
32 Foote D, Hudson H, Parker EB. Telemedicine in Alaska: The ATS-6
satellite biomedical demonstration. Springfield, VA: National Technical Information Service (NTIS). US Department of Commerce; 1976.
33 Norton SA, Burdick AE, Phillips CM, et al. Teledermatology and
underserved populations. Arch Dermatol. 1997;133:197–200.
34 Burgiss SG, Julius CE, Watson HW, et al. Telemedicine for dermatology care in rural patients. Telemed J. 1997;3:227–233.
35 Ritchie C. British Army establishes telemedicine unit in Bosnia.
Lancet. 1998;352:46.
36 Lyden B. Telemedicine goes to sea. Nord Med. 1998;113:120–121.
37 MacDonald A, McNicholl BP, Wootton R. Transmission of medical data from an aircraft. J Telemed Telecare. 1998;4:62.
38 Giovas P, Papadoyannis D, Thomakos D, et al. Transmission of
electrocardiograms from a moving ambulance. J Telemed Telecare. 1998;4(suppl 1):5–7.
39 Piccolo D, Smolle J, Wolf IH, et al. Face-to-face diagnosis vs telediagnosis of pigmented skin tumors: a teledermoscopic study.
Arch Dermatol. 1999;135:1467–1471.
40 Piccolo D, Smolle J, Argenziano G, et al. Teledermoscopy–results of a multicentre study on 43 pigmented skin lesions. J
Telemed Telecare. 2000;6:132–137.
41 Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the
Internet. J Am Acad Dermatol. 2003;48:679–693.
42 Moreno-Ramirez D, Ferrandiz L, Galdeano R, et al. Teledermatoscopy as a triage system for pigmented lesions: a pilot study.
Clin Exp Dermatol. 2006;31:13–18.
43 Massone C, Hofmann-Wellenhof R, Ahlgrimm-Siess V, et al. Melanoma screening with cellular phones. PLoS One. 2007;2:e483.
Aklarus Multi-Wavelength
Phototherapy System
The Aklarus is a multi-wavelength phototherapy
system FDA cleared and medically proven
to provide a drug-free and pain-free treatment
for acne, photodamaged skin and wrinkles.
HA90D Chair
Shown with
optional contour
seat and back
and removable
fold-down
armrests.
See Before and After
Photos and Video at
www.HillTherapy.com
The Versatile, Affordable
Chair for Dermatology!
$9650
$8150
Includes cart,
treatment arm
and accessories
Working closely with
a leading dermatologist,
Hill Laboratories has
designed the ideal chair
for dermatology...
• Electrically controlled height - 22”-35”
• Standard width - 26” or 24”
• Length - 70"
• 2.5” Foam top
• Electric Lift back
• Full-width foot section-locks at any angle
• Adjustable headrest
• Standard Flat Cushions
• Paper retainer on headpiece
• Paper roll bracket on foot cushion
• One-year warranty
Starts at
$3495
HA90D fully reclined with
matching stool
Since 1945
Optional
Tapered-Top
& headpiece
90ALB for Aesthetics
starts
$3175
Call Today!
1-877-445-5020
www.HillLabs.com
Optional
Removable, FoldDown Armrests
May/June 2010
Volume 8 • Issue 3
Historical Vignette
Charles Steffen, MD, Section Editor
The Advent of a Novel Diagnosis:
Melanoma Through the Ages
Navid Ezra, BS; Jason Rabie, BS
In the 1930s, the risk of contracting melanoma was only 1:1500; however, by 1996 this number had risen to 1:87 and has been increasing ever
since. To better understand the nature of melanoma, books, journals, and scholarly literary works were searched and contributors of this disease
were studied in greater detail. Antiquity of melanoma is said to be approximately 2400 years old based on observations made on 9 Peruvian Inca
mummies in the 1960s that showed apparent signs of melanoma. René Théophile Hyacinthe Laënnec, the inventor of the stethoscope, first
described melanoma as a disease entity. William Norris, an English general practitioner, gave the first English language report of this disease.
There are many other physicians from France, England, and the United States who had an active role in the discovery of melanoma.
A
nalysis of the history of melanoma shows a dramatic increase in the lifetime risk of melanoma in recent years.
In 1996, the lifetime risk of contracting melanoma was
1:87, significantly greater than the risk of 1:1500 in the 1930s.1
Some have speculated that this rapid increase in the development
of melanoma results from increased life expectancy, whereas others attribute this to increased levels of ultraviolet radiation in the
atmosphere due to destruction of the ozone layer.2
Melanoma is a malignant tumor of melanocytes found predominantly in the skin, but also the gastrointestinal tract and eyes.
Although melanoma of the skin is one of the rarest types of
skin cancer, it causes the majority of skin cancer–related deaths.
Once the cancer has metastasized, the survival rate is often measured in months rather than years. This is due to the fact that
melanoma is able to cloak itself from the body’s immune system,
leaving it to grow relatively unchallenged. Based on information
retrieved from the National Cancer Institute’s (NCI’s) Surveillance Epidemiology and End Results (SEER) Cancer Statistics
Review, it has been estimated that 59,940 persons (specifically
33,910 men and 26,030 women) were diagnosed with melanoma and 8110 men and women died of melanoma of the skin
in the year 2007.3
This paper examines the development of the concept of melanoma in the 19th century to better understand the origins of
this disease and how the medical community has realized its
significance, ultimately leading to it becoming widely studied
and researched.
The Origins of Melanoma
Although melanoma is not a new disease, evidence for its occurrence in the past is still rather scarce. Nine Peruvian Inca mummies that were studied in the 1960s showed apparent signs of
melanoma and provided evidence for the presence of melanoma
in past centuries. Upon examination, these specimens displayed
melanotic masses in the skin and metastases in the bones, especially in the skull and extremities. Through the use of radiocarbon-14 dating, it has been determined that melanoma originated approximately 2400 years ago.4
The 19th Century as the Age of Discovery
Discoveries of Melanoma in France: 19th Century
French physician René Théophile Hyacinthe Laënnec (February 17, 1781–August 13, 1826) first described melanoma as a
disease entity. He is also considered to be the first to recognize
that melanotic lesions were different from the black tuberculosis
granulomas or carbon deposits commonly found in the lungs.
In describing these tumors, Laënnec coined the term melanose,
which means “black” in Greek.5
Jean Cruveilhier, a French pathologist, anatomist, and physician,
wrote several important works on pathologic anatomy. Between
1829 and 1842 he published an atlas of pathology, Anatomie
Pathologique du Corps Humains (“Pathological Anatomy of the
Human Body”), which included the original descriptions of melanoma of the hand, foot, and vulva, and metastatic melanoma of
the heart and bowels.6
From the David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA 90095
Address for Correspondence: Navid Ezra, BS, David Geffen School of Medicine at UCLA, Box 951720, 12-159 CHS, Los Angeles, CA
90095-1720 • E-mail: navid.ezra@ucla.edu
172
May/June 2010
HISTORICAL VIGNETTE
Discoveries of Melanoma in England: 19th Century
In 1820, William Norris, an English general practitioner from
Stourbridge, United Kingdom, presented the first English language report of melanoma. In retrospect, Norris published what
he called “the first genuine good case of melanoma,” although
his original term was fungoid disease.7 In this case, a changing
mole developed into a fungating tumor with subsequent satellitosis. When removed, it recurred rapidly.7 Later, in 1857, he
used the case studies of 8 patients with diagnosed melanoma
to conclude that there is a familial predisposition for development of melanoma. He suggested that the etiology of melanosis
may be the product of the industrial revolution.4 Norris indicated that melanoma often arises from preexisting moles, that
individuals with melanoma are most likely to have fair skin and
light-colored hair, and that trauma to the tumor may aggravate
it and increase its rate of growth.8
In characterizing the pathologic features of melanoma, Norris
stated that: (1) melanoma is not always black and can be amelanotic; (2) the tumor is sometimes characterized by pigment
spreading about it, but is sometimes nodular or polyploidy; (3)
satellite deposits may develop about the primary melanoma; (4)
widespread subcutaneous deposits may develop; and (5) dissemination is possible to every site in the body.8
After providing treatment to his patients, Norris also documented the results. He found that limited local excision of the melanotic tumor may result in its local recurrence. Wider excision of
the tumor and surrounding unaffected skin was more effective at
preventing the tumor’s recurrence.8
Robert Carswell, the researcher who first coined the word melanoma in 1838, used chemical analysis to suggest that melanoma
resulted from degradation of blood.9 Oliver Pemberton (1858)
detailed the characteristics and sites of metastases, early pathologic
studies, and the earliest descriptions with statistics on prognosis.10
He studied 60 cases of melanoma and made the first report of
melanoma in a black person.8 Jonathan Hutchinson (1890) described lentigo maligna, which now bears his name, as “infective
senile freckles” that he believed to be infective because of their
slow progressive growth.11 He also described melanotic whitlow in
1886.12 Moreover, in 1903, 74 years after Jean Cruveilhier’s case
of hand melanoma, Frederick Eve, a surgeon at London Hospital,
described and illustrated a melanoma of the palm of the hand.13
Herbert Snow (1847–1930), a London surgeon with a particular
interest in melanoma, was a controversial advocate of the method
of anticipatory gland excision before the general acceptance of elective lymph node dissections that are currently employed.14 Snow
suggested “…it is essential to remove, whenever possible, those
lymph glands which first receive the infective protoplasm, and bar
its entrance into the bloodstream, before they have increased in
bulk.”15 Lymph node dissection was extremely controversial during Snow’s era, and his theories on cancer were widely criticized.16
Like the general public, though, he believed that a microorganism could not cause cancer. Even though the seminal theories on
cancer-gene theory were not yet evident, in 1890, Snow wrote
that to prevent the recurrence of cancer, the surgeon must achieve:
Figure. René Théophile Hyacinthe Laënnec, Jonathan Hutchinson,
and John Hunter.
…the perfect eradication of every cell nucleus or nuclear
particle which has thus apparently become endowed with
independent vitality; and which, therefore, if undestroyed,
must continue its independent growth at the expense of
the remainder of the organism, and must multiply in a
constantly increasing ratio.17
John Hunter (1728–1793), the founding father of British pathology, is reported to be the first to operate on metastatic melanoma
in 1787.18 During his career, Hunter excised a tumor from behind
the angle of the jaw of a 35-year-old man. Although Hunter did
not exactly know what it was, he described the lesion as “cancerous fungus excrescence.”19 In 1968, microscopic examination of
the specimen revealed that it was, in fact, metastatic melanoma.19
While the first case of cutaneous malignant melanoma ever
treated was by John Hunter in 1787,20 the first logical approach
to surgical therapy was not investigated until 1905.21 William
Handley, a research fellow at Middlesex Hospital, London, spent
2 years researching the metastatic dissemination of breast cancer.
In 1905, he began to analyze the lymphatic spread of a secondary melanoma deposit on a woman’s leg. On the basis of this
one case of metastatic melanoma, he suggested the removal of 2
inches of subcutaneous tissue down to the level of muscle fascia
along with a radical removal of lymph nodes. His work, published in The Lancet in 1907, set the rules for the surgical management of malignant melanoma for 50 years.21
In 1908, Handley delivered the famous Hunterian Lectures on
malignant melanoma to the Royal College of Surgeons of England.21 He acknowledged that he never treated a case of primary
malignant melanoma and based all his recommendations on a
single autopsy of a patient who had died of disseminated melanoma. Nevertheless, his recommendations became the standard
of treatment for most of the 20th century. Handley suggested
excising a 1-inch circumference of skin and a 2-inch margin
of subcutaneous tissue around the melanoma. That recommendation was later expanded to 5 centimeters, and even 15
173
May/June 2010
HISTORICAL VIGNETTE
centimeters in select cases.22 Several investigators supported this
trend by reporting premalignant changes in the skin within several centimeters of the primary lesion.23,24
Altérations Morbides dont le Corps Humain est Susceptible. Vol 1
(livriason xix) (1829–1842). Paris, France: JB Baillière; 1878.
7 Norris W. Case of fungoid disease. Edinb Med Surg J.
1820;16:562.
8 McLeod GR, Davis NC. Historical overview of melanoma. Clin
Dermatol. 1992;10:5–7.
Discoveries of Melanoma in the United States:
19th Century
In 1840, Samuel Cooper was the first person to formally acknowledge that advanced melanoma is untreatable. He stated that the
“...only chance for benefit depends upon the early removal of the
disease....”25 This situation currently remains largely unchanged.
Besides Cruveilhier’s descriptions of melanoma in his pathology
text, there were other early reports of melanoma in the United
States. Isaac Parrish26 in 1837 and Montgomery27 in 1844 described melanoma of the foot. The report by Parrish is the first
case report of melanoma in North America. The book on the
melanoma of the foot by Montgomery included a rare case of
melanoma in a black patient. Furthermore, in 1837, John Warren reviewed several cases of melanoma in his book on tumors.28
The identification and treatment of melanoma has come a long
way from the initial discoveries in the 19th century. Although
the medical community has made great leaps, there is still much
we do not know about these cancerous lesions. Melanoma is currently a hot topic of research, and because of the dramatic increase in its rate of development, investigations will persist until
a treatment is devised that can lead to a better recovery, a decent
prognosis, and a higher survival rate.
References
1 Rigel DS, Friedman RJ, Kopf AW. The incidence of malignant
melanoma in the United States: issues as we approach the 21st
century. J Am Acad Dermatol. 1996;34:839–847.
2 Fears TR, Scotto J, Schneiderman MA. Skin cancer, melanoma,
and sunlight. Am J Public Health. 1976;66:461–464.
3 Horner MJ, Ries LAG, Krapcho M, et al., eds. SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. Bethesda,
MD. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER Web
site, 2009. Accessed May 14, 2010.
4 Urteaga O, Pack G. On the antiquity of melanoma. Cancer.
1966;19:607–610.
9 Carswell R. Illustrations of the Elementary Forms of Disease. Vol
9. London, England: Longman, Orme, Brown, Green, and Longman; 1838.
10 Pemberton O. Observations on the History, Pathology, and
Treatment of Cancerous Diseases. Part I: Melanosis. London,
England: Churchill; 1858:1–38.
11 Clemmensen O. Hutchinson’s freckle. Am J Dermatopathol.
1982;4:425–428.
12 Hutchinson J. Melanosis often not black: melanotic whitlow. Brit
Med J. 1886;1:491.
13 Eve F. A lecture on melanoma. Practitione. 1903;70:165.
14 Neuhaus SJ, Clark MA, Thomas JM. Dr. Herbert Lumley Snow, MD,
MRCS (1847–1930): the original champion of elective lymph node
dissection in melanoma. Ann Surg Oncol. 2004;11:875–878.
15 Snow H. Twenty-Two Years’ Experience in the Treatment of Cancerous and Other Tumours. London, England: Tindall and Cox; 1898.
16 Snow H. Obituary. Lancet. 1930;ii:1212–1213.
17 Snow H. On the Re-appearance (Recurrence) of Cancer After Apparent Extirpation: With Suggestions for Its Prevention, and General Remarks on the Operative Treatment of Malignant Growths.
London, England: J&A Churchill; 1890.
18 Kobler J. The Reluctant Surgeon. A Biography of John Hunter.
Garden City, New York; Doubleday & Company Inc: 1960.
19 Nahabedian MY. Melanoma. Clin Plast Surg. 2005;32:249–259.
20 Davis NC. William Norris, M.D.: a pioneer in the study of melanoma. Med J Aust. 1980;1:52–54.
21 Handley W. The pathology of melanotic growths in relation to
their operative treatment. Lancet. 1907;1:927–933.
22 McNeer G, Cantin J. Local failure in the treatment of melanoma.
The Janeway lecture, 1966. Am J Roentgenol Radium Ther Nucl
Med. 1967;99:791–808.
23 Wong CK. A study of melanocytes in the normal skin surrounding
malignant melanomata. Dermatologica. 1970;141:215–225.
24 Olsen G. The malignant melanoma of the skin. New theories based on a study of 500 cases. Acta Chir Scand Suppl.
1966;365:1–222.
25 McCarthy WH, Shaw HM. The surgical treatment of primary
melanoma. Hematol Oncol Clin North Am. 1998;12:797–805.
5 Roguin A. Rene Theophile Hyacinthe Laennec (1781–1826): the
man behind the stethoscope. Clin Med Res. 2006;4:230–235.
26 Parrish I. Case of melanosis. Am J Med Sci. 1837;20:266.
6 Cruveilhier J. Anatomie Pathologique du Corps Humain; ou, Descriptions, avec Figures Lithographiées et Coloriées, des Diverses
28 Warren JC. Surgical Observations on Tumours With Cases and
Operations. Boston, MA; Crocker and Brewster: 1837.
174
27 Montgomery A. Melanoma in black mail. Lancet. 1844;ii:280.
May/June 2010
Volume 8 • Issue 3
New to the Clinic
Noah S. Scheinfeld, MD, JD, Section Editor
Vibativ (Telavancin) for Complicated Skin and
Skin Structure Infections
Pedram Ghasri, BA;1 Noah S. Scheinfeld, MD, JD2
O
n September 11, 2009, the US Food and Drug Administration (FDA) approved telavancin (Vibativ; Theravance,
Inc, San Francisco, CA, and Astellas Pharma US, Inc,
Deerfield, IL) for the treatment of complicated skin and skin structure infections (cSSSIs) caused by methicillin-resistant Staphylococcus aureus, methicillin-sensitive Staphylococcus aureus, Streptococcus
pyogenes, Streptococcus agalactiae, Streptococcus anginosus group,
and vancomycin-susceptible Enterococcus faecalis.1 Telavancin, a lipoglycopeptide derivative of vancomycin, has a 2-fold mechanism
of action: (1) it inhibits polymerization and cross-linking of peptidoglycans during bacterial cell wall synthesis, and (2) it alters cell
membrane permeability by disrupting the membrane potential.2 It
is currently approved for intravenous infusion at a dosage of 10 mg/
kg over 60 minutes every 24 hours, although it is recommended
that the dosage be decreased to 7.5 mg/kg every 24 hours and 10
mg/kg every 48 hours in patients with a creatinine clearance of 30
to 50 mL/min and 10 to 29 mL/min, respectively.3
The efficacy of telavancin was initially examined in 2 randomized,
double-blind, active-controlled phase II trials (FAST I and FAST
II studies), which demonstrated that intravenous administration of
telavancin was as effective as standard therapy for the treatment of
cSSSIs.4,5 Ultimately, its approval by the FDA was based on 2 parallel, randomized, double-blind phase III clinical trials (the Assessment
of Telavancin in Skin and Skin Structure Infections [ATLAS] I and
ATLAS II studies).6 In these large-scale studies spanning 21 countries,
a total of 1867 adult patients were randomized to 2 treatment groups:
10 mg/kg of telavancin administered intravenously every 24 hours
or 1 g of vancomycin administered intravenously every 12 hours. Of
those patients, 1410 were considered to be clinically evaluable. The
types of infections treated most commonly were major abscesses, deep
cellulitis, infected wounds, infected ulcers, and infected burns. After 1
to 2 weeks of the last administered dose, 88% and 87% of the patients
had successful responses to telavancin and vancomycin, respectively
(95% confidence interval for the difference, –2.1 to 4.6). Among 579
patients infected with methicillin-resistant Staphylococcus aureus, 91%
of patients in the telavancin group and 86% of patients in the vancomycin group were effectively treated (95% confidence interval for the
difference, –1.1 to 9.3). Overall, the study demonstrated a comparable efficacy and tolerability profile to vancomycin for the treatment
of cSSSIs caused by Gram-positive bacteria.
Adverse effects of telavancin include taste disturbances, nausea,
and vomiting. Furthermore, it is classified as a category C drug,
stemming from developmental toxicology studies that demonstrated a decrease in fetal weight and a low incidence of limb
defects in rats treated with telavancin.7 There have also been reported incidences of reversible renal dysfunction and prolonged
corrected QT interval, although no major cardiovascular events
or arrhythmias have been reported.8,9 Given these findings, clinicians are advised to perform a pregnancy test and monitor renal
function during treatment with telavancin.
The mounting epidemic of antibiotic-resistant cSSSIs has
prompted clinical investigations for the development and approval of telavancin, a novel antibiotic with a substantial efficacy
and tolerability profile. The superiority of telavancin over traditional antibiotics stems from its rapid, concentration-dependent
bactericidal activity, as well as a 2-fold mechanism of action that
helps curb the development of resistance. It also has considerable
economic practicality compared with the use of vancomycin,
owing primarily to its once-daily dosing and a lack of need for
monitoring its serum concentrations. There is a lack of evidence
purporting its clinical effectiveness over vancomycin, however,
which has been a mainstay of treatment options for cSSSIs and
is associated with substantially less renal toxicity.
Therefore, although telavancin may hold promise as a timely supplement to current treatment options for cSSSIs amidst their growing
incidence, further clinical investigations should be implemented to
explore its efficacy against vancomycin-resistant bacteria.
From the University of California, Irvine School of Medicine, Irvine, CA;1 and the Department of Dermatology, Columbia University, School of Medicine,
New York, New York2
Address for Correspondence: Noah S. Scheinfeld, MD, JD, Columbia University, College of Physicians and Surgeons, Department of Dermatology,
150 West 55th Street, New York, NY 10019 • E-mail: scheinfeld@earthlink.net
175
May/June 2010
NEW TO THE CLINIC
References
1 Food and Drug Administration. FDA labeling information. http://www.
accessdata.fda.gov/drugsatfda_docs/label/2009/022110s000lbl.
pdf (2009) Accessed March 1, 2010.
2 Higgins DL, Chang R, Debabov DV, et al. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and
cell membrane integrity in methicillin-resistant Staphylococcus
aureus. Antimicrob Agents Chemother. 2005;49:1127–1134.
3 Telavancin (Vibativ) for gram-positive skin infections. Med Lett
Drugs Ther. 2010;52:1–2.
4 Stryjewski ME, O’Riordan WD, Lau WK, et al. Telavancin versus standard
therapy for treatment of complicated skin and soft-tissue infections due
to gram-positive bacteria. Clin Infect Dis. 2005;40:1601–1607.
5 Stryjewski ME, Chu VH, O’Riordan WD, et al. Telavancin versus
standard therapy for treatment of complicated skin and skin
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
176
structure infections caused by gram-positive bacteria: FAST 2
study. Antimicrob Agents Chemother. 2006;50:862–867.
6 Stryjewski ME, Graham DR, Wilson SE, et al. Telavancin versus
vancomycin for the treatment of complicated skin and skinstructure infections caused by gram-positive organisms. Clin
Infect Dis. 2008;46:1683–1693.
7 Guskey MT, Tsuji BT. A comparative review of the lipoglycopeptides: oritavancin, dalbavancin, and telavancin. Pharmacotherapy. 2010;30:80–94.
8 Smith WJ, Drew RH. Telavancin: a new lipoglycopeptide for grampositive infections. Drugs Today (Barc). 2009;45:159–173.
9 Barriere S, Genter F, Spencer E, et al. Effects of a new antibacterial, telavancin, on cardiac repolarization (QTc interval duration) in healthy subjects. J Clin Pharmacol. 2004;44:689–695.
May/June 2010
Volume 8 • Issue 3
MYTHS AND MISCONCEPTIONS
Ronni Wolf, MD, Section Editor
Mothers With Anogenital HPV Should
Avoid Breastfeeding: Myth or…?
Ronni Wolf, MD;1 Danny Wolf, MD;2 Batya Davidovici, MD1
A
28-year-old pregnant (week 27 of gestation) patient came
for routine follow-up examination of recurrent condylomata acuminata. Examination of the outer anogenital area
with a clear and powerful light detected no lesions suspicious for
condyloma. She did, however, have a verrucose lesion on her nipple
that was removed and histologically diagnosed as condyloma acuminatum. She was instructed not to breastfeed her baby.
Discussion
The issue of extragenital human papillomavirus (HPV), eg, in oral
mucosa and breast, is not new, but most of the relevant studies
focus on a possible etiologic link between HPV and breast cancer.
Since 1992, 11 of 15 published studies that searched HPV DNA
in breast carcinomas by polymerase chain reaction observed the
presence of HPV DNA genes in breast tumors at a prevalence of
12% to 86%.1,2 Normal breast tissue controls were available for 4
of these studies, and HPV sequences were identified in only 1 of
89 controls (1.1%).2 Neither the patients nor the controls were
tested for the presence of genital HPV and so these studies cannot
provide any indication about the rates of transmission of HPV
from the genital area to the breast. Furthermore, all but one study3
examined breast tissue and not the skin of the mamilla.
The issue of transmission of HPV from parent to child has been
poorly investigated. Most of the studies on transmission have focused on genital HPV, while the role of extragenital HPV (oral and
breast) has been neglected. There is enough evidence today that
perinatal transmission of genital HPV from mothers to infants does
occur.4–7 The mechanisms of HPV transmission between parents
and children are of great interest but remain poorly understood.8,9
Breastfeeding and Risk of HPV Transmission
We are aware of only one very recent study that addressed this
issue.10 The investigators tested HPV by polymerase chain reaction in 223 milk samples 3 days postpartum, and HPV DNA
was detected in 10 (4.0%) of the samples, of which 9 were positive for HPV-16. HPV carriage of the milk was not related to the
genital or oral HPV DNA status of the mother. Rather, it was
significantly correlated with the oral HPV carriage of the spouse
at months 6 and 12 after childbirth, but not at the time of delivery. The researchers made every effort to avoid contamination
of the milk from the mother’s hands and skin surrounding the
nipple. This approach is obviously correct if the aim is to analyze
the milk, but it does not allow an examination of the real risk of
contamination involved in the entire breastfeeding process, eg,
the possibility of acquiring the virus from the nipple and areolar
tissues. There is some evidence today (we are aware of only one
study3) of the occurrence of HPV in nipple and areolar tissues,
and it is assumed that the HPV reached the milk from these tissues in a retrograde manner.10 Consequently, we might assume
that the transfer of HPV from the skin of the nipple is possible
even if the milk itself is not infected. Furthermore, the length of
time the baby’s oral mucosa is in contact with the skin is many
times longer than it is with the milk, thus increasing the risk of
virus transmission from the skin to the baby’s oral mucosa.
The Bottom Line
We are not aware of any investigations of the rate of breast HPV
infection among women with genital or oral HPV infection.
Transmission of viral particles from the mother’s nipple and areola
or her milk to the baby’s oral cavity is certainly a very plausible
possibility. Taken together, we conclude that until more information on the subject is available, in spite of the important health
benefits, it would be wise to err on the side of caution and recommend that HPV-positive mothers refrain from breastfeeding.
References
1 Liang W, Tian H. Hypothetic association between human papillomavirus infection and breast carcinoma. Med Hypotheses.
2008;70:305–307.
From the Dermatology Unit, Kaplan Medical Center, Rechovot, affiliated with the Hebrew University – Hadassah Medical School, Jerusalem,
Israel;1 and the Pediatric Outpatient Clinic, Sherutei Briut Clalit, Hasharon Region, Israel2
Address for Correspondence: Ronni Wolf, MD, Dermatology Unit, Kaplan Medical Center, 76100 Rechovot, Israel • E-mail: wolf_r@netvision.net.il
177
May/June 2010
MYTHS AND MISCONCEPTIONS
2 Lawson JS, Gunzburg WH, Whitaker NJ. Viruses and human
breast cancer. Future Microbiol. 2006;1:33–51.
3 de Villiers EM, Sandstrom RE, zur HH, Buck CE. Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res. 2005;7:R1–R11.
4 Pakarian F, Kaye J, Cason J, et al. Cancer associated human
papillomaviruses: perinatal transmission and persistence. Br J
Obstet Gynaecol. 1994;101:514–517.
rate and mode of delivery. Obstet Gynecol. 1998;91:92–96.
7 Cason J, Kaye JN, Jewers RJ, et al. Perinatal infection and persistence of human papillomavirus types 16 and 18 in infants. J
Med Virol. 1995;47:209–218.
8 Syrjanen S, Puranen M. Human papillomavirus infections in children: the potential role of maternal transmission. Crit Rev Oral
Biol Med. 2000;11:259–274.
5 Puranen MH, Yliskoski MH, Saarikoski SV, et al. Exposure of an
infant to cervical human papillomavirus infection of the mother
is common. Am J Obstet Gynecol. 1997;176:1039–1045.
9 Rintala MA, Grenman SE, Puranen MH, et al. Transmission of
high-risk human papillomavirus (HPV) between parents and infant: a prospective study of HPV in families in Finland. J Clin
Microbiol. 2005;43:376–381.
6 Tseng CJ, Liang CC, Soong YK, et al. Perinatal transmission of
human papillomavirus in infants: relationship between infection
10 Sarkola M, Rintala M, Grenman S, et al. Human papillomavirus DNA
detected in breast milk. Pediatr Infect Dis J. 2008;27:557–558.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
178
May/June 2010
Volume 8 • Issue 3
CASE STUDY
Vesna Petronic-Rosic, MD, MSc, Section Editor
A Rare Manifestation of Nail Changes
With Docetaxel Therapy
Christian R. Halvorson, MD; Corinne L. Erickson, MD; Anthony A. Gaspari, MD
A 60-year-old African American man presented to the dermatology clinic for evaluation of skin and nail changes associated with docetaxel
therapy for adenocarcinoma of the prostate. Previous treatment cycles of docetaxel had resulted in hyperpigmented patches and linear streaks
on his arms where he received infusions, as well as nail changes, including longitudinal melanonychia and onycholysis of his great toenails.
At his latest visit, he reported recently noticing a new bluish discoloration of his lunulae that had developed during the past 3 months, the
time corresponding to his most recent docetaxel treatment cycle (Figure). The patient’s history did not reveal any recent medication changes,
ingestion of known lunula-discoloring agents, or any silver-containing compounds. On examination, the patient’s observation was confirmed, as the lunulae on both his right and left thumb were found to have a marked blue discoloration to them. The patient was treated with
observation as the discoloration was not bothersome to him, and on routine follow-up for an unrelated skin complaint, the patient’s nail
beds had returned to their normal color, approximately 3 months after discontinuation of the docetaxel therapy, according to the patient.
D
ocetaxel is a taxane chemotherapeutic agent approved
for a wide variety of neoplastic conditions, including
breast cancer, non–small-cell lung cancer, head and neck
cancers, and androgen-independent metastatic prostate cancer.1
Common side effects of docetaxel therapy vary according to treatment schedules and most commonly include myelosuppression
when dosed every 3 weeks, and malaise, fatigue, excessive tearing,
and skin and nail changes with weekly treatment.1,2 Skin changes
classically include erythematous macular and papular eruptions
with desquamation, nonpruritic erythema, and the hand-foot
syndrome, occurring in as many as 50% to 70% of patients.2,3
Docetaxel-induced nail changes are also very common, occurring
in up to 44% of patients and include paronychia, onycholysis,
subungual hemorrhage, subungual abscess, nail pigmentation,
splinter hemorrhages, nail bed purpura, Beau’s lines, and nail bed
pigmentation.2 One nail change that has yet to be reported in the
literature, however, is blue lunulae. Here we describe a case of a
patient with blue lunulae developing in the context of docetaxel
therapy for treatment of adenocarcinoma of the prostate.
DISCUSSION
Acquired blue lunulae are commonly induced by systemic
medications, with zidovudine being perhaps the most frequent
causative agent.4 Combination chemotherapies have also been
shown to cause this reaction, with different combinations of cy-
clophosphamide, vincristine, doxorubicin, dacarbazine, 5-fluorouracil, vinblastine, dactinomycin, and bleomycin being implicated.5 Additional causes of blue lunulae include the intentional
or inadvertent ingestion of silver-containing products (argyria),
hemoglobin M disease, Wilson’s disease, hereditary acrolabial
telangiectasia, and the medication phenolphthalein purgative.4,5
To our knowledge, this bluish lunulae discoloration in a patient being treated with docetaxel is unique and has yet to be
reported in the literature. Accordingly, we present this case so
that physicians can recognize this finding as a potential, harmless reaction to docetaxel therapy and avoid unnecessary tests
and referrals. As seen in this case, this discoloration is not a precursor to the more severe and often painful nail changes associated with docetaxel therapy and should not prompt a change to
a different dosing schedule or chemotherapeutic agent, which
could lead to therapeutic failure.
Acknowledgments: Christian Halvorson, MD, Corinne Erickson,
MD, and Anthony Gaspari, MD, had full access to all of the data
in the study and take responsibility for the integrity of the data and
the accuracy of the data analysis. All authors contributed to the study
concept and design; analysis and interpretation of data; drafting of
the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; and administrative, technical,
or material support; and obtained funding.
From the Department of Dermatology, University of Maryland School of Medicine, Baltimore, MD
Address for Correspondence: Christian R. Halvorson, MD, University of Maryland School of Medicine, 419 West Redwood Street, Suite 160,
Baltimore, MD 21201 • E-mail: chalv001@umaryland.edu
179
May/June 2010
CASE STUDY
Figure. Clinical presentation of blue lunulae resulting from docetaxel therapy. The patient’s bilateral thumbs reveal a bluish
discoloration of the lunulae (A). A return to normal nail bed coloration after discontinuing docetaxel therapy (B).
References
1 Engels FK, Sparreboom A, Mathot RA, et al. Potential for improvement of docetaxel-based chemotherapy: a pharmacological review. Br J Cancer. 2005;93:173–177.
3 Poikonen P, Sjöström J, Klaar S, et al. Skin toxicity as a risk
factor for major infections in breast cancer patients treated with
docetaxel. Acta Oncol. 2004;43:190–195.
2 Roh MR, Cho JY, Lew W. Docetaxel-induced onycholysis: the
role of subungual hemorrhage and suppuration. Yonsei Med J.
2007;48:124–126.
4 Kalouche H, Watson A, Routley D. Blue lunulae: argyria and hypercopprecaemia. Australas J Dermatol. 2007;48:182–184.
5 Cohen PR. The lunula. J Am Acad Dermatol. 1996;34:943–953.
Formulary of Dr George C. Andrews
Perspiration Corrective
• Talcum – 4.00
• Zinc stearate – 25.00
• Boric acid – 8.00
• Benzzoid acid – 5.00
• Carbitol – 58.00
• Ol. Violet – q.s. for odor
Submitted by Douglas D. Altchek, MD, New York, NY
180
May/June 2010
Volume 8 • Issue 3
CASE STUDY
Faun Tail:
A Rare Cutaneous Marker of Spinal Dysraphism
Muhterem Polat, MD;1 Fazli Polat, MD;2 Pinar Öztaş, MD;1 Canan Kaya, MD;1 Nuran Alli, MD1
A 10-year-old girl who was admitted to the urology department with complaints of urinary incontinence was referred to our dermatology outpatient clinic because of a congenital, circumscribed, hypertrichotic area on the lumbosacral region. Cutaneous examination revealed a circumscribed area of coarse, dark terminal hair measuring 25×15 cm overlying the lumbosacral area with normal underlying skin (Figure 1). There were
erythematous macular lesions on the superior of the hairy area. The lesion had been present since birth, and no other family member had similar
lesions. Her history revealed back pain and a long history of urinary incontinence. On neurologic examination, no motor weakness or sensory
changes were observed. Babinski reflex was positive on the left. Magnetic resonance imaging (MRI) findings included diastematomyelia between
T12 and L1 levels and slight flattening of lumbar lordosis (Figure 2). A diagnosis of faun tail with underlying spinal dysraphism was made. There
was also urinary incontinence as late sequelae of spinal dysraphism.
S
pinal dysraphism refers to incomplete fusion of midline structures of the embryonal dorsal median region that may affect
any combination of somatic ectoderm, neuroectoderm, or
mesoderm. Since both skin and nervous tissue are of ectodermal
origin, anomalies of these tissues may simultaneously occur.1 Faun
tail nevus is an abnormal lumbar hypertrichosis represented by tufts
of coarse terminal hair, several inches in length that usually manifests as a lozenge or triangular patch in the lumbosacral area and
may overlie an occult spinal abnormality.2,3
A midline cutaneous posterior anomaly is often a clue for an underlying
occult spinal dysraphism. The cutaneous signs of spinal dysraphism are
seen in 50% of cases of occult spinal dysraphism.4 Other studies have
documented an even higher prevalence of cutaneous lesions up to 76%
(43%–95%).5 The cutaneous lesions that should raise a higher degree
of suspicion include hypertrichosis, dimples, aplasia cutis, lipoma,
hemangioma, dermoid cyst or sinus, acrochordons, true tail, pseudotail, and congenital scarring. Lesions with a low index of suspicion include telangiectasia, capillary malformations (port wine stain), hyperpigmentation, hypopigmentation, melanocytic nevi, connective tissue
nevus, hypertrophic skin, hamartomas, teratoma, and neurofibroma.1
Sacral hypertrichosis (faun tail nevus) is the most common skin lesion
evident at birth,6 as was seen in our patient. Hairy patches are most
frequently associated with tethered cord and diastematomyelia.4 Our
patient had a hairy patch in association with diastematomyelia.
gular or lozenge-shaped patch of coarse hair, usually several inches long.
Faun tail is a rare entity. Mild hypertrichosis on the back, including the
lumbar area, is a common finding in certain ethnic groups, such as persons found in the Middle East. It is usually not sharply circumscribed
and is never as long or thick7 as in the case described herein.
The term spinal dysraphism encompasses many congenital abnormalities, including dermal sinuses, dermoid cysts, diastematomyelia,
fibrous bands, filum terminale, intraspinal lipomas, lipomyelomeningoceles, and myelomeningoceles. Among them, diastematomyelia
presents as an embryologic defect that is characterized by the division
of the spinal cord or cauda equina into two separate portions.2,3
Abnormal lumbar hypertrichosis may present as a “faun tail” or “silky
down.” Silky down is represented by tufts of hair with the texture of
fine, soft, nonterminal, or lanugo hair. A faun tail is a wide, often trian-
Figure 1. Tufts of coarse terminal hair in the lumbosacral area.
From the First Dermatology Department, Ankara Numune Education and Research Hospital,1 and the Department of Urology, Çağ Hospital,2 Ankara, Turkey
Address for Correspondence: Muhterem Polat, MD, Çukurambar mah, 41. cad. No: 2/8, Ankara, Turkey • E-mail: drmuhterempolat@mynet.com
181
May/June 2010
CASE STUDY
ment was performed, and she was advised to see a child psychiatrist.
The patient was referred to our outpatient clinic by a urologist for
the hypertrichotic area on the lumbosacral area. MRI was performed
and diastematomyelia was detected. Early diagnosis of spinal dysraphism is important, because spinal correction can prevent irreversible
neurologic damage. When neurologic deterioration starts, complete
recovery may not be guaranteed, and if the cases remain untouched,
sensorimotor deficits and bladder and bowel dysfunction may occur.2,11 In our patient, urinary incontinence occurred as late sequelae
of spinal dysraphism.
Surgical intervention should be early after entire neural axis screening by MRI.6 In occult spinal dysraphism, neurosurgical treatment
methods differ from patient to patient. Laminectomy of the lumbar
and lumbo-sacral spine with excision of intraspinal lipoma, excision
of bony or cartilaginous spur in diastematomyelia, and detethering
of the conus medullaris and cauda equina can be successful.12 Significant improvements can be achieved with a judiciously timed division of the spinal tethered cord.13 There are studies showing that
tethered cord release is beneficial in terms of clinical and urodynamic
outcomes.13 In one study,6 the most common postoperative complications were pseudomeningocele, wound infection, and meningitis.
Conclusions
Dermatologists should be familiar with faun tail as it can easily be
overlooked by physicians other than dermatologists. We think that
the recognition of the cutaneous stigmata of spinal dysraphism may
decrease the long-term morbidity of this condition. The aim of this
article is to draw attention to faun tail and to emphasize the responsibility of physicians in the early recognition of lumbar paraspinal
skin lesions so that appropriate early treatment may be given for
underlying anomalies before irreversible sequelae develop.
References
1 Gupta R, Singal A, Pandhi D. Faun tail naevus: a cutaneous marker of spinal dysraphism. Indian Pediatr. 2005;42:67–69.
2 Thursfield WR, Ross AA. Faun tail (sacral hirsuties) and diastematomyelia. Br J Dermatol. 1961;73:328–336.
Figure 2. Diastematomyelia between T12 and L1 levels.
Faun tail is very rare, and it is not only an aesthetic problem, because
its presence may be one of the cutaneous stigmata of occult spinal
dysraphism like other midline posterior cutaneous anomalies.1,7–11
Although both the cutaneous and neurologic lesions are usually asymptomatic until the child is older, a delay in diagnosis may result in
neurologic, urologic, and orthopedic complications. This emphasizes
the need for increased awareness of these cutaneous signs. Patients
with well-localized paraspinal hypertrichosis must undergo neurologic and radiologic assessment to exclude spinal abnormalities. Our
patient had been to various first-step health care clinics for the complaint of urinary incontinence, but no neurologic or radiologic assess-
182
3 McAtee-Smith J, Hebert AA, Rapini RP, et al. Skin lesions of the
spinal axis and spinal dysraphism. Arch Pediatr Adolesc Med.
1994;148:740–748.
4 Tavafoghi V, Ghandchi A, Hambrick GW, et al. Cutaneous signs
of spinal dysraphism. Report of a patient with a tail like lipoma and review of 200 cases in the literature. Arch Dermatol.
1978;114:573–577.
5 Scatliff JH, Kendall BE, Kingsley DPE, et al. Closed spinal dysraphism:
analysis of clinical, radiological and surgical findings in 104 consecutive patients. AJR Am J Roentgenol. 1989;152:1049–1057.
6 Singh SN, Kumar R. Spinal dysraphism: trends in northern India.
Pediatr Neurosurg. 2003;38:133–145.
7 Kaya TI, Kokturk A, Guleryuz A, et al. Faun tail: a rare cutaneous
stigmata of spinal dysraphism. Int J Dermatol. 2002;41:119–120.
May/June 2010
CASE STUDY
8 Calikoglu E, Oztas P, Anadolu RY, et al. Faun tail with aplasia cutis congenita and diastematomyelia. Dermatology. 2004;209:333–334.
vus as cutaneous markers of spinal dysraphism. Clin Exp Dermatol. 2002;27:645–648.
9 Arora WC, Rawat CS, Banerjee LC. Faun tail nevus: a case report. MJAFI. 2006;62:286–287.
12 Bajpai M, Kataria R, Gupta DK, et al. Occult spinal dysraphism.
Indian J Pediatr. 1997;64:62–67.
10 Birol A, Bademci G. Faun tail: diagnosis of occult spinal dysraphism
with a rare cutaneous marker. J Dermatol. 2004;31:251–252.
13 Balkan E, Kiliç N, Avşar I, et al. Urodynamic findings in the tethered spinal cord: the effect of tethered cord division on lower
urinary tract functions. Eur J Pediatr Surg. 2001;11:116–119.
11 Antony FC, Holden CA. Diffuse hypertrichosis and faun-tail nae-
Wax Moulage
Tinea facialis, Trichophytia superficialis. Moulage Nr. 1198, made by Ruth Willi in the Dermatology Clinic in Zurich in the
1950s. Museum of Wax Moulages Zurich, www.moulagen.ch.
Courtesy of Michael Geiges, MD
183
May/June 2010
Volume 8 • Issue 3
CASE STUDY
Werner Syndrome in an Iranian Family
Zahra Hallaji, MD; Massumeh Barzegari, MD; Katrin Kiavash, MD
A 49-year-old man was first seen in our department for the evaluation of scleroderma-like skin changes and a nonhealing ulcer on his leg from 2
years before referral. His medical history was of long duration. His growth was stunted at the age of 12. At 21 years of age, the patient noted graying of the scalp hair, most prominent on his temples, and the process was progressively completed by the age of 23. At the same age, atrophy and
thinning of the skin and loss of subcutaneous fat resulted in a tense, shining, and adherent appearance of his skin, most obvious on his face and extremities. Soon after, he developed a high-pitched, hoarse voice. He had undergone bilateral cataract surgery at the age of 30. Around the age of 46,
he developed a unilateral nonhealing chronic leg ulcer (Figure 1). He had separated from his wife because of infertility. He was the first offspring of
his second-degree healthy relative parents. The other 3 siblings had similar signs and symptoms. Our patient gave the history of premature graying
of the hair of his younger brother at the age of 18 and his 2 younger sisters at the age of 12 and 16. His brother had recently received diagnoses of
bilateral cataract and diabetes mellitus. All of the siblings had ceased growth from early adolescence. On physical examination, our patient’s weight
was 48 kg and his height was 150 cm. He had normal intelligence. He was speaking with a high-pitched and childish voice. He had a bird-like
appearance with a beak-shaped nose. Mottled and diffuse pigmentation and poikiloderma appearance was conspicuous on his neck (Figure 2). The
entire skin was smooth, shiny, and scleroderma-like, and a marked decrease in the subcutaneous fat was noted over the extremities. A deep cutaneous ulcer was evident on his slimmed leg. Digital ulcers were not found, and radial and dorsalis pedis pulses were palpable. Clinodactyly of the toes
were conspicuous. His nails were dystrophic and he had used dentures from the age of 20. On examination of the external genitalia, his testes were
smaller than normal. In the biopsy taken from the leg ulcer, there were no signs of malignancy. There were no signs of osteomyelitis on x-ray. Biopsy
of the normal skin revealed atrophic epidermis and thick dermis with hyalinization of the collagen fibers and absence of pilosebaceous structures
(Figure 3). The patient’s scalp hair was thin and sparse and there were few axillary and pubic hairs. His fasting plasma glucose level was normal.
W
loss of the WRN gene product, which is required for genomic stability.9 Consanguinity of our patient’s parents and occurrence of the
same disease in his siblings point to an autosomal-recessive pattern
of inheritance; unfortunately, genetic analysis was not available.
Werner syndrome occurs worldwide, but 75% of patients are Japanese.4 Few cases have been reported from the Middle East.5 Werner syndrome is an autosomal-recessive disorder that belongs to the
category of human premature aging disorders.6 Disorders such as
Rothmund-Thomson syndrome, progeria, Cockayne syndrome, xeroderma pigmentosum, and Hutchinson-Gilford progeria belong to
this disease category.7 Werner syndrome is caused by a mutation in
the RecQ-type DNA helicase gene (WRN gene), which was identified in 1996.8 It appears that the syndrome is the result of complete
Since Werner’s original report in 1904,10 several authors have attempted to define more accurate diagnostic criteria of the Werner
syndrome to be distinguished from other premature aging syndromes. Thannhauser presented a list of principal features for accurate diagnosis of this syndrome in 1945.11 According to diagnostic
criteria from the Werner Syndrome International Registry, Werner
syndrome can be diagnosed as definite, probable, or possible based
on number of positive cardinal and further signs and symptoms.12
Cardinal signs and symptoms include: (1) bilateral cataract; (2) characteristic dermatologic pathology (tight skin, atrophic skin, pigmentary alterations, ulceration, hyperkeratosis, regional subcutaneous
atrophy) and characteristic facies (“bird” facies); (3) short stature;
(4) parental consanguinity (3rd cousin or greater) or affected sibling;
(5) premature graying and/or thinning of scalp hair; and (6) positive
24-hour urinary hyaluronic acid test (when available). Further signs
and symptoms include: diabetes mellitus; hypogonadism (secondary
sexual underdevelopment, diminished fertility, testicular or ovarian
erner syndrome, or adult progeria, is a rare inherited
disorder in which the aging process is accelerated.1 Patients show normal growth in childhood that usually
arrests at puberty. It is followed by balding and premature graying of hair, scleroderma-like skin changes, poikiloderma, atrophy,
and cataracts in the late 20s to 30s.2 Other features include muscle
wasting, especially in the extremities, giving rise to spindle-shaped
legs, chronic leg ulcer, hypogonadism and impotence, high-pitched
voice, osteoporosis, premature arteriosclerosis, diabetes mellitus,
and increased rate of malignancy, especially fibrosarcoma.3 Our patient’s history was compatible with Werner syndrome, and many
features of this syndrome were also found in his siblings.
From Razi Hospital, Tehran University of Medical Sciences, Tehran, Iran
Address for Correspondence: Katrin Kiavash, MD, Razi Hospital, Tehran University of Medical Sciences, Vahdate-Eslami Sq 11966, Tehran, Iran •
E-mail: kiavashkatrin@yahoo.com
184
May/June 2010
CASE STUDY
Figure 1. Nonhealing chronic leg ulcer of the patient.
Figure 2. Aged appearance and poikiloderma of the neck.
atrophy); osteoporosis; osteosclerosis of distal phalanges of fingers
and/or toes (x-ray diagnosis); soft tissue calcification; evidence of
premature atherosclerosis (eg, history of myocardial infarction); mesenchymal neoplasms, rare neoplasms, or multiple neoplasms; voice
changes (high-pitched, squeaky, or hoarse voice); positive 24-hour
urinary hyaluronic acid test (when available); and flat feet.12 Since
our patient had all of the cardinal features (except for a positive result
of 24-hour urinary hyaluronic acid test, which was not available) and
two further features (hypogonadism and voice changes), diagnostic
group assignment of our patient is “definite.”
Our patient was referred to our clinic because of his leg ulcer. Unusual ulcers are a feature of Werner syndrome but they are not always
recognized as being a part of this syndrome.13 In this case, the clinical
feature led us to suspect Werner syndrome rather than a simple ulcer.
Correct diagnosis is important since these patients must be followed
for possible occurrence of malignancies. Study of patients with Werner syndrome may also help in understanding the process of aging.7
Figure 3. Atrophic epidermis, thick hyalinized dermis, and
sparse appendages (hematoxylin and eosin stain, original
magnification ×40).
References
3 Björnberg A. Werner’s syndrome and malignancy. Acta Derm
Venereol. 1976;56:149–150.
1 Epstein CJ, Martin GM, Schultz AL, et al. Werner’s syndrome a
review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process.
Medicine (Baltimore). 1966;45:177–221.
4 Shiratori M, Sakamoto S, Suzuki N, et al. Detection by epitopedefined monoclonal antibodies of Werner DNA helicases in the
nucleoplasm and their upregulation by cell transformation and
immortalization. J Cell Biol. 1999;144:1–9.
2 Duvic M, Lemak NA. Werner’s syndrome. Dermatol Clin.
1995;13:163–168.
5 Kocabay G, Ozturk S, Ecder T. Werner syndrome associated
with renal involvement. Saudi Med J. 2006;27:1768–1769.
185
May/June 2010
CASE STUDY
6 Pesce K, Rothe MJ. The premature aging syndromes. Clin Dermatol. 1996;14:161–170.
7 Opresko PL, Cheng WH, von Kobbe C, et al. Werner syndrome and the function of the Werner protein; what they can
teach us about the molecular aging process. Carcinogenesis.
2003;24:791–802.
8 Yu CE, Oshima J, Fu YH, et al. Positional cloning of the Werner’s
syndrome gene. Science. 1996;272:258–262.
9 Rossi ML, Ghosh AK, Bohr VA. Roles of Werner syndrome protein in
protection of genome integrity. DNA Repair (Amst). 2010;9:331–344.
10 Werner O. Uber katarakt in werbindung mit sclerodermie. Keil:
Schmidt and Klauning, 1904.
11 Tannhauser SJ. Werner’s syndrome (progeria of the adult) and Rothmund’s syndrome: two types of closely related heredofamilial atrophic
dermatoses with juvenile cataracts and endocrine features: a critical
study with five new cases. Adv Exp Med Biol. 1985;190:15–56.
12 International Registry of Werner syndrome. Diagnostic Criteria for Werner Syndrome. Available at: http://www.pathology.
washington.edu /research /werner /registry/diagnostic.html.
Accessed October 1, 2007.
13 Cohen M, Shelley WB. Ankle ulcer sign of Werner’s syndrome.
Arch Dermatol. 1963;87:86–88.
HISTORICAL DIAGNOSIS & Treatment
Diagnosis and treatments have advanced over the past century. This feature depicts conditions from a collection of steroptic cards published
in 1910 by The Stereoscopic Skin Clinic, by Dr S. I. Rainforth.
Synonyms: Pediculosis capillitii; Phthiriasis capitis; Head-lousiness
186
May/June 2010
Volume 8 • Issue 3
CASE STUDY
Ulcerative Granulomatous Mycosis Fungoides
Sareeta R.S. Parker, MD;1,4 Carmen Traywick, MD;1 Jack L. Arbiser, MD, PhD1,2,3
A 42-year-old white male military recruit presented with a 2-year history of painful ulcerations on the skin of his flanks and thighs. Prior skin
biopsies were nondiagnostic but raised the suspicion of an infectious etiology due to the presence of a granulomatous infiltrate. His medical history was significant for herpes zoster and eczema, and, on review of systems, he had a 1-year history of progressive fatigue and night sweats. Examination revealed approximately one dozen 1- to 5-cm indurated, dusky violaceous plaques on his trunk and lower extremities. Several of the
plaques, including one on his right flank, had overlying deep ulcerations (Figure 1A and 1B). Bilateral inguinal lymphadenopathy was present.
L
aboratory studies revealed normal complete blood cell count
and lactate dehydrogenase values. Computed tomography
scans of his chest, abdomen, and pelvis confirmed the bilateral
inguinal lymphadenopathy, but were otherwise unremarkable. Skin
histopathology showed an atypical lymphocytic infiltrate involving
the full thickness of the dermis and extending into the subcutis (Figure 2). While focal epidermotropism was present, folliculotropism
was more prominent. Admixed with the lymphocytes were numerous
histiocytic multinucleated giant cells, consistent with granulomatous
mycosis fungoides (MF). In immunoperoxidase-stained sections,
most of the lymphocytes stained positive for leukocyte common antigen. The majority of lymphocytes were CD3 positive. No organisms were identified in either acid fast– or periodic acid Schiff–stained
sections. Skin tissue culture results were negative. Biopsy of an enlarged inguinal lymph node also revealed histopathologic features
of granulomatous MF, with partial effacement of the normal nodal
architecture. Flow cytometric immunophenotyping of both the skin
and lymph node samples revealed an aberrant clonal population of
lymphoid cells expressing CD2, CD3, CD4, and CD5 and partial
CD45 and CD25. The cells failed to express CD7. Peripheral blood
flow cytometric analysis showed no aberrant clone. Blood, skin, and
lymph node analysis for T-cell receptor (gamma chain) gene rearrangements (TCRg) by polymerase chain reaction (PCR) were negative. Bone marrow histology, flow cytometry, and molecular analysis
showed no evidence of involvement.
Based on these results, the patient was diagnosed with cutaneous Tcell lymphoma (CTCL), granulomatous MF variant, stage IVA (T3,
N3, M0, B0). Therapy with subcutaneous interferon α (2b) at 4 million units 3 times per week and acitretin 25 mg daily was initiated.
The ulcerated lesions were irradiated with electron beam. The patient’s
lesions responded well to radiotherapy (left ankle [21.5 Gy], right
posterior thigh [33 Gy], and right flank [33 Gy]) (Figure 3A and 3B).
He has been maintained on acitretin and interferon α, with resolution
of his systemic symptoms and lymphadenopathy, minimal skin findings, and no evidence of disease progression for 30 months.
DISCUSSION
CTCLs are a heterogeneous group of primary cutaneous lymphomas characterized by malignant, skin-homing abnormal Tcell clones. MF, the most common type of CTCL, is frequently
difficult to diagnose. This is due to the wide variation in clinical
manifestations,1 and also to the heterogeneity of histopathologic
findings. In addition, assays for the detection of monoclonality in
the cutaneous infiltrate, although useful adjuncts, are occasionally
inconclusive or negative, particularly in the early stages of disease.
Granulomatous MF is a rare variant of MF originally described in
1970.2 Since that time there have been only a few dozen cases of
granulomatous MF published in the English literature.3–5 Granulomatous MF is distinct from granulomatous slack skin, as the
latter condition has a distinctive clinical presentation characterized
by multiple, pendulous, and lax skin folds. In addition, it has been
suggested that elastolysis is a prominent histopathologic feature
of granulomatous slack skin, but it is less of a characteristic feature in granulomatous MF.6 The two conditions have significant
histological overlap, however,4,7 and they likely represent different
forms of the same disease.
According to previously published reports, patients with granulomatous MF may present with a variety of skin lesions, similar to that
of classic MF. These include localized or widespread patches, papules, plaques, and tumors. Ulcerations, erythroderma, and ichthyotic and poikilodermatous lesions have also rarely been reported.3,4,8
From the Departments of Dermatology1 and Pathology,2 Emory University School of Medicine; Atlanta VA Medical Center;3 and Grady Memorial Hospital,4 Atlanta, GA
Address for Correspondence: Sareeta R.S. Parker, MD, The Emory Clinic, 1365 Clifton Road, NE, Atlanta, GA 30322 • E-mail: srsingh@emory.edu
188
May/June 2010
CASE STUDY
Figure 1. Ulcerations on the right flank (A) and right posterior thigh (B) before therapy.
Apart from granuloma formation, no other histological features are
unique to granulomatous MF as compared with other forms of MF.
The presence of granulomas in cutaneous infiltrates of MF is a rare
phenomenon and was once ascribed as a favorable prognosis.2 It
has since been acknowledged, however, that the granulomatous response in MF is not necessarily protective, nor is it of clear prognostic relevance.3 In fact, there are rare reports of granulomatous MF
characterized by progression to visceral involvement and subsequent
death.9,10 In a recent review from Europe of 15 patients with granulomatous MF, the overall prognosis appeared to be less favorable
than those with classic MF.4
importance of relying most heavily on the clinical and histopathologic findings rather than on molecular assays in establishing the
diagnosis of cutaneous lymphoma. In addition, lesional tissue flow
cytometric analysis is a useful adjunct in establishing the diagnosis
in some cases. On flow cytometric analysis of our patient’s skin and
nodal tissue, a phenotypically aberrant population of T cells was
clearly demonstrable, thus supporting the diagnosis.14
Conclusions
Prognosis of patients with granulomatous MF, as with other
subtypes of CTCL, depends on overall stage of disease.15 No
In the case of our patient, the presence of granulomas in the infiltrate prompted a thorough investigation in search of microbial
pathogens, particularly in light of his extensive travel history.
When presented with granulomatous infiltrates, tissue cultures
should be performed, since they are generally more sensitive than
direct tissue examination (even with special stains), for pathogens
such as deep fungi. Even if the histology is supportive of granulomatous MF, examination for infectious agents is critical, as patients with lymphoid malignancies often manifest immune dysregulation predisposing them to infection.11
An interesting and notable feature in our patient was the absence
of a detectable clone by PCR analysis for TCRg chain gene rearrangements in both the skin and the lymph node. The reason for
this is unclear. It is possible that the particular rearrangement occurring in our patient was not detectable by the molecular assay used
in our laboratory. Published studies using PCR suggest the range
of detection of a clonal (TCRg) rearrangement from involved skin
of patients with CTCL is 74% to 90%.12,13 The potential inability
to detect a clonal population by molecular testing underscores the
189
Figure 2. Lesional skin biopsy showing an atypical
lymphocytic infiltrate with focal epidermotropism and
scattered multinucleated histiocytic giant cells (hematoxylin
and eosin, original magnification ×10).
May/June 2010
CASE STUDY
Figure 3. Right flank (A) and right posterior thigh (B) after radiation therapy and initiation of acitretin and interferon α.
particular therapeutic regimen has been shown to be superior for
patients with the granulomatous variant, and the optimum therapeutic approach remains to be determined. Previously reported
effective therapies include retinoids, interferon, chemotherapy,
and radiation. Because treatment with a combination of modalities seems to offer benefit over any single agent in advanced cases
of MF,16,17 we employed skin-directed therapy (radiation) along
with systemic immunomodulatory agents acitretin and interferon α. With this approach, our patient reported herein with stage
IVA disease has remained alive and well for 30 months without
evidence of disease progression.
References
1 Zackheim H, McCalmont T. Mycosis fungoides: the great imitator. J Am Acad Dermatol. 2002;47:914–918.
2 Ackerman A, Flaxman B. Granulomatous mycosis fungoides. Br
J Dermatol. 1970;82:397–401.
3 Fischer M, Wohlrab J, Audring T, et al. Granulomatous mycosis
fungoides: report of two cases and review of the literature. J Eur
Acad Dermatol Venereol. 2000;14:196–202.
4 Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous
mycosis fungoides and granulomatous slack skin. Arch Dermatol. 2008;144:1609–1617.
5 Hazrati LN, Bril V, Nag S. Muscle and nerve involvement in granulomatous mycosis fungoides. Muscle Nerve. 2007;36:860–865.
6 LeBoit P, Zackheim H, White C Jr. Granulomatous variants of cutaneous T-cell lymphoma: the histopathology of granolomatous
mycosis fungoides and granulomatous slack skin. Am J Surg
Pathol. 1988;12:83–95.
7 Metzler G, Schlagenhauff B, Krober S, et al. Granulomatous mycosis fungoides: report of a case of histopathologic features of granulomatous slack skin. Am J Dermatopathol. 1999;21:156–160.
190
8 Eisman S, O’Toole E, Jones A, et al. Granulomatous mycosis fungiodes presenting as an aquired ichthyosis. Clin Exp Dermatol.
2003;28:174–176.
9 Dabski K, Stoll H Jr. Granulomatous reactions in mycosis fungoides. J Surg Oncol. 1987;34:217–229.
10 Gomez-De La Fuente E, Ortiz P, Vanaclocha F, et al. Aggressive
granulomatous mycosis fungoides with clinical pulmonary and
thyroid involvement. Br J Dermatol. 2000;142:1026–1029.
11 Axelrod PI, Lorber B, Vonderheid EC. Infections complicating mycosis fungoides and Sezary syndrome. JAMA. 1992;267:1354–1358.
12 Tok J, Szabolcs MJ, Silvers DN, et al. Detection of clonal T-cell
receptor gamma chain gene rearrangements by polymerase
chain reaction and denaturing gradient gel electrophoresis
(PCR/DGGE) in archival specimens from patients with early cutaneous T-cell lymphoma: correlation of histologic findings with
PCR/DGGE. J Am Acad Dermatol. 1998;38:453–460.
13 Wood GS, Tung RM, Haeffner AC, et al. Detection of clonal Tcell receptor gamma gene rearrangements in early mycosis
fungoides/Sezary syndrome by polymerase chain reaction and
denaturing gradient gel electrophoresis (PCR/DGGE). J Invest
Dermatol. 1994;103:34–41.
14 Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis
and therapy of cutaneous T cell lymphoma. J Clin Invest.
2005;115:798–812.
15 Kim YH, Liu H, Mraz-Gernhard S, et al. Long-term outcome of
525 patients with mycosis fungoides and Sezary syndrome:
clinical prognostic factors and risk for disease progression.
Arch Dermatol. 2003;139:857–866.
16 Duvic M, Apisarnthanarax N, Cohen DS. Analysis of long-term
outcomes of combined modality therapy for cutaneous T-cell
lymphoma. J Am Acad Dermatol. 2003;49:35–49.
17 Suchin KR, Cucchiara AJ, Gottleib SL, et al. Treatment of cutaneous T-cell lymphoma with combined immunomodulatory therapy: a 14-year experience at a single institution. Arch Dermatol.
2002;138:1054–1060.
May/June 2010
Volume 8 • Issue 3
CASE STUDY
Contact Dermatitis Due to Temporary Henna Tattoos
Jana Kazandjieva, MD;1 Maria Balabanova, MD;1 Kamelia Kircheva, MD;2 Nikolai Tsankov, MD1
Case 1: A 19-year-old woman presented with an allergic contact dermatitis on her back. Skin lesions developed exactly where a henna tattoo had been applied 3 days earlier. She noticed that the henna artist placed some kind of occlusive dressing on her back for 30 minutes.
The artist explained that this is made to keep the tattoo on her skin. During the first 2 days after the application, the patient experienced
severe pruritus in the tattooed area. One day later, multiple small vesicles developed within the tattoo design (Figure 1). The eruption was
palpable. The skin lesions had risen, sparing the unpainted areas. The histologic investigation revealed spongiosis in the epidermis with dense
lymphohistiocytic infiltrates in the dermis (Figure 2). Epicutaneous tests were positive for henna paste and paraphenylene diamine (PPD).
Epicutaneous test results with henna powder was negative, confirming the lack of allergy to henna. Treatment with topical steroids (class IV)
resulted in gradual improvement, with resolution in several weeks.
Case 2: During a holiday on the coast of the Black Sea, a 14-year-old boy applied on his back a nonpermanent henna tattoo in the shape of a dragon
(Figure 3). His mother noticed that the henna artist used a syringe to apply the henna mixture. Two days later an acute erythematous and edematous
pruritic eruption developed where the skin paint was originally applied. The skin lesions improved after treatment with local corticosteroids, but a
residual postinflammatory hyperpigmentation remained, tracing the tattoo design occurring in the form of a dragon. Patch tests were performed
with the European Standard Series and commercial and natural henna. The patient revealed positive reactions (3+) to PPD 1%, but not to henna.
I
n ancient times, henna was used to treat psoriasis, eczema, and
even ringworm because of its mild anti-inflammatory effect.
The flowers, pulverized leaves, fruit, and bark were all used
as a remedy. The ancient Egyptians identified 7 types of henna
and used henna even before mummification. Because of the sweet
smell of the henna flowers, the distilled water made from them
was applied into cosmetics in the Orient. The powdered leaves
have been used all over the world for dying hair, nails, and skin.
lites, which, in turn, may lead to the formation of immunogenic
hapten-protein conjugates and are then presented to the immune system. PPD may be oxidized to benzoquinone diimine,
which, in turn, may form the trinuclear dye called Bandrowski’s
base. Researchers5 reported that Bandrowski’s base is responsible
for the common allergic reactions of PPD.
There are only a few reports concerning allergic reactions due to
pure henna.1,2 Sensitization to henna is common in hairdressers.3
The typical symptoms consist of sneezing, runny nose, cough,
and shortness of breath, with a lack of skin reactions.
The mixtures applied to the skin for creation of temporary tattoos
do not contain only henna. The painting with pure henna gives a
red color. The henna paste used by “henna artists” contains natural
henna and different coloring agents to darken the color. The following ingredients might be found in henna paste4: PPD, coffee, black
tea, lemon juice, eucalyptus, clove, mustard oil, and fenugreek seeds.
PPD is in the “top 10” of the most frequent contact allergens. A
skin eruption is the major outbreak of allergic reactions to PPD.
The molecular mechanism behind the recognition of PPD by the
immune system has not been fully elucidated. According to classic studies, such small molecules need to form reactive metabo-
Figure 1. Case 1: Contact dermatitis 1 week after application
of a henna tattoo.
From the Department of Dermatology, Medical University, Sofia;1 and Poliklinik Albena, 9620 Albena,2 Bulgaria
Address for Correspondence: Jana Kazandjieva, MD, Medical University, Department of Dermatology, 1, St. Georgi Sofiiski Street, 1431
Sofia, Bulgaria • E-mail: janaderm@abv.bg
191
May/June 2010
CASE STUDY
The use of PPD and other diaminobenzenes is allowed in the
European Union for hair dyes in maximum concentration of 6%
freebase in the final product and 10% diaminotoluens. The same
directive forbids the use of PPD for dying eyelashes, eyebrows,
or skin. According to the literature, PPD was present in the
black henna tattoo mixture at a concentration of 15.7%, which
is significantly higher than commercial hair dye preparations.7
More than 100 cases of contact dermatitis due to temporary
tattoos have been reported.8 In all cases, PPD was confirmed to
be the key factor for the development of allergic contact dermatitis. In the present 2 cases, there are some special features:
the use of a syringe and an occlusive dressing by the henna
tattooist. According to our previous investigations,1,9 the occlusive dressing used enhanced the penetration and thus could
explain the short incubation period and severity of the allergic
reaction. In the second case, the syringe needle penetrated the
stratum corneum and this way the antigen was directly presented in the Langerhans cells. Because of this, the upper layer
of the skin was bypassed and a very short incubation period
was observed (only 2 days).
Figure 2. Case 1: Histologic examination revealed spongiosis in
the epidermis with dense lymphohistiocytic infiltrates in the dermis.
Persons with known reactions to PPD or cross-reacting allergens
with a para-positioned amino group in the benzene ring should
be cautious of temporary tattoo application.10 This fact is especially important for such substances as sulfonamides, para-amino
benzoic acid, sulfonylureas, dapsone, azo dyes, and benzocaine.8
References
1 Garcia Ortiz JC, Terron M, Bellido J. Contact allergy to henna. Int
Arch Allergy Immunol. 1997;114:298–299.
2 Nigam P, Saxena A. Allergic contact dermatitis from henna. Contact Dermatitis. 1988;18:55–56.
3 Bolhaar ST, Mulder M, van Ginkel CJ. IgE-mediated allergy to
henna. Allergy. 2001;56:248.
4 Lestringant GG, Bener A, Frossard PM. Cutaneous reaction to henna and associated additives. Br J Dermatol. 1999;141:598–600.
5 Krasteva M, Nicolas JF, Chabeau G, et al. Dissociation of allergenic and immunogenic functions in contact sensitivity to para-phenylenediamine. Int Arch Allergy Immunol. 1993;102:200–204.
Figure 3. Case 2: Allergic contact dermatitis 3 days after
application of a temporary tattoo.
PPD is the most widely used agent in hair dye formulae. This
compound is also used as a photographic developing agent and
as an intermediate in the manufacture of azo dyes, antioxidants,
and accelerators for rubber vulcanization.
Patch testing of our patients showed a strongly positive response
to PPD. PPD is added to henna paste to speed up the process (to
minutes) and intensify the color of the dye.6 The risk of allergic reaction to PPD escalates due to a lack of a neutralizing agent, long
duration of skin contact, and high concentration of the allergen.
192
6 Onder M, Atahan CA, Oztas P, et al. Temporary henna tattoo
reactions in children. Int J Dermatol. 2001;40:577–579.
7 Brancaccio RR, Brown LH, Chang YT, et al. Identification and
quantification of para-phenylenediamine in a temporary black
henna tattoo. Am J Contact Dermat. 2002;13:15–18.
8 Gallo R, Ghigliotti G, Cozzani E, et al. Contact dermatitis from
para-phenylene diamine used as a skin paint: a further case.
Contact Dermatitis. 2000;40:57.
9 Kazandjieva J, Grozdev I, Tsankov N. Temporary henna tattoos.
Clin Dermatol. 2007;25:383–387.
10 Kluger N, Raison-Peyron N, Guillot B. Temporary henna tattoos:
sometimes serious side effects. Presse Med. 2008;37:1138-–142.
Locoid Lipocream® Cream, 0.1%
(hydrocortisone butyrate 0.1% cream)
For Topical Use Only
Rx Only
BRIEF SUMMARY
INDICATIONS AND USAGE
Locoid Lipocream is a topical corticosteroid indicated for: relief of the inflammatory
and pruritic manifestations of corticosteroid-responsive dermatoses in adults and
the treatment of mild to moderate atopic dermatitis in patients 3 months to 18 years
of age.
WARNINGS AND PRECAUTIONS
Reversible hypothalamic-pituitary-adrenal (HPA) axis suppression may occur, with
the potential for glucocorticosteroid insufficiency. Consider periodic evaluations for
HPA axis suppression if Locoid Lipocream is applied to large surface areas or used
under occlusion. If HPA axis suppression is noted, reduce the application frequency,
discontinue use, or switch to a lower potency corticosteroid.
Systemic effects of topical corticosteroids may also include manifestations of
Cushing’s syndrome, hyperglycemia, and glucosuria.
Pediatric patients may be more susceptible to systemic toxicity due to their larger
skin surface-to-body-mass ratios.
Initiate appropriate therapy if concomitant skin infections develop.
Discontinue use if irritation develops.
ADVERSE REACTIONS
The most common adverse reactions (>1%) are HPA axis suppression and
application site reactions.
The following additional local adverse reactions have been reported infrequently
with topical corticosteroids, and they may occur more frequently with the use of
occlusive dressings and higher potency corticosteroids. These reactions included:
irritation, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis,
allergic contact dermatitis, secondary infection, skin atrophy, striae, miliaria and
telangiectasia.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Category C. Corticosteroids have been shown to be teratogenic in
laboratory animals when administered systemically at relatively low dosage levels.
Some corticosteroids have been shown to be teratogenic after dermal application
in laboratory animals.There are no adequate and well-controlled studies in
pregnant women. Therefore, Locoid Lipocream should be used during pregnancy
only if the potential benefit justifies the potential risk to the fetus.
Please refer to full prescribing information for detailed information regarding
systemic embryofetal development studies.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could
suppress growth, interfere with endogenous corticosteroid production, or cause
other untoward effects. It is not known whether topical administration of
corticosteroids could result in sufficient systemic absorption to produce detectable
quantities in human milk. Because many drugs are excreted in human milk, caution
should be exercised when Locoid Lipocream is administered to a nursing woman.
Pediatric Use
Safety and efficacy in pediatric patients below 3 months of age have not been
established.
Because of higher skin surface-to-body-mass ratios, pediatric patients are at a
greater risk than adults of HPA axis suppression when they are treated with topical
corticosteroids. They are therefore also at a greater risk of glucocorticosteroid
insufficiency after withdrawal of treatment and of Cushing’s syndrome while on
treatment.
Eighty-six (86) pediatric subjects (5 months to less than 18 years of age) with
moderate to severe atopic dermatitis affecting at least 25% of body surface area
(BSA) treated with Locoid Lipocream three times daily for up to 4 weeks were
assessed for HPA axis suppression. The disease severity (moderate to severe
atopic dermatitis) and the dosing regimen (three times daily) in this HPA axis study
were different from the subject population (mild to moderate atopic dermatitis) and
the dosing regimen (two times daily) for which Locoid Lipocream is indicated.
Five of the 82 evaluable subjects (6.1%) demonstrated laboratory evidence of
suppression, where the sole criterion for defining HPA axis suppression was a
serum cortisol level of less than or equal to 18 micrograms per deciliter after
cosyntropin stimulation. Suppressed subjects ranged in age from 5 months to
16 years and, at the time of enrollment, had 25% to 95% BSA involvement. These
subjects did not develop any other signs or symptoms of HPA axis suppression.
At the first follow up visit, approximately one month after the conclusion of
treatment, cosyntropin stimulation results of all subjects had returned to normal,
with the exception of one subject. This last subject recovered adrenal function by
the second post treatment visit, 65 days post-treatment.
Cushing’s syndrome, linear growth retardation, delayed weight gain, and
intracranial hypertension have also been reported in pediatric patients receiving
topical corticosteroids. Manifestations of adrenal suppression in pediatric patients
include low plasma cortisol levels to an absence of response to ACTH stimulation.
Manifestations of intracranial hypertension include bulging fontanelles, headaches,
and bilateral papilledema.
Geriatric Use
Clinical studies of Locoid Lipocream did not include sufficient numbers of subjects
aged 65 and over to determine whether they respond differently from younger
subjects.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies were conducted to determine the photococarcinogenic or dermal
carcinogenic potential of Locoid Lipocream.
Hydrocortisone butyrate revealed no evidence of mutagenic or clastogenic
potential based on the results of two in vitro genotoxicity tests (Ames test and
L5178Y/TK+ mouse lymphoma assay) and one in vivo genotoxicity test (mouse
micronucleus assay).
No evidence of impairment of fertility or effect on mating performance was
observed in a fertility and general reproductive performance study conducted in
male and female rats at subcutaneous doses up to and including 1.8 mg/kg/day
(0.7X maximum topical human dose [MTHD]). Mild effects on maternal animals,
such as reduced food consumption and a subsequent reduction in body weight gain,
were seen at doses ≥0.6 mg/kg/day (0.2X MTHD).
PATIENT COUNSELING INFORMATION
Patients using Locoid Lipocream should receive the following information
and instructions:
Apply a thin layer to the affected skin two or three times daily for corticosteroidresponsive dermatoses in adults. Consult with your physician to determine if
treatment is needed beyond 2 weeks. Apply a thin film to the affected skin areas
two times daily for atopic dermatitis in patients 3 months of age and older.
Safety of Locoid Lipocream in pediatric patients has not been established beyond 4
weeks of use.
Rub in gently.
Avoid contact with the eyes.
Do not bandage, otherwise cover, or wrap the affected skin area so as to be
occlusive unless directed by your physician.
Do not use Locoid Lipocream in the diaper area, as diapers or plastic pants may
constitute occlusive dressings.
Do not use Locoid Lipocream on the face, underarms, or groin areas unless
directed by your physician.
If no improvement is seen within 2 weeks, contact your physician.
Do not use other corticosteroid-containing products while using Locoid Lipocream
without first consulting your physician.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled
Room Temperature]. Protect from freezing. Keep out of the reach of children.
Manufactured for: Triax Pharmaceuticals, LLC
Cranford NJ 07016
By: Ferndale Laboratories, Inc.
Ferndale MI 48220
Locoid Lipocream is a registered trademark of
Astellas Pharma Europe BV licensed to
Triax Pharmaceuticals, LLC.
Marketed and Distributed By:
Triax Pharmaceuticals, LLC
Cranford NJ 07016
www.Locoid.com
131B301
Rev 11/09
Now younger eczema
patients have something
to smile about
Now approved for use in children
down to 3 months of age
The power of an ointment with the elegance of a cream
Locoid Lipocream is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses,
including the treatment of mild to moderate atopic dermatitis in patients 3 months of age and older.
Safety and effectiveness in pediatric patients below 3 months of age have not been established.
Reversible HPA axis suppression may occur, with the potential for corticosteroid insufficiency. Consider periodic evaluations for HPA
axis suppression if applied to large surface areas or used under occlusion. Systemic effects of topical corticosteroids may also include
manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria. Pediatric patients may be more susceptible to systemic toxicity
due to their large skin surface-to-body-mass ratios. Initiate appropriate therapy if concomitant skin infection develops. Discontinue use
if irritation develops. Please see full Prescribing Information on adjacent page.
Visit us at www.locoid.com
(hydrocortisone butyrate 0.1%) Cream
©2010 Triax Pharmaceuticals, LLC. All rights reserved. Locoid is a registered trademark of Astellas Pharma Europe B.V. licensed to Triax Pharmaceuticals, LLC. LOC-0410-01