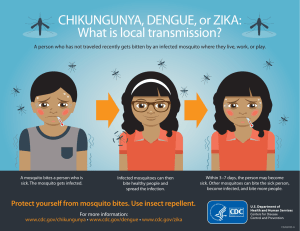
Mathematical Modeling of Dengue - Temperature Effect on Vectorial
advertisement

Mathematical Modeling of Dengue Temperature Effect on Vectorial Capacity Jing Helmersson 2012 Supervisor: Joacim Rocklöv Abstract Background As climate change and globalization continues, the vector (mosquito) borne disease - dengue - has changed its pattern, spreading from tropical and subtropical region to more temperate areas and become a threat to Europe. Therefore a better understanding of how the transmission of dengue is affected by climate is an important research subject in public health. Objective This study is to develop a theoretical framework in mathematical modeling of dengue and to explore the relation of dengue vectorial capacity with temperature – both average and daily variation. Methods This thesis has reviewed the basic and some sophisticated theoretical frameworks in mathematical modeling of infectious disease with focus on dengue modeling. Temperature effect on dengue transmission was explored from two different models and literature search for vector and virus transmission parameters. Relative vectorial capacity for dengue transmission between humans was estimated for different mean temperatures and diurnal temperature range variation. Results & Discussion The study showed that the relative vectorial capacity peaks around mean temperature of 28-30 0C and reduces at both low and high mean temperature. Large daily temperature fluctuation increases the dengue transmission at low mean temperature and decreases the dengue transmission at high mean temperature. As a result, daily temperature fluctuation reduces greatly the gap in dengue transmission between warmer and cooler region. As global warming continues with increased temperature and temperature variation especially in temperate countries, this result is important in considering dengue potential and risk assessment based on climate data. Sensitivity analysis indicated that the mosquito’s mortality rate was the most important vector parameter in affecting the value of the relative vectorial capacity especially at low temperatures. Conclusion This study showed that a simple model can give powerful insight into the dengue spreading. The generalizability of the model depends on the vector parameters used. A systematic review of vector parameters is in great need in mathematical modeling of dengue. Both choosing the right mathematical model with proper complexity and the vector parameters are crucial to make modeling useful in understanding, predicting and guiding dengue control. Key words: dengue, mathematical modeling, vectorial capacity, daily temperature fluctuation, Aedes aegypti, climate change, Europe. - ii - Acknowledgements This master thesis could not be possible without the understanding and loving support of my family, Sven, Erik and Lena Helmersson. Special thanks to Professor Ragnar Andersson from Department of Public Health from Karlstad University, whose suggestion and encouragement has made my decision to study Public Health and to get a Master’s degree in Umeå University. It is not easy to follow other’s ideas after having a Ph.D. in physics and leading a research group in Physics for over 10 years in California State University Long Beach. It is especially hard for me to go back to use mathematics after deciding to quit a professor career in physics in USA. It is my supervisor Joacim Rocklöv’s persistence and gentle encouragement that have made me choosing mathematical modeling for my thesis and near future research. It is Lancet chief editor, Richard Horton’s impressive public lecture given in Umeå University Dec. 2011 with strong emphasis on modeling and its importance in affecting public health that gave me the enthusiasm in pursuing mathematical modeling. Dr. Horton said as I can best recall “…Do modeling instead! We, as scientists, need to give politicians and decision makers confidence by providing scientifically based evidence so that it can make a difference in public health!” Lancet is the world’s top research journal on general medicine and public health. Thus, many thanks go to Dr. Joacim Rocklöv and Dr. Richard Horton. Special thanks to all of my teachers in Public Health in Umeå University, especially Nawi Ng, for his rich knowledge in epidemiology, great lectures and lessons, hard working and productive research, which have made me to be humble again. Thanks for all of my classmates in 2011/2012 public health master’s program for friendship and for understanding of our differences. Especially thank Charlotte Reding for her spoken of her heart and gave me a chance to improve myself. Life is a learning process. I thank all of those who have taught me valuable knowledge and lessons from public health to life along the path to finish this thesis. - iii - - iv - Content Abstract .......................................................................................................................................ii Acknowledgements.....................................................................................................................iii Content .......................................................................................................................................iv List of Tables and Figures...........................................................................................................vi List of Abbreviations and Mathematical Symbols.....................................................................vii Introduction.............................................................................................................................1 Objective …………………………………………………………………………………………………………………..4 Methods ……………………………………………………………………………………………………………………5 1. Basic Concepts in Mathematical Modeling of Infectious Diseases 1.1 Mathematical Modeling and Public Health Policy ……………………………………................. 5 1.2 Development of Mathematical Modeling ………………………………………………….…………... 6 1.3 The Basic Concepts in Mathematical Modeling of Infectious diseases…………................ 6 1.4 A Simple Epidemic SIR Model of Infectious Diseases - Humans only…………................. 7 1.5 A Single Epidemic Outbreak, the Reproduction number…..……………………….…………….10 2. Theoretical Frame Work for Dengue Infection 2.1 Dengue transmission process ………….……………………………….…………………….…………... 13 2.2 A SIS Model - Humans and Vectors, the Vectorial Capacity ……………..…….….………….. 13 2.3 Effects of Temperature in Dengue – Seasonality 2.3.1 Sinusoidal Variation of Transmission Rate………….…………………………….…………. 18 2.3.2 A Modified SIR model – Humans, Vectors and Eggs..……………………………………..19 2.4 Effects of Temperature in Dengue – Daily Fluctuation.…………….………………..…….….... 21 Results .....................................................................................................................................24 1. Combined Effects of Temperature on Dengue Relative Vectorial Capacity …………..….. 24 1.1 Effect of Mean Temperature on Dengue Relative Vectorial Capacity ……….…….….24 -v- 1.2 Effect of Daily Temperature Variation on Dengue Relative Vectorial Capacity ……………………………………………………………………………………………………… 27 2. Sensitivity Analysis of Vector Parameters on Dengue Vectorial Capacity ………………….30 Discussion ..............................................................................................................................34 Limitations of this study and suggestions ……………………………………………………………...36 Conclusions ..........................................................................................................................38 References ............................................................................................................................39 - vi - List of Tables and Figures Table 1 Vector mortality rate (µM) and survival probability (SV).......................................26 Table 2 Comparison of relative change in vectorial capacity V*........................................31 Figure 1 1a: Average Earth surface temperature measured during the 20th and projected for 21th century; 1b: Aedes mosquito (WHO, 2003)………………….….…….……….....1 Figure 2 Flow Diagram of the SIR model……………………………………….……………………….....8 Figure 3 Flow diagram of dengue infection including humans and mosquitoes……………14 Figure 4 Simulation of a system with sinusoidal transmission rate ………………………...…19 Figure 5 Flow diagram of dengue infection including humans, mosquitoes and eggs…. 20 Figure 6 Vector infection and transmission probabilities as a function of DTR………….. 22 Figure 7 Vector parameters for Aegypti as a function of mean temperature……………..…26 Figure 8 Relative vectorial capacity as a function of mean temperature……….……………. 27 Figure 9 Vector mortality rate as a function of DTR for two dengue viruses….…………….28 Figure 10 Relative vectorial capacity as a function of DTR…………………………………….……29 Figure 11 The effect of different Extrinsic Incubation Period n on relative vectorial capacity………….……….……………………………………………………………………………...32 Figure 12 The effect of different mortality rate on relative vectorial capacity…..…………...33 - vii - List of Abbreviations and Mathematical Symbols DENV Dengue virus DTR Diurnal Temperature Range EIP Extrinsic Incubation Period SIR Mathematical model: Susceptible-Infectious-Recovered SIS Mathematical model: Susceptible-Infectious-Susceptible WHO World Health Organization A Amplitude of annual temperature variation FI(t) Seasonal variation in mosquito production from infected eggs Fs(t) Seasonal variation in mosquito production from susceptible eggs IE Number of Infective mosquito eggs I, IH Number of Infective humans IM Number of Infective mosquitoes LM Number of Latent mosquitoes NM Total female mosquito population NH Total human host population R Number of Recovered humans Re Effective reproduction number R0 Basic Reproduction number S Number of Susceptible humans Sth Threshold number of Susceptible humans Sv The vector survival probability S* Endemic number of Susceptible humans - viii - T Mean or ambient temperature V Vectorial capacity V* Relative vectorial capacity, V/m a Average number of mosquito bites per person per day bH Probability of viral transmission from the mosquito to human per bite bM Probability of viral transmission from the human to mosquito per bite f Frequency in a yearly cycle of sinusoidal function g Proportion of infected eggs laid by infected female mosquitoes m Number of female mosquitoes per person m* Critical or threshold number of female mosquito per person for dengue transmission n Duration of the extrinsic incubation period p Vector’s daily survival probability pc Fraction of critical vaccination coverage pI Per capita infected mosquito egg hatch rate ps Per capita susceptible mosquito egg hatch rate pT Probability of infection transmission per contact r Per capita human birth rate rM Oviposition rate of mosquito eggs t Time α, αH Dengue induced human mortality rate βH Viral transmission rate to humans βM Viral transmission rate to vectors λ, λH Force of infection to humans λM Force of infection to vectors µ, µH Human natural mortality rate - ix - µM Mosquito natural mortality rate µE Mosquito egg natural mortality rate γ, γH Per capita human recovery rate from dengue φ Phase in a sinusoidal function -x- Introduction Global warming is a fact. During the twentieth century, the average Earth surface temperature increased by approximately 0.6 ºC, of which 0.4 ºC has occurred since 1975 as shown in Figure 1 below. During this century, the average Earth surface temperature is expected to increase about 2-30C which exceeds the safe threshold above preindustrial average temperature (WHO, 2003). The consequences of climate change to health are both direct and indirect with some being already experienced and others yet to come. For example, heat waves, extreme weather with consequences, such as flood and other natural disasters, were already seen, and the changes in the geographical and temporal transmission patterns of infectious diseases has just started to be observed and more to be expected to come. Fig. 1. 1a: Average Earth surface temperature measured th th during the 20 and projected for 21 century (WHO, 2003). 1b: Aedes mosquito (WHO, 2012) As stated in the summary of the book Climate change and human health - risks and responses (WHO, 2003): “The first detectable changes in human health may well be alterations in the geographic range (latitude and altitude) and seasonality of certain infectious diseases – including vector-borne infections such as malaria and dengue fever, and food-borne infections (e.g. salmonellosis) which peak in the warmer months.” Dengue is a mosquito-borne viral infection that is usually found in tropical and sub-tropical regions around the world. According to WHO (2012), “Dengue causes a severe flu-like illness, and sometimes a potentially lethal complication called dengue haemorrhagic fever” About 2.5% of those that are infected by dengue die since dengue has neither treatment nor vaccination. Dengue has become a major international public health concern. According to WHO (2012), the incidence of the dengue has increased drastically in the recent decades. For example, dengue cases increased from 1.2 million in 2008 to over 2.2 million in 2010 across the Americas, South-east Asia and Western Pacific – 55% increase in two years! Now almost half of the world population - over 2.5 billion people is at risk from dengue. Dengue Haemorrhagic Fever or severe dengue was first recognized in the 1950s and has become a -1- leading cause of hospitalization and death among children in most Asian and Latin American countries. WHO currently estimates that every year there may be 50 - 100 million dengue infections worldwide and about 500 000 people with severe dengue require hospitalization, a large proportion of them are children. In addition, global mobility and trade have facilitated dengue spread to areas that were not previously considered important – e.g. Europe, and potential climate change is likely to contribute to more spread and more suitable conditions in those areas. In 2010, dengue cases were reported for the first time in France and Croatia, and imported cases were detected in three other European countries. In Sweden, there are about 30-60 cases every year from travelers to overseas (Heddini et al., 2009). Dengue virus is transmitted to human by the two mosquitoes called, Aedes aegypti and Aedes albopictus. The dengue mosquitoes are typically proliferating in certain tropical and subtropical climate regimens. However since introduced to Europe from Asian through global trade and travel, the Aedes albopictus mosquito has learned to adapt to temperate climate and diapauses (overwinter) during the winter season. As global warming continues and global traffic and trade steadily increases, it is currently spreading northward in Europe and is even expected to reach Sweden in the year of 2030s (ECDC Technical Report, 2009). However, our understanding of the dengue transmission overall, and in particularly in temperate climate regimes is very limited, especially the effect of temperature and temperature daily variability. Therefore a better understanding of how the transmission of dengue is affected by climate especially temperature and its variation is an important research subject in public health. It is important to monitor and model the spread of dengue to vulnerable areas where people have no or little immunity, such as, Europe. Mathematical modeling can help our understanding and assessment of the present and future risk areas on spread of infectious diseases based on climate data as shown in the case of the malaria cartography (Gething et al., 2011). Mathematical modeling uses a set of mathematical equations derived from a theoretical framework and calculates the threshold condition such as, the vectorial capacity for transmitting virus and/or incidences of dengue as a function of time for a particular area. In other words, mathematical modeling can help us not only understand and predict the future spread of infectious diseases but also evaluate strategies on combating dengue (Burattini et al 2008). Using computer simulation from mathematical modeling one can produce estimates of disease transmission, e.g. disease incidences under certain assumptions, and threshold for epidemic outbreaks. The accuracy of modeling a real situation depends on the assumptions of the theoretical framework and parameters used to describe the relations between human and mosquito populations, mosquito and virus interaction in the virus transmission and disease spreading process. The first step for modeling dengue is to develop a good theoretical framework to describe the dengue transmission in a given environment. The theoretical framework should capture all the key variables and make approximations on other less important variables. Temperature is a key environmental determinant in shaping the landscape of dengue, while it is often not incorporated explicitly in disease models at the present, especially daily temperature variation. However, diurnal temperature variation can be higher in temperate countries compared to tropical countries and is therefore essential to be incorporated in -2- order to understand dengue transmission and quantify risks in vulnerable areas such as Europe. Today most studies on modeling of infectious diseases are based on theoretical frameworks that consider only constant or average temperature (Macdonald, 1952; Diekmann et al., 1990). As shown recently both the daily (Lambrechts et al., 2011) and seasonal (Massad et al., 2011) temperature fluctuations have important impact on some factors in the transmission of the dengue virus. However, both studies have either neglected or treated the other vector parameters as temperature independent in the dengue transmission. Thus, there are no studies in the dengue modeling taking into account of the temperature effect of all the important parameters in the chain of events of causing dengue transmission. Within the DengueTools research program carried out in the department of Public Health at Umeå University as part of the global project, studies are conducted to better understand the risks of dengue infection in Europe through both empirical and modeling studies. This Master thesis is the part of the modeling effort of the program that intends to fulfill part in one of the three gaps described in DengueTool project (Annelies et al., 2012): “3. Lack of predictive models for the risk of establishment of dengue in uninfected regions (in particular Europe), taking into account global travel networks and climate change” Thus, this thesis intends to review and develop a theoretical framework for dengue mathematical modeling and to estimate the potentials risk to vulnerable areas with focus on Europe. -3- Objective The objective of this thesis is to develop a general theoretical framework for mathematical modeling of dengue transmission potential based on temperature. Through reviewing the existing dengue mathematical models, this study aims at finding the best way to incorporate temperature effect on dengue transmission. Through reviewing vector data, it intends to specify the vectorial capacity as a function of the daily average temperature and daily temperature variations experienced to an entirely susceptible population. -4- Methods This section reviews the commonly used mathematical modeling frameworks for infectious diseases first. Then it will zoom in to dengue modeling specifically. Emphasis will be given to those dengue models that incorporate temperature effect. These are bases for developing the theoretical framework for this thesis. The results of the modeling are generally obtained numerically through sophisticated computer programming. For the scope of this Master’s thesis, mainly analytical solutions are presented. 1. Basic Concepts in Mathematical Modeling of Infectious Diseases 1.1 Mathematical Modeling and Public Health Policy The goal of public health research is more than just knowledge quest. It aims at making a difference in public health. The frustration that faced many scientists in public health is that their research results were not taking into account in the decision making process. That is why we have making health policy as part of our curriculum in public health master program. In public health, we may divide the research into two types of studies: empirical and mathematical modeling. Empirical study consists of 1) designing the study – quantitative or qualitative, 2) collecting data, 3) analyzing data and 4) reporting results as seminars and publications. This is the main stream of study in public health. In the last few decades, modeling starts to enter in public health. Its sophistication in attacking research problems increases with the capacity of computer’s development. Modeling consists of 1) developing a theoretical framework – transfer a research problem into a set of mathematic equations, 2) finding relevant parameters to connect with health reality from empirical data, 3) computing the solutions of the equations in numerical and/or graphic form, and 4) reporting results as seminars and publications. One of the important differences between these two study types is the relevant time frame at focus: empirical study is data based and data is from events that happened before. Thus the focus of study is what has occurred in the past. One may predict future events with limitations to the same conditions as in the past events. Whereas, mathematical modeling focuses directly on the prediction of past or future events based on a set of assumptions and past events data and projected future conditions. A mathematical disease model constitutes a set of causal pathways involved in the exposure to disease process and simulates disease transmission over time and space. The importance of mathematical modeling in public health policy is strongly stressed by the Lancet chief editor, Richard Horton, during his public lecture given in Umeå University 2011 (Horton, 2011). In fact, Mathematical (computer) modeling has been used in evaluating social and economic policies. It can be used to evaluate health policies as well. As quoted by Aron J. (2007): “Properly used, computer models can improve the mental models upon which decisions are actually based and contribute to the solution of the pressing problems we face.” -5- Although it is not easy to include all the socioeconomic and demographic factors beside climate factors, mathematical modeling gives an tool to facilitate consensus and action with an iterative and incremental approach to making decisions (Aron J., 2007). 1.2 Development of Mathematical Modeling Using mathematical language to describe the transmission and spread of infectious diseases is not new (Kretzschmar and Wallinga, 2010). As early as 1766, Daniel Bernoulli used mathematical life table analysis to describe the effects of smallpox variolation (a precursor of vaccination) on life expectancy (Dietz and Heesterbeek, 2000). However, only in the twentieth century, the nonlinear dynamics of infectious disease transmission was better understood. Hamer (1906) was one of the first to recognize that the decreasing density of susceptible persons alone could stop the epidemic. The 1902 Nobel prize winner, Sir Ronald Ross, developed mathematical models to investigate the effectiveness of various intervention strategies for malaria. In 1927, Kermack and McKendrick derived the celebrated threshold theorem (Kermack & McKendrick, 1927). They found that a threshold quantity is needed in order for an infectious disease to spread in a susceptible population. This leads to the concept of herd immunity. The threshold theorem has been very valuable during the eradication of smallpox in the 1970s (CDC, 2012). Mathematical modeling became more widespread toward the end of 20th century. Especially in public health policy making at strategic and tactical levels, modeling approaches have the advantage of predicting the future courses of an epidemic and the consequences associated to different scenarios to identify the most effective preventions strategies (Anderson & May, 1991). Such models have successfully been used to better surveillance and control of the AIDs pandemic in 1980-90s, the UK Foot & Mouth disease livestock epidemic 2001 (Keeling M.J., 2005) and the outbreak of the SARS virus 2003 (Wallinga and Teunis, 2004). 1.3 The Basic Concepts in Mathematical Modeling of Infectious diseases Models help us to understand reality because they simplify it. In a sense, a model is always “wrong” since it is not reality (Aron J., 2007). However, a model may be a useful approximation, permitting conceptual experiments that would otherwise be difficult or impossible. Models need to capture essential behavior of interest and incorporate essential processes. Making models explicit mathematically clarifies thinking and allows others to examine them. Thus mathematical models allow precise, rigorous analysis and quantitative prediction. Therefore, it is not necessary that the more complex model is always the best. Complicated and detailed models are usually better in fitting the data than simpler models, but they can obscure the understanding and the mechanism responsible for the result. Simpler models are more transparent which provide insight and guide thought better (Aron J., 2007). “The choice of the optimal level of complexity obeys a trade-off between bias and variance (Burnham & Anderson, 2002). A model should only be as complex as needed, depending on the questions of interest. This philosophy is referred to as Occam’s razor or the principle of parsimony and can be summarized as the simplest explanation is the best” (Choisy et al., 2007). -6- There are two types of mathematical models: deterministic (or transmission) and stochastic (or statistical). Deterministic models are those in which there is no element of chance or uncertainty. As such, they can be thought to account for the mean trend of a process and more suitable for disease propagation in large population. Stochastic models, on the other hand, account not only for the mean trend but also for the variance structure around it. This is proper when the population is small and random events cannot be neglected (Choisy et al., 2007). In this thesis, I will focus only deterministic model assuming that a large population will be our concern for the dengue infection. In a deterministic model, the time evolution of an epidemic is described in mathematical terms. It connects the individual level process of transmission with a population level description of incidence and prevalence of an infectious disease. Thus, modeling builds on our understanding of the transmission process of an infection in a population, such as, the prevalence of infectious individuals, the rate of contact between individuals, infectiousness of the infected individuals or vectors, etc. Thus, the following factors are important: 1) population demography, e.g. age, sex, population density, birth and death rate; 2) natural history of infection, e.g. latency, infectious period, immunity; 3) transmission of infection, e.g. direct or indirect, contact rate. The transmission process is generally a dynamic process where the individual’s risk of infection can change over time. It requires dynamic models, e.g. modeling state variables as a function of time, and can be used for prediction and analysis of disease spread and preventative programmes. Modeling generally consists of four steps. First, a flow diagram represents the natural history and transmission of infection. Based on this diagram, we can then write a set of mathematical equations to express the transmission process. The third step is to find proper values for the parameters used in the equations. Finally, we need to solve the equations algebraically or numerically with help of computer simulation programs. Thus, mathematical modeling involves many disciplines, from clinic medicine, biology, mathematics/physics, computer science, zoology, to environmental and social science. Due to short time limit of this thesis, I will focus on the first three steps and carry out the last step of calculation only for simple cases. 1.4 A Simple Epidemic SIR Model of Infectious diseases – Humans Only Let us consider first a simple case of transmission process of an infectious disease (Kretzschmar and Wallinga, 2010), such as measles. As mentioned earlier, some of the most important quantities can be learnt from simple models. Analysis of this model helps us to develop a deeper understanding of the phenomenon of epidemic spread and disappearance. Here individuals in the population can be classified into three compartments: a) Susceptible to the disease (Susceptibles)– S, b) Currently Infectious (Infectious or Infectives) – I, -7- c) Recovered and immune (Recovered or Removals) – R. Here S, I, R, represent the number of individuals in each compartment. The total host population is N = S + I + R. The transmission process is represented by the flow diagram shown in Figure 2. This is called SIR model. Here we have neglected details including vectors and considered just human population. Susceptible Infectious Recovered & Immune S I R birth, r Recovery, γ Infection, λ death, µ death, µ, α death, µ Fig. 2. Flow Diagram of the SIR model Each arrow represents the flow rate at which individuals enter or leave a compartment per unit time, that is, the incidence rate. The number of susceptible individuals (S) is increased by birth (rate r) and decreased by natural (non-diseased) death (rate µ) and by transmission of infection events of susceptible (rate λ, the force of infection). The number of Infectious individuals (I) is increased by infection events of susceptible, and decreased by natural death (rate µ) plus disease-induced death (rate α) and by recovery (rate γ) of infected individuals into immunity. The number of recovered individuals (R) is increased from recovered infectious individuals and decreased by natural death (rate µ). All the parameters r, µ, α, λ and γ are per-capita rates. The population flow rate is the percapita rate (r, µ, α, λ or γ) multiplied by the number of individuals subjected to that percapita rate (N, S, I, or R). For example, in-flow “birth” to compartment of “susceptible” is rN, the per capita birth rate r times the total population N of the system, assuming all individuals give birth at the same rate r which is averaged over males and females. The population recovery rate is γI, the per capita recovery rate γ times the number of infected I. Thus, the population flow rate as each arrow shown in the flow diagram is summarized below: Birth = rN. Infection = λS, Recoveries = γI. Deaths of S = µS. Deaths of I = (µ+α)I. Deaths of R = µR. -8- The next step is to write equations based on the flow diagram to express change of state variables: dS/dt, dI/dt, dR/dt. Here t is time. dS/dt = birth – Infection – deaths (of S) dI/dt = Infection – Recoveries – deaths (of I) dR/dt = Recoveries – deaths (of R) Every term is a rate quantity and has the unit of 1/time. Using the parameters and population flow rates described above, we have the mathematical expression of the transmission process in form of differential equations: dS/dt = rN – λS – µS (1-1) dI/dt = λS – γI – (µ+α)I (1-2) dR/dt = γI – µR (1-3) N=S+I+R (1-4) Here the only non-constant parameter is λ, the force of infection, which is the per-capita rate of infection of susceptible and describes the risk that a susceptible individual will get infected per unit time. λ depends on the rate of contact with other individuals, c, the probability of transmission when an infectious individual contacts a susceptible, pT, and the infectious proportion in the population, I/N . λ can be expressed as: λ = pT c I/N, or = βI/N. (1-5) where β=pT c is the total transmission rate in the population. Put equation (1-5) into equation (1-1 & 1-2), we have a set of four non-linear differential equations, Eqs. (1-1)-(1-4) for the transmission process. Here S, I, R, N are state (or dependent) variables that change with time. They describe the state of an epidemiological system – population in each compartment in this case. Their dependence on time varies intrinsically and can be simulated in model using a computer program based on the equations given (1-1) – (1-5). They are not manipulated directly. On the other hand, r, µ, α, β (or c & pT) and γ are parameters that do not change with time. Once their values are specified, they stay constant during the calculation as the computer program runs. They are chosen either based on estimates from epidemiological data, or based on assumptions. Assume that the values of parameters are specified based data and assumption made for a specific infectious disease and a specific population, we move to the final step of modeling – solving the equations. Solving the equations will give us the time evolution of state variables. Most of the models cannot be solved algebraically. Numerical integration using a computer is -9- normally the standard methods. We need to specify an initial state and the computer will run the program to solve the set of equations (1-1) – (1-5) as an iterative procedure over time. However, under special conditions, some of the analytical solutions can be obtained. Here, some of these analytical solutions will be listed, especially those related to the threshold conditions. 1.5 A Single Epidemic Outbreak, the Reproduction Number We can gain some insight and learn the most important concept from the SIR model on a simple case – a single epidemic outbreak. During a single epidemic outbreak, the time span is normally short so that any demographic change can be neglected (Iannelli 2005). That is, we assume that no birth and death occur during this time. In other words, the birth and death rate are zero on the scale of average duration of infectivity (1/γ), or µ+α << γ. The total population (N) is constant. In addition, we assume that the whole population N is susceptible at the beginning: S0 = N, where S0 is the initial value of S at time t=0. Eq. (1-2) can be rewritten while putting in Eq. (1-5) as: dI/dt = λS – γI = βSI/N – γI = (βS/N – γ)I = γ(Re(t) − 1)I (1-6) Where Re(t) is defined as: Re(t) = βS/(Nγ) = R0 S/N, (1-7) and R0 is defined as R0 = β/γ . (1-8a) Here, Re(t) is known as the effective reproduction number and R0 is known as the basic reproduction number. They are sometimes called reproduction ratio or reproduction rate (Aron J., 2007). Since both β and γ have the same unit of 1/time, Re and R0 are dimensionless numbers. Either number or ratio are accurate description for this quantity. Here this thesis adopted the conventional name – reproduction number, although reproduction ratio is just as good or may be more clear in term of its meaning as explained later. In order for an epidemic to take place, the number of infected persons (I) must increase. This means mathematically that dI/dt > 0, or Re(t) > 1. - 10 - Eq. (6) also shows that if Re(t) = R0 S/N < 1, then dI/dt < 0 - the number of infectious population is decreasing. If Re(t) = R0 S/N = 1, then dI/dt = 0. (1-9) dI/dt = 0 or Re(t) = 1 means that the number of infectious (I) reaches its maximum or stays constant. This is the threshold value. From Eq. (1-9), the threshold number of susceptible (Iannelli 2005) is needed to sustain the infection is when R0 S/N = 1 or Sth = N/R0 (1-10a) At the beginning when a new case was introduced to a totally susceptible population (S0 = N), the transmission process can be described by Re(t=0) = R0. If R0 > 1, the rate at which susceptibles become infectives exceeds the rate at which infectives are recovered, or the number of new infectious increases at first. As time goes on, a part of the population is infected and become immune. The number of available susceptible individuals (S) decreases. As S becomes less than the threshold value Sth = N/R0, Re(t) become less than 1. The epidemic dies out as it runs out of susceptible individuals. If initially the population is only partially susceptible due to intervention or immunity from past recovery of the same disease, still Re > 1 is needed to have the epidemic spread and Re < 1 is the criterion for the epidemic to stop. Both R0 and Re are threshold quantities that predict the occurrence of an epidemic. The value of either Re or R0 is greater than or less than one respectively determine the prevalence of infection to increase or decrease for a totally (R0) or partially (Re) susceptible population (Cintron-Arias et al., 2009). While the basic reproduction number (R0) is defined by Macdonald (1952) as the number of secondary infections produced by a single infective in a completely susceptible population, the effective reproduction number (Re) describes the number of secondary cases produced per index case in a population that is only partially susceptible. Since S ≤ N, Re (t) ≤ R0. The effective reproduction number Re depends on the state variable S/N and is changing with time which makes it less easy to be used in the prediction of an epidemic without computer simulation. On the other hand, the basic reproduction number R0 is a constant that depends on parameters only. From Eq (1-8a), we see that R0 = β/γ = pT c/γ. (1-8b) As shown, the basic reproduction number is determined by three parameters: the average rate of contact between susceptible and infected individuals (c), the probability of infection being transmitted during a contact (pT), and the duration of infectiousness (1/γ). R0 is the central quantity in infectious disease epidemiology. R0 defines the threshold value for an epidemic to occur in a completely susceptible population. If R0 > 1 or a single case introduced into a susceptible population generates more than one new case, the number of cases is increasing and an epidemic will spread. If R0 <1 or a single case introduced into a susceptible population generates less than one new case, the number of cases decreases and an outbreak - 11 - will die down. However, to successfully eliminate a disease from a population, the effective reproduction number Re <1 needs to be maintained even for a completely susceptible population initially. R0 can in principle be determined for every infectious disease based on model and can be estimated for every infectious disease although it is not easy in the beginning of an epidemic (Iannelli 2005). The great contribution of the SIR model is that the value of R0 and Re can be used to analyze the dynamics of transmission, both when infection has just been introduced (R0) into a population and when infection has long been endemic (Re). Endemic means that the chain of transmission from infective host to susceptible host is maintained in a population or infectious population reaches an equilibrium constant: dI*/dt = 0, or Re(t)* = R0 S*/N = 1. Here endemic state variables are labeled with *. This means that S* = N/R0, or S*/N = 1/R0 (1-10b) Thus, at endemic state, a single case will generate only 1 new case (Re* = 1) while at the beginning of epidemic, a single case generates, on average, R0 new cases when everyone is susceptible. This means that only a fraction, 1/R0, of the possible contacts is susceptible at the endemic equilibrium as shown in Eq. (1-10b) (Aron J., 2007). In other words, not all susceptible population would be infected before an epidemic stops. The larger the R0, the less the fraction of population is left uninfected. This fact can be used to describe the herd immunity in the SIR model. Since at endemic state, there will be certain susceptible left: S* = N/R0. Thus, to prevent an epidemic spread out, we need to vaccinate only a fraction of the population: pc = (N-S*)/N = 1 – S*/N = 1 – 1 /R0 (1-11) pc is the critical vaccination coverage fraction (Kretzschmar and Wallinga, 2010; D´ebarre, 2012). For example, for an influenza with R0=2 (that is, one infected person will infect two people during his/her infectious time) the fraction of the population needed to be vaccinated would be, pc= 0.5. On the other hand, for smallpox, R0 is about 5 and pc is then around 0.8. This means that vaccination of 50% population is enough to prevent flu epidemic, while for smallpox, 80% population needs to be vaccinated. The fact that not everybody is needed to be vaccinated in order to eliminate an infectious disease is known as herd immunity. - 12 - 2. Theoretical Frame Work for Dengue Infection 2.1 Dengue transmission process According to WHO (2012), dengue virus is transmitted to humans through the vector of mosquitoes - the infected female mosquito bites. After a person is infected, he/she became the main carrier and amplifying host of the virus, serving as a source of the virus for uninfected mosquitoes for 4-5 days; maximum 12 days. Thus, mosquitoes acquire the virus mainly from biting an infected person. Once infective, a mosquito is capable of transmitting the virus to humans for the rest of its life through biting. In addition, an infective female mosquito can transmit the virus to its eggs in the ovaries through vertical transmission route as shown in Aedes albopictus mosquitoes with 75% probability (Shroyer, 1990). In contrast, only a few percent viral transmission is through vertical route in Aedes aegypti mosquito and the main virus transmission route is horizontal – from mosquitoes to mosquitoes through biting infected humans. Among the two types of dengue mosquitoes: Aedes aegypti and Aedes albopictus, the first is the primary vector of dengue in warm climate. However, Aedes albopictus is the one that can adapt and survive in cooler, even below freezing, temperate regions of Europe (ECDC Technical Report, 2009). There are four different dengue viruses - DENV-1, DENV-2, DENV-3 and DENV-4. Although recovery from one type of virus provides lifelong immunity against the virus, it does not give immunity against other types of viruses. In fact, “Subsequent infections by other serotypes increase the risk of developing severe dengue…At present, the only method of controlling or preventing dengue virus transmission is to combat the vector mosquitoes.” (WHO, 2012). Based on this information, the modeling of dengue can be divided into two ways, considering: 1) only one type of virus exists – infected will either die or recover and immune (SIR model as shown in Sec. 1); 2) more than one type of virus exists - infected will either die or become susceptible again (SIS model which will be shown next). Other vector information is also very important, such as latency, the time delay between being infected and becoming infectious, daily biting rate which pertains to female mosquitoes only and strain of viruses. Since temperature influences greatly the vector’s survive, latency and transmission capability of dengue virus, the modeling needs to consider temperature effect for both cases. There are different ways of incorporating temperature effect to the modeling: from average yearly temperature, to seasonal, or to daily variation. The most important quantity that we are interested in finding out is still the basic reproduction number in order to determine the threshold value for dengue transmission. With a vector as the part of the transmission of dengue between humans, the process and the mathematical expressions become more complicated compared to the one that we have discussed earlier. 2.2 A SIS model - Humans and Vectors, the Vectorial Capacity In this modified Ross-Macdonald model, the recovered individuals become susceptible again and there is no permanent immunity for humans (Smith et al., 2012). This is referred to as a - 13 - SIS model – susceptible to infected and back to susceptible again. For vectors, the infected mosquitoes are assumed to remain infectious for the rest of their lives. Thus, only two compartments exist for humans and for vectors: the number of susceptible - SH, SM, and the number of infected - IH and IM, where the subscript H denotes for humans and M for mosquitoes. In addition, for simplicity latency is not taken into account initially. This process can be a simplified case of either dengue or malaria where the mosquito is the vector. The flow diagram for this infectious transmission process is shown in Figure 3. Here the top row is the transmission process for humans and the bottom is for vectors. The susceptible humans (SH) may be moved out of its compartment through infection at the per capita rate λH or through natural death at a per capita rate µH. The increase of susceptible humans is due to recovery of infected humans. Here the human birth is neglected since birth rate is generally small compared to the recovery rate. The infected humans are increased from infection of susceptible and decreased through death due to both natural cause and disease as well as through recovery. Similar process goes for mosquitoes except that there is no recovery for infected mosquitoes. death, µH, αH death, µH Human vector to human infection βH=mabH Infection, λH SH IM Recovery, γH Infection, λM IH SM human to vector infection βM=abM Mosquitoes death, µM death, µM Fig. 3. Flow diagram of dengue infection including humans and mosquitoes. Here λH & λM are force of infection to humans and vectors separately and are defined as the per capita rate at which susceptible humans & vectors are infected. λH (λM) depends on the number of mosquito bites in the human population per unit of time, NM a, where a is the average daily biting rate per fly on humans, the infectious proportion in the female mosquito (human) population, IM/NM (IH/NH), and the probability of viral transmission from the mosquito (human) to human (mosquito) per bite, bH (bM). They can be expressed as: λH = (NM a bH IM/NM)/NH = mabH IM/NM = βH IM/NM (2-1) λM = (NM abM IH/NH)/NM = abH IH/NH - 14 - = βM IH/NH (2-2) Thus, the equivalent total transmission rate as used in SIR model from vector to the human population is βH= mabH and from human to vector is βM= abM where m = NM/NH is the vector (female mosquito) to human population ratio or the number of female mosquitoes per person. Based on the flow diagram, the relevant mathematical equations are those related to infected human and vector populations, although the other equations are also important in order to find solutions to the whole systems: dIH/dt = λHSH – γH IH – (µH+ αH) IH = λHSH – (γH + µH+ αH) IH (2-3) dIM/dt = λMSM – µM IM (2-4) NH = S H + IH (2-5) NM = S M + IM (2-6) For an invasion of infection to take place, both infected humans and vectors must increase. Humans: dIH/dt > 0, or λHSH – (γH + µH+ αH) IH > 0; (2-7a) Vectors: (2-8a) dIM/dt > 0, or λMSM – µM IM > 0. Using Eqs. (2-1), (2-2), (2-5) & (2-6) and the conditions at the beginning of an epidemic: SH = NH, SM = NM, Eqs. (2-7a) & (2-8a) can be rewritten as: Humans: mabH (IM/NM) NH > (γH + µH+ αH) IH; or mabH (IM/NM) > (γH + µH+ αH) IH/ NH. Vectors: (2-7b) abM (IH/NH) NM > µM IM ; or IH/NH > µM IM / (abMNM). (2-8b) Combining Eqs. (2-7b) & (2-8b), we have the condition: mabH (IM/NM) > (γH + µH+ αH) µM IM / (abMNM); or ma > (γH + µH+ αH) µM / (abHbM); (2-9) ma is the mosquito biting rate per person which must meet the condition shown in Eq. (2-9) in order for an epidemic to grow. - 15 - For an infection to stop, the number of both infected humans and vectors must decrease. So the threshold conditions for epidemic to take place are when infected humans and vectors reach constant values, or in mathematical forms: dIH/dt = 0, & dIM/dt = 0. The threshold condition can be rewritten as: ma = (γH + µH+ αH) µM / (abHbM); or mabH abM/[(γH + µH+ αH) µM] = 1. For dengue, µH (~10-5/day) & αH (~10-3/day) are normally small and negligible relative to γH (~10-1/day) (Massad et al., 2011). This threshold condition defines the basic reproduction number R0 as R0 = ma2bHbM/(γH µM) (2-10a) This is the relation obtained by Macdonald in his classical paper (Massad et al., 2011) on malaria, except that the parasite latency was not incorporated here. To incorporate latency, let n represents the pathogen (virus for dengue or parasite for malaria) extrinsic incubation period (EIP) in days, which is the time for the vector between being infected to becoming infective to the vertebrate host. In the experiments, it is usually measured from the time that the mosquitoes ingested the infected human blood to the time that the virus is found in its salivary gland or in legs and other body tissues as proxy. Also let p be the vector’s daily survival probability. Assuming that the vector’s probability of daily survival decreases exponentially with time, in one day p = e-µM, or µM = - ln (p). (2-11) Here the per capita mortality rate of mosquito µM is measured in unit of day-1. The exponent in p is µM (1/day) · 1 day = µM, so that there is no unit. The same goes in the expression of µM = - ln (p) where p has no unit and implicitly µM = µM (day-1) · Time (=1 day) using day-1 as the unit for µM. Thus, the probability of surviving the whole latency period of n days is, pn = e-µM·n. This is the fraction of susceptible mosquitoes that will survive the extrinsic incubation period (n days) and become infective. Using pnSM to replace SM in Eq. (2-4) and solving for the threshold condition as it described above, the only thing changes in Eq. (2-10a and 10b) is to replace bH by bH pn or bH e-µM·n. Using expression for p and µM in Eq. (2-11), Eq. (2-10a) can be rewritten as R0 = mabHe-µM·n/µM · abM/γH = mabHpn/[- ln (p)] · abM/γH - 16 - = ma2bHbMpn/[- γH ln (p)] We can divide R0 further as consisting of two parts, one from vector to human population and one from human to the vector population. Using the same expression R0 = β/γ from Eq. (18a) as in SIR model – the transmission rate β multiplies the infectious period (1/γ) of the infectee, taking into account of latency R0 can be rewritten as: R0 = R0 M->H · R0 H->M = βH e-µM·n /µM · (βM /γH) = mabHe-µM·n/µM · (abM/γH), or = mabHpn/[- ln (p)] · (abM/γH). (2-10b) Here R0 M->H = βHe-µM·n/µM = mabHe-µM·n/µM = mabHpn/[- ln (p)], and R0 H->M = βM/γH = abM/γH. As discussed in Section 1, the basic reproduction number R0 represents the number of secondary cases in the first generation produced by one primary case during his/her entire infectious period. This applies to not only humans but also vectors. For vectors, it is during the vector’s lifetime (1/µM) since we have assumed that the vector stays infectious for life once it is infected and past incubation time. For humans, the infectious period is 1/γH. Therefore, R0 M->H represents the number of humans infected by one infectious mosquito during its lifetime after being introduced to an entirely susceptible human population. Similarly, R0 H->M represents the number of infected mosquitoes produced by one infectious human during his/her infectious period after being introduced to fully susceptible vector population. Since there is no recovery in mosquitoes, the vector population are assumed to be susceptible. Combining these two parts, the basic reproduction number means that the number of new human cases generated by one infective human during his/her infectious time (about 10 days for dengue) after being introduced to a fully susceptible human population, through vectors who have survived incubation time after being infected by the infective human. As before, R0 > 1 is the condition for an epidemic to take place. This condition does not require either R0 M->H or R0 H->M to be larger than 1 but the product of them. Thus, if the transmission rate from human to vector (βM) is low or the recovery rate (γH) is high so that R0H->M < 1, the epidemic can still take place if the transmission rate from vector to human (βH) is high due to a large vector to human ratio or a low vector mortality rate so that R0 M->H >1. Another quantity called Vectorial Capacity, V, is often used to characterize the vector’s ability in transmission disease. It is defined as V = R0·γH = R0 M->H ·R0 H->M · γH - 17 - = ma2bHbM e- µM·n /µM, or = ma2bHbMpn/[-ln (p)] (2-12) From this equation, we may say that only the second part of R0, R0 H->M, is averaged by the duration of infectiousness of the infected person – D=1/γH , that is, R0 H->M/D. The first part is intact, not averaged over vector’s lifetime. This means that V represents the number of infected vectors per unit time, e.g. a day, when an infected person is introduced to a susceptible vector population multiplies the number of new cases in humans produced during each infected vector’s life time after being introduced to a fully susceptible human population. In other words, the vectorial capacity is the average new cases generated per unit time by one infected case introduced in a fully susceptible population during his/her infectious period. As suggested by Garrett-Jones & Grab (1964, p83) using day as the unit of time, the vectorial capacity represents the average daily number of secondary cases generated by one primary case introduced in a fully susceptible host. Hence, he called the term "vectorial capacity” the "daily reproduction rate, that is, the daily fraction of the basic reproduction rate.” Here the basic reproduction rate refers to the basic reproduction number R0 used in this thesis. Thus, the vectorial capacity can be called also the basic daily reproduction number. From Eq. (2-10b), it is possible to estimate the critical density of female mosquitoes (number of female mosquito per host), m*, below which the disease will naturally disappear by setting R0 = 1: m* = - γH ln (p) /a2bHbMpn (2-13) 2.3 Effects of Temperature in Dengue – Seasonality 2.3.1 Sinusoidal Variation of Transmission Rate Temperature affects the behavior of vectors: its population NM, biting rate a, biting capacity – bV and bH, incubation time n, daily survival probability p or mortality rate µM, and eggs hatching rate, etc. Thus, temperature affects the basic reproduction number or the vectorial capacity. The temperature effect has been seen in the dengue cases where they increase during summer and disappear during winter. As climate changes over the last few decades, the ambient temperature increase has possibly contributed to the drastic increase of the dengue cases, such as in Singapore – a more than 10-fold increase between 1989 and 2005 (Massad et al., 2011). Thus, both average temperature and its variation are important factors to be taken into account in modeling. A simple way to incorporate the seasonal change of temperature may be to reconsider the SIR model of section 1. The temperature effect on vector’s behavior may be simplified and reflected on the disease transmission rate, β(t), as a time dependent sinusoidal function with a period of one year (Coutinho et al., 2006). β(t) = β[(T − A sin(2π f t + φ)] · θ[T − A sin(2π f t + φ)], - 18 - (2-14) where T, A, f, and φ are constant parameters to represent the temperature with varying amplitude A around a constant temperature T, and the frequency f which is 1/365 days-1 and phase φ to set initial transmission rate. θ(x) is the Heaviside function with a value of 1 when x is positive and 0 when x is negative, to guarantee that β(t) stays positive. The threshold condition (Re=1) as expressed by the effective reproduction number, Re, in Eq. (1-7) is now also a periodic function of time, Re(t) = β(t)S(t)/(N(t)γ) As R(t) > 1, the number of infected individuals (I) increases after a time delay. As Re(t) < 1, the number of infected individuals decrease. Thus, as Re(t) varies periodically from below 1 to over 1 within one year period, the infected individuals also varies periodically as shown in Fig. 4 based on Eqs. (1-6) and (2-14), a simulation done by Coutinho et al. (2006). In addition, the maxima of I increase as the average (over one year) Re-avg(t) > 1 for the first 11 years and decrease as the average Re-avg(t) < 1 after that. Fig. 4. Simulation of a system with sinusoidal transmission rate (Coutinho et al., 2006). The time unit is in days. 2.3.2 A Modified SIR model – Humans, Vectors and Eggs A more advanced Ross-Macdonald model to incorporate seasonal temperature variation is given by Massad et al. (2011) & Coutinho et al. (2006). In this model, they have considered three components in the dengue transmission process: humans, mosquitoes and their eggs which include other intermediate stages like larvae and pupae. It is the mosquito eggs who survive the winter. This component is necessary in the model in order to explain the observed dengue phenomena: overwintering without assuming unreasonably high biting rate (Coutinho et al., 2006). The flow diagram is shown below which is created based on the mathematical equations given by Massad et al. (2011) & Coutinho et al. (2006). The relevant equations are those describing the changes of Infected humans (IH), Infected female mosquitoes (IM), Infected eggs (IE), and latent mosquitoes (LM), although to solve the equations, a full set of 9 differential equations are needed – one for each compartment and - 19 - one for the total human population which is not constant (Coutinho et al. (2006; Massad et al., 2011). dIH/dt = λHSH – (γH + µH+ αH) IH (2-15) dLM/dt = λMSM – e - µM·τ λM(t-τ)SM(t-τ) – µM IM (2-16) dIM/dt = e - µM·τ λM(t-τ)SM(t-τ) – µM IM + pI ·FI t) (2-17) dIE/dt = grM IM – µE IE – pI ·FI(t) (2-18) death, µH, αH death, µH Human Infection λH SH vector to human infection βH=mabH Infection e IM - µM·τ λM(t-τ) IH Recovery γH Infection λM Latent LM lay egg rM Mosquitoes µM lay egg grM Eggs egg hatch pI·FI (t) IE µM SE (1-g)rM death, µE death, µH RH human to vector infection βM=abM SM µM egg hatch ps·Fs t) death, µE Fig. 5 Flow diagram of dengue infection including humans, mosquitoes and eggs. Here λH = abH IM/NH (= mabH IM/NM) and λM = abH IH/NH are the same as in Eqs. (2-1) & (22). τ is the Latent period (n) which is used to express the time delay from infection to transmission. Term e- µM·τ expresses the fraction of mosquitoes that survive the latency period. pI is the per capita egg hatch rate, and FI(t) = [(T − A sin(2π f t + φ)] · θ[T − A sin(2π f t + φ)]. (2-19) FI(t) is the seasonal variation in mosquito production from infected eggs. g is the proportion of infected eggs laid by infected female mosquitoes. rM is the oviposition rate of eggs which depends on the density of eggs due to limited breading places: rM = rM0 [1- (SE + IE)/kE] (2-20) Here, rM0 and kE are constants. - 20 - As shown by Massad et al. (2011), to solve the approximated threshold condition we can assume that some disease is introduced at time = 0 and then ‘freeze’ the system at time t. The effect of a small amount of infected cases introduced on the stability of the frozen equilibrium is investigated - solving the four differential equations (2-15) to (2-18). The threshold condition is obtained if the effective reproduction number meets the criterion: Re(t) = abM abH SM(t − τ)·exp (−μMτ)·SH(t)/[(γH + αH + μH) μM NH(t − τ)·NH(t)] + pI ·FI(t) g rM/ [μM· (μE + FI(t))] (2-21) If we look at the initial condition (t=0) for a whole susceptible population: SM(t − τ) =NM, SH(t) = NH, αH & μH << γH, the first term is the same as the basic reproduction number R 0 expressed in Eq. (2-10b). The second term takes into account the effect of seasonal variation in temperature on the mosquito behavior through the egg population. Thus, when Re(t) > 1, the system is unstable and the epidemic takes off. The basic reproduction number alone (the first term) is not sufficient in determining the threshold of epidemic after a small amount of disease is introduced at time t = 0. With this seasonal dependent reproduction number Re(t), Coutinho et al. (2006) has successfully explained the dengue overwintering and the observed delay of a few months between mosquitoes population density and the peak in dengue cases. Their simulation showed that the vertical transmission through eggs is more likely responsible for annual returning sustained transmission than particularly long-lived female mosquitoes for overwintering (and dry season). This model is useful and relevant to our interest in dengue suitability in Europe where the Aedes albopictus mosquitoes is the main concern for its survival ability in cooler European climate and its high vertical virus transmission probability through eggs (Shroyer, 1990). Here the work presented for dengue has taken into account only seasonal changes of temperature. The seasonal temperature effect is accounted through the change of egg population only. All other adult mosquito behavior parameters in Re have been assumed constant in this model by Coutinho et al. (2006) & Massad et al. (2011). 2.4 Effects of Temperature in Dengue – Daily Fluctuation Recent empirical study by Lambrechts et al. (2011) on Aedes aegypti mosquito showed that daily temperature variation also had strong effect on the mosquito’s behavior. They did both experiments and computer simulations using thermodynamic modeling. The dependence of diurnal temperature range - DTR or daily temperature variation amplitude - on four parameters were examinated: the probability of infection from human to vector - bM, the probability of transmission from vector to human - bH, Extrisic Incubation Period (EIP) - n, and the survival probability, Sv(t) which is related to the vector mortality rate - µM. In their laboratory experiments, Lambrechts et al. (2011) have varied the daily temperature around an average of 26 0C with 3 amplitudes (diurnal temperature range DTR): 0, 10 and 20 0C where a sinusoidal rising and exponential decay are used to imitate observed daily temperature fluctuation (12 hours above mean and 12 hours below mean temperature) in Thailand during high (DTR=10 0C, summer) and low (DTR=20 0C, winter) - 21 - season of dengue. After feeding the female mosquitoes with infected blood from Thailand of virus DENV-1 and DENV-2 separately, they measured bM by checking the prevalence of midgut infection of the vector, and viral dissemination from midgut to other body tissue which is equivalent to bH, Sv(t) the probability of vector survival by counting how many died every 1-3 days, and n the pathogen EIP duration by the time required for viral dissemination from the midgut to other tissues. Their results showed that the average infected female mosquitoes over the entire time course (32 days) were 97.1, 94.9 and 78.9% for DENV-2 under DTRs of 0, 10 and 20 0C, and 97.0 and 88.4% for DENV-1 under DTRs of 0 and 20 0C. Increasing DTR reduces the likelihood that a female became infected (bM). This finding is confirmed by their simulation using thermodynamic model to investigate the same effect (bM) over a wider range of temperature (mean temperature 14 ≤ T ≤ 28 0C and 0 ≤ DTR ≤ 22 0C). They also found that at a mean temperature lower than about 19 0C, large DTRs increase infection probability, while in environments higher than 19 0C, large DTRs reduce infection probability. From the 3-d map of the dependence of bM on T and DTR, I have extrapolated bM values for two mean temperatures: T= 14 and 26 0C which is shown in Figure 6. Fig. 6. Vector infection and transmission probabilities as a function of DTR based on simulation result (Lambrechts et al., 2011). The same simulation on the transmission probability (related to bH) has shown a stronger nonlinear response to temperature than infection probability (bM). Similar to infection probability, the probability of transmission showed also sign reverse depending on the mean temperature. At a mean temperature lower than 18 0C, the large the DTR is, the more increased the transmission probability is; while above 18 0C, the large the DTR is, the more reduced the transmission probability is. At mean temperature T=14 °C, a 2.7-fold increase (from 0.11 to 0.297) is found for transmission probability (bH) and 2.4-fold increase is found for infection probability (bM) as DTR increases from 0 to 20 °C. However, at T=26 °C, the temperature fluctuation DTR is more dramatic to bH than to bM: as DTR increases from 0 to 20 °C, a 2.1-fold decrease is found for transmission probability (bH, from 0.95 to 0.45) and only 1.3-fold decrease for infection probability (bM). Figure 6 shows the simulation results for two mean temperatures discussed here. However, this DTR dependence of bH was not found in their experiment from the measurement of the prevalence of dissemination (bH). Here bH is the probability of infection to human per bite. The experiment measures the prevalence of dissemination which is related but not exactly the same as bH. This may account for the differences between measurement and simulation. - 22 - In their experiments on survival analysis, a significant difference was observed of DTR on overall survival probability, Sv, although mean survival time (27.3-27.9 days) was similar across different DTRs for DENV-2. At the end of experiments (32 days), 30% females under DTR of 20 °C survived compared to 50 and 70% at DTR of 10 and 0 °C for DENV-2. Whereas for DENV-1 virus, about 10% vs. 60% survived in the end at DTR of 20 and 0 °C, and the mean survival times were different and corresponded to 22.2 and 28.5 days. No effect of DTRs on EIP or n, was observed in their experiment. From their simulation a decreasing function of n on mean temperature is found which is supported by other studies (Gubler, 1998). - 23 - Results 1. Combined Effects of Temperature on Dengue Relative Vectorial Capacity So far, we have seen a description of two Ross-Macdonald mathematical models (SIS and modified SIR) on dengue transmission process, and two examples of temperature effects – seasonal and daily fluctuation - on reproduction number and on some of the dengue vector parameters. In each of these two examples the model is limited since each considered part of the temperature effect and neglected the other part. This section will focus on a combined effect of temperature on dengue suitability by using the results of mathematical modeling presented in Methods Section 2.2 & 2.4 and connecting the data from empirical studies both in Section 2.4 and other literature. Without using a computer program to simulate the temperature (seasonality) change on dengue through mosquito’s egg hatching behavior, the focus here is on how each parameter depends on temperature and their effect on the dengue transmission. The seasonality can be viewed as being built in the effect of temperature on adult female mosquito’s parameters in both its mean value – low in winter and high in summer, and in daily fluctuation range which has shown large in winters and small in summers in sub-tropical regions, such as, Thailand (Lambrechts et al., 2011). To predict the start of an epidemic, the vectorial capacity, V, is often used for vector-borne infections instead of the basic reproduction number R0. They differ by the human recovery rate from the infection that is normally temperature independent. Thus, vectorial capacity depends on temperature the same way as the basic reproduction number but involving only vector parameters. As expressed in Eq. (2-12), vectorial capacity depends on, m, the vector to human population ratio, or the number of female mosquitoes per person, which varies from location to location. To get a general idea without limiting to a specific location, the relative vectorial capacity, V/m, will be used in the estimation of dengue risk based on temperature. The expression of Eq. (2-12) can be rewritten as: V/m = a2bHbM e-µM·n/µM (2-22) In principle, every parameter here depends on temperature and particular species of mosquito and virus. The mostly studied dengue mosquito is Aedes aegypti whose data will be used here, although ideally both dengue mosquitoes, Aedes aegypti and Aedes albopictus, need to be parameterized and modeled. There is no distinction between different viruses due to lacking of data. 1.1 Effect of Mean Temperature on Dengue Relative Vectorial Capacity Through literature search, I have found and listed below the dependence of all five vector parameters, a, bH, bM, n, and µM on temperature for Aedes aegypti mosquito, where T is either mean or ambient temperature depending on studies. 1) Biting rate a - 24 - According to experimental study by Scott et al. (2000) in Thailand, the blood feeding frequency of female Aedes aegypti collected weekly in Thailand (1990-1992) showed the following linear regression fitting: a = 0.03T + 0.66. (2-23) The blood feeding increases significantly (p=0.05) with temperature for the range 21 °C ≤ T ≤ 32 0C measured and the unit is 1/week. From this relation, the average daily biting rate a (dividing the equation by 7) increases slowly with T at the values from 0.18/day at T = 21 °C to 0.23/day at T = 32 0C. They showed many measurement data. In addition, different locations, e.g. Brazil, showed different linear fitting – independent of temperature. Given this fact and the variation of a on temperature is small across the measured range, we may extend the relation to lower temperature. For example, at T = 12.4 and 14 °C, a is calculated to be 0.147 and 0.154/day based on Eq. (2-23), where the values of a at these low temperatures are not very different from the experiment data observed on higher temperatures, e.g. 0.18/day at T = 21 °C. 2) The probability of infection from human to vector per bite - bM Based on empirical data from many studies in the range of 12.4 °C ≤ T ≤ 32.5 °C, Lambrechts et al. (2011) modeled both infection and transmission probabilities and found the following relation from fitting the data: in the range of 12.4 °C ≤ T ≤ 26.1 °C, bM increases linearly with T as bM =0.0729T −0.9037 (2-24) until it reached one and then remained equal to 1 for 26.1 < T < 32.5 °C. The empirical data that they used were chosen from different studies that measured the highest proportion of infected and transmitting vectors at various constant temperatures and for the flaviviruses used: West Nile virus, Murray Valley encephalitis virus, and St. Louis encephalitis virus. 3) The probability of infection from vector to human per bite - bH From the same study as above, Lambrechts et al. (2011) modeled and found a nonlinear relation of bH on T: bH increases almost linearly with T for 12.4 ≤ T < 26 0C, decreases sharply when T> 28 0C and to zero at T =32.5 0C. The fitting relationship between bH and T is described by the following equation: bH = 0.001044T (T – 12.286) (32.461 - T)1/2. (2-25) 4) Extrinsic incubation period - n Time required by DENV and many other mosquito-borne viruses to complete extrinsic incubation in the vector is temperature sensitive (Watts et al., 1987). Using an enzyme kinetics model to fit experimental data, Focks et al. (1995) showed a decreasing relationship: from about 40 to 5.4 days as T increases from 12 to 36 °C. The simulation is based on - 25 - experimental data range from 12 ≤ T ≤ 35 °C. Since there is no analytical equation given, I used an exponential model to fit their data and obtained the following relation: n = 4+e(5.15-0.123T). (2-26) Since the temperature range is less than one order of magnitude, the fitting relation Eq. (226) with 3 constants is not unique. Other relations may work also, such as, polynomial. The exponential function was chosen because it has been used in other modeling for n in malaria mosquitoes (Massad et al., 2011). Within the range of temperature used in this estimation it is likely not so affected by the model assumptions, 5) Mortality rate - µM The per capita female Aedes aegypti mosquito’s mortality rate (e.g. the incidence rate) was studied by Yang et al. (2009). Their experimental data measured in the temperature range of 10.54 °C ≤ T ≤ 33.4 °C showed that the mortality rate µM ranged from 0.027 to 0.092/day. The mortality rate is the highest at both low (T < 14 °C) and high temperature (T > 32 °C) and remains nearly constant in between. The lowest mortality rate 0.27/day occurs at T = 27.6 °C. To fit the data, they found a 4th order polynomial function: µM = 0.8692 – 0.1590 T + 0.01116 T2 – 3.408x10-4 T3 + 3.809x10-6 T4. (2-27) When putting all the analytical relations in the spread sheet, the dependence of the five parameters mentioned above on temperature is calculated. Their dependence on mean or ambient temperature is shown in Figure 7. Fig. 7. Vector parameters for Aegypti as a function of mean temperature. (Notice, different scales are used to bring all the parameters under one graph.) - 26 - From these data input, the relative vectorial capacity, V/m, is then calculated using Eq. (222). The result of V/m as a function mean or ambient temperature is shown in Fig. 8. A nonlinear relation is observed. When temperature is in the range 12.4 ≤ T < 28 0C, a monotonic increasing dependence on temperature is observed and when T> 28 0C up to 32.5 0C, a sharp decreasing relation is found. Thus, at both high and low ends of the mean temperatures, the vector Aedes aegypti mosquito’s dengue transmission capability is reduced. While around 28-30 0C, the relative vectorial capacity reaches its peak. Thus, 28-30 0C is the optimal mean temperature for Aedes aegypti mosquito to transmit dengue. At temperature T = 14 0C, the vectorial capacity is reduced by 770 times from its peak value around 28-30 0C. As mean temperatures reduces under 26 0C, all the five vector parameters contribute to the reduction of the relative vector capacity; while at high mean temperatures (over 30 0C), only two vector parameters contribute to the reduction of the relative vector capacity - the mosquitoes’ mortality rate and the probability of transmission from vector to human. 1.4 V/m (1/days) 1.2 1 0.8 0.6 0.4 0.2 0 12 16 20 24 T (ºC) 28 32 Fig. 8. Relative vectorial capacity as a function of mean temperature. 1.2 Effect of Daily Temperature Variation on Dengue Relative Vectorial Capacity The temperature results in Fig. 8 were obtained without considering the daily temperature variation. Here, we will incorporate diurnal temperature range – DTR in the vectorial capacity calculation. However, the work in this area on vector parameters is scarce and the work by Lambrechts et al. (2011) seems the only available study of dengue on DTR. As shown in Methods section, Lambrechts et al., (2011) found in their experiments and at T = 26 0C that DTR had strong influence on the two vector parameters: the probability of infection per bite to vectors (bM) and survival probability Sv(t) which is related to mortality rate – see below. In addition, they have simulated two parameters, bH and bM, as a function of mean temperature and DTR in a 2-d map from 14 0C ≤ T ≤ 28 0C & 0 0C ≤ DTR ≤ 22 0C. Using their simulation map, the dependence of bM and bH on DTR can be extrapolated for each mean temperature. Two of the DTR dependent relations at mean temperatures of 14 and 26 0C were shown in Figure 6. These data will be used as examples to show the DTR influence on V/m at these two mean temperatures. - 27 - In the measurement of DTR dependence on EIP– n, Lambrechts et al. found no variation over DTR from 0 to 20 0C. From temperature dependent relations Eq. (2-26), the extrinsic incubation period (n) is found to be 34 days at T=14 0C and 12 days at T=26 0C. This result – 12 days - is similar to their and others’ experimental findings of 8-14 days at T=26 - 30 0C (Gubler, 1998). In the survival analysis at T = 26 0C, as mentioned in Methods section 2.4, Lambrechts et al., (2011) found quite different values for the survival probability, Sv(t), at the end of experiment (duration of 32 days) depending on the DTRs and virus types, see Table 1. From this data, the daily average mortality rate, µM, can be estimated based on the definition through the following equation: µM = Ndead/(Ntotal*D) = (Ntotal - Nsurvival) /(Ntotal*D) = (1 - Sv)/D. (2-28) Here Ndead is the number of dead mosquitoes during the observation time - D = 32 days and Ntotal is the total mosquitoes observed where Ntotal = Ndead + Nsurvival. The survival probability, Sv = Nsurvival/Ntotal. For the two types of viruses studied, DENV-2 and DENV-1, the calculated vector daily mortality rates for Aedes aegypti mosquito at T = 26 0C are listed in Table 1. Table 1. Vector mortality rate (µM) and survival probability (Sv) measured for Aedes aegypti mosquito at mean temperature 0 T = 26 C and different diurnal temperature range (DTR) (Lambrechts et al., 2011). DENV-2 0 DENV-1 -1 -1 DTR ( C) Sv (%) µM (days ) Sv (%) µM (days ) 0 70 0.00938 60 0.0125 10 50 0.01563 20 30 0.02188 10 0.02813 Fig. 9. Vector mortality rate as a function of DTR for two dengue viruses based on data in Table 1. - 28 - As shown in Figure 9, the vector mortality rate increases linearly as the daily temperature fluctuation increases for both types of viruses. At mean temperature T = 26 0C, the larger the daily temperature fluctuates, the less likely the mosquito is going to survive. The value at highest DTR is very close to the experimental study by Yang et al. (2009), where the mortality rate is 0.03/day at T = 26 0C and 0.04/day at T = 14 0C as obtained from their fitting to the measured data for Aedes aegypti mosquitoes in Brazil. As mentioned in Result section 1.1, their range of measurement for mean temperature is from 10.5 to 33.4 0C at a constant DTR for each T. Specifically, a fixed two-step temperature per day is used to simulate natural photo period for day and night. For example, at mean temperature T = 15 0C, the daily temperature is 17.9 0C for 10 hours and 12.1 0C for 14 hours to imitate winter; at T = 25 0C, the daily temperature is 27.6 0C for 13 hours and 22.4 0C for 11 hours to imitate Spring/Fall. Thus, a constant value of µM = 0.04/day will be used for T = 14 0C to calculate V/m. Due to lacking of data, a constant average daily biting rate a - independent of DTR - will be used. From temperature dependent relations Eq. (2-23), the value of a is 0.21/day at 26 0C and is 0.15/day at T=14 0C assuming that it is still valid at this temperature (Scott et al., 2000). These values are similar to that used by Massad et al. (2012) for dengue in Thailand 0.164/day, but less than that for dengue in Singapore – 1.2/day (Massad et al., 2011). 0.014 4 0.012 3.5 3 0.01 T = 14 ºC V/m (1/day) V/m (1/day) When putting all the values of the parameters mentioned here in Eq. (2-22) where mortality rate for DENV-2 is used at T = 26 0C, V/m is calculated as a function of daily temperature fluctuation – diurnal temperature range (DTR). At mean temperature T = 14 and 26 0C, the results of V/m are shown in Figure 10. 0.008 0.006 0.004 T = 26 ºC 2.5 2 1.5 1 0.002 0.5 0 0 0 5 10 15 Daily temperature variation DTR (ºC) 20 0 5 10 15 Daily temperature variation DTR (ºC) 0 20 0 Fig. 10. Relative vectorial capacity as a function of DTR at two mean temperatures, 14 C (left) and 26 C (right). A reversed dependence of relative vectorial capacity on DTR is observed in Figure 10 at the two mean temperatures. At T=14 0C, large daily temperature fluctuation amplitude, will enhance the vector’s ability to transmit dengue infection between humans. For example, at DTR=20 0C which corresponds to that temperature changes daily from 4 to 24 0C, 6.4 times increase is expected when comparing to a constant daily temperature of 14 0C. In contrast, at high temperature T=26 0C, the vector’s ability to transmit dengue decreases with the range of temperature fluctuation. For example, at DTR=20 0C which means that daily temperature changes from 16 to 36 0C, V/m decreases by a factor of 7.3 relative to the constant of 26 0C daily temperature. The exact reason for this temperature behavior is unclear. The suspected - 29 - reason may be “deleterious effects of low and/or high temperatures on key steps of the progression of virus infection in the mosquito (Lambrechts et al., 2011).” In addition, the ratio of V/m between the two mean temperatures (V/m at T=26 0C over V/m at T=14 0C) showed a strong dependence on DTR - from 1881 at DTR=0 0C to 40 at DTR=20 0C. Thus, in a place with mild summer (T=14 0C) but large daily temperature variation such as in Sweden, the dengue transmission may not be as small as it would be if the daily temperature variation is small. At T=26 0C the value of relative vectorial capacity obtained in Fig. 8 without considering temperature fluctuations specifically is V/m = 0.95/day, which is a factor of 1.8 greater than that at DTR=20 0C and 4 times less than that at DTR=0 0C. It corresponds to the DTR around 14 0C. 2. Sensitivity Analysis of Vector Parameters on Dengue Vectorial Capacity Since different values have been used for Dengue in the literature for the same parameter in calculating vectorial capacity (V) or basic reproduction number (R0), it would be interesting to see how much those values affect the result. Thus, a sensitivity analysis is performed here and illustrated by some commonly used values for the parameters. The sensitivity of V/m can be shown mathematically through partial derivative, ∂(V/m)/∂x to each parameter x, in Eq. (2-22), assuming that other parameters are constant (Massad et al., 2011). Let V*= V/m to simplify the expression. ∂V*/∂a = 2abHbM e-µM·n/µM = 2V*/a (2-29a) ∂V*/∂(bH) = a2bMe-µM·n/µM = V*/bH (2-30a) ∂V*/∂(bM) = a2bHe-µM·n/µM = V*/bM (2-31a) ∂V*/∂n = - µM a2bHbM e-µM·n/µM = - µM V* (2-32a) ∂V*/∂µM = - n a2bHbM e-µM·n/µM - a2bHbM e-µM·n/µM2 = - (n+µM-1)V* (2-33a) From these equations, it is clear that parameters a, bH, bM affect V* positively, n and µM affect V* negatively. In other words, as either a, bH or bM increases, V* increases as well. However, as either n or µM increases, V* decreases as the negative signs in Eqs. (2-32a & 33a) show. Here, the magnitude of change in V* relative to the change of each parameter can be compared by re-arranging Eqs. (2-29a) to (2-33a): ∂V*/V* (relative to a) = 2 ∂a/a (2-29b) ∂V*/V* (relative to bH) = ∂bH/bH (2-30b) ∂V*/V* (relative to bM) = ∂bM/bM (2-31b) - 30 - ∂V*/V* (relative to n) = - µM ∂n = - µMn ∂n/n (2-32b) ∂V*/V* (relative to µM) = - (n+µM-1) ∂µM = - (µMn +1) ∂µM/µM (2-33b) If the relative change of each parameter ∂x/x is 1%, the effect on V* - the relative change of vectorial capacity, ∂V*/V* (= ∂V/V), would be those values as shown in Table 2. Table 2. Comparison of relative change in vectorial capacity V* (= V/m) as each vector parameter varies 1%. Relative change of parameter ∂a/a = 1% ∂b /b = 1% ∂b /b = 1% ∂n/n = 1% ∂µM/µM = 1% ∂V*/V* (%) 2 1 1 - µM n -(µMn +1) H H M M Since n is on the order of 10 or more, µM n >1 if µM is larger than 0.1/day. However, for the values used for plotting Figure 8 & 10 (Table 1), µM n < 1. Thus, the magnitude of relative change in V* is the largest when the biting rate a changes. In other words, the biting rate a is the most sensitive parameter in determining vectorial capacity providing µM n < 1. The next sensitive parameter is the mortality rate µM. The least sensitive parameter is the EIP – n. This ranking is based on the theoretical assumption that each parameter can change relatively the same amount and µM n < 1. However, in reality the range of change for each parameter varies and this range is just as important as the theoretical analysis. Let us see some of the examples. 1) Daily biting rate a Figure 8 & 10 used the calculated values of vector parameters based on temperature relation obtained from experimental study (Scott et al., 2000). If varying a from 0.206/day – the value measured by Scott et al. (2000) at T=26 0C - to 0.164/day as used by Massad et al. (2012) for dengue study in Thailand – 1.25-fold reduction in a, the V/m decreases by a factor of 1.57 throughout the whole DTR range. The same way of varying a at T=14 0C from 0.154 to 0.164/day – 1.06-fold increase in a, V/m increases 1.13 times for the whole DTR range. A square depending relation is confirmed. Thus, an accurate measurement of a is important in principle but not so critical in reality on the value of vectorial capacity due to both the its less sensitive dependence on temperature and the relative small range of value used in the different modeling of dengue. 2) Extrinsic Incubation Period – n Different values of the extrinsic incubation period (EIP) n for dengue vector has been reported and used in modeling in the literature based on different studies. Although the general reported values are between 8-14 days for mean temperature T= 30 to 26 0C, n = 7 days has been used also for dengue study in Singapore (Massad et al., 2011), where the mean temperature is within this range – from 27.3 to 28.1 0C during 1989 to 2005. These range of values will be used here to show the comparison of n= 7 - 14 days on V/m at mean temperature of 26 0C. n= 12 days is the one used in Figure 8 & 10. As n increases by a factor - 31 - of 2, V/m decreases from 7% to 17% depending on the value of DTR. As expected from theoretical analysis shown in Table 2, the relative change of n has little effect on relative change of V* since a small mortality rate (see Table 1) is used in this case. However, if a large mortality rate, such as, µM = 0.263/day (instead of 0.01 - 0.02/day as used in Fig. 10) would be used as discussed in the next section, a relative larger effect of 6.3 time decrease on V* would result. However, this is still small considering a factor of 13-26 fold increase in µM. V/m (1/days) At T= 14 0C, the n used in Figure 8 & 10 is 34 days. If n varies 10 days in each direction – a factor of 1.8 time increase from the lowest to the highest value, the effect on V/m is a factor of 2.2 reductions as shown in Figure 11 where mortality rate is fixed at µM =0.04/day. The effect of n at T= 14 0C is much bigger than that at T= 26 0C. This is because T= 14 0C, µM n= 1.36 which is larger than 1 and much larger than the values of µM n = 0.12 – 0.24 at T= 26 0C (the variation is for the range of DTR). Thus, as expected from the theoretical analysis in Table 2, n is not a very sensitive parameter to the vectorial capacity at low µM. Its sensitivity increases as µM increases and needs to be considered as µM n > 1. 1 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 n=24 days n=34 days n=44 days 0 T = 14 ºC 5 10 15 Daily temperature variation DTR (ºC) 20 0 0 Fig.11. The effect of different Extrinsic Incubation Period n on relative vectorial capacity, V/m. Left: T= 14 C. Right: T= 26 C µM varies with DTR as shown in Table 1. 3) Mosquito mortality rate µM In the mathematical modeling studies, a constant value µM = 0.263/day were used by Massad et al. (2011; 2012) for dengue studies in both Thailand and Singapore. Thus, the value of µM=0.263/day will be used here to compare with the values used in Figure 10. Figure 12 shows the dependence of V/m on µM for the two mean temperatures. At T=14 0C, as the per capita vector mortality rate µM changes between the two constant values: from 0.04/day to 0.263/day – a factor of 6.6 times increase, about 13,000 - fold decrease is found in V/m! At T=26 0C, as µM changes from a range of values: 0.01 to 0.02/day (Table 1 DENV-2) to a constant value of 0.263/day – a factor of 26 to 13 times increase, V/m decrease by a factor from 588 to 217 times as DTR changes from 0 to 20 0C. Thus, the vector mortality rate is the most sensitive vector parameter affecting the value of vectorial capacity. This is because that V depends on µM exponentially. At small value of µM and n (e.g. at T= 26 0C), it is small change on V but at large value of µM and n, the effect is quite big as shown here at T=14 0C as well as illustrated in table 2. Thus, to identify the proper value of µM and its dependence on - 32 - temperature is the most important step to take for improving the accuracy of modeling on predicting the dengue risk. Fig. 12. The effect of different mortality rate on relative vectorial capacity. Varying µM means that it varies according to daily survival probability as shown in table 1. - 33 - Discussion We have seen in the Results section that the relative vectorial capacity, V/m, depends on temperature, both its mean value T and its daily fluctuation amplitude – DTR through the five vector parameters as expressed in Eq. (2-22): V/m = a2bHbM e-µM·n/µM As mean temperature increases, the vectorial capacity increases slowly at low temperature and speeds up as temperatures approaches 26 0C. After reaching its peak around 28-30 0C, the vectorial capacity decreases sharply. The ratio of V/m at peak T = 28 0C relative to 12.40C is over 7 orders of magnitude. Thus, practically speaking, there is little dengue risk when T < 14 0C, based on the result of this study from combined temperature effect. As DTR varies, the calculated result showed that V/m increases with DTR at low mean temperature T=14 0C and decreases with DTR at high mean temperature T=26 0C. The value of V/m at T=26 0C is about 1881 times greater than that at T = 14 0C when there is no daily temperature fluctuation. However, at DTR = 20 0C this ratio is decreased to about 40. Thus, large daily temperature fluctuation increases the dengue transmission at low mean temperatures and reduces the gap between warmer and cooler region drastically, e.g. over 46 fold as DTR increases from 0 to 20 0C between two areas with mean temperatures of 14 and 26 0C. This result is important considering the ongoing climate change, which comes with large temperature fluctuation in additional to overall temperature increase. These two mean temperatures (14 and 26 0C) used in the example could describe two different seasons in one place, for example the spring or fall and summer in a city in Southern Europe, or two different places in the same season of summer, such as, one in Thailand and one in Europe. Thus, the cooler region may have higher risk than expected based on warmer region’s data, such as, Thailand where the typical daily temperature fluctuation is around 10 0C. This daily temperature fluctuation is not as large as in temperate region of Europe, such as, Sweden. This result also helps us to understand when a place in Europe may be at risk of dengue epidemics, e.g. the time of the year and how long this risk period potentially may be, according to the climate data. These results reflect the dengue vector, Aedes aegypti’s behavior in dengue transmission, given in the parameters measured for carrying certain type and amount of virus. These parameters depend on the biological condition and physical environment experienced in the experiment or data collection in the nature. However, dengue transmission seems also to depend on where the virus is from and which sub species are used as viruses also change over time (Hii, 2012). Thus, we need to be careful in generalizing this result to specific places. The local temperature and proper vector and pathogen data need to be used in the model. Sensitivity analysis indicates that not all the parameters affect vectorial capacity the same way. The most sensitive vector parameter is the mortality rate especially when it is larger than 0.1/day. Small variation in mortality rate makes a large difference in vectorial capacity, especially if combined with longer extrinsic incubation time, n. Vector biting rate is in - 34 - principle a sensitive parameter but in reality it seems not varying so much with temperature. The less sensitive parameter is extrinsic incubation period, n, especially its direct effect to relative vectorial capacity at high mean temperatures. However, it amplifies the effect of mortality rate through the vector’s survive probability of EIP, e-µM·n. At low temperatures where both vector’s mortality rate and EIP are large, their combined effect is large to reduce the relative vectorial capacity. Thus these three parameters need to be measured as accurately as possible and to be carefully checked before being used in the modeling. As we have seen, it is quite challenging to incorporate the temperature effect in dengue transmission either through the basic or effective reproduction number, or through the vectorial capacity. It is a balance between building a sophisticated model that accounts for the detailed vector behavior and using a simple model to give clear insight into the effect of each important factor. The two sub-sections (2.3 and 2.4) in Methods have shown the two ways of describing dengue suitability based on temperature. In the sophisticated model that takes into account of seasonality, the yearly temperature variation is incorporated as an separate term RE in the effective reproduction number, Re(t) = R1 + RE. Here RE describes the effect of seasonality through mosquito Egg’s population (see Eq. (221)), which is zero at low dengue season – winter, and non-zero at high dengue season summer. At t =0, R1 = R0 for a totally susceptible population and Re(t) = R1 = R0 when no egg hatching, such as in winter. Thus, the effective reproduction number Re will be used instead of R0 as the criterion for epidemic. This type of modeling is important since it is relevant to Europe’s temperate climate that egg overwinter is the most crucial survival mechanism for the mosquito population. The disadvantage of this model is that no simple insight can be drawn without computer simulation involved. In addition, it has assumed temperature independent parameters in R1. As we have seen in the Results section, every vector parameter in V/m depends on temperature for both its mean value T and its daily fluctuation amplitude, DTR. Thus, even this sophisticated model cannot take into account all the important factors about vector behavior. Also it relies on seasonality as to be similar in different places, while the seasonality of temperature between different climates can vary greatly. For example, some climate for example may have two transmission seasons per year. A more universal representation of seasonality may be based on the seasonality in observational temperature time series. In contrast, the simple SIS model in section 2.2 has illustrated the important quantity in describing vector-borne infection, vectorial capacity, V. Using this quantity and the parameters found through literature search, the result of dengue relative vectorial capacity based on temperature was calculated. In choosing the type of modeling, SIS was chosen although two types of models are both used for infectious diseases, SIR and SIR. SIR is used for the situation where only one type of dengue virus exists and where recovered becomes immune, and SIS is used for the situation where more than one type of virus coexists and where recovered from one type virus is still - 35 - susceptible to other types of viruses. In principle, each virus type should be considered separately since all the vector parameters are in principle different for each virus, therefore only SIR model may be used. However, since the data is limited for different viruses in dengue vector parameters, the model did not account for the detailed path difference between different viruses. In fact, in terms of initial threshold criterion for an epidemic to spread in a totally susceptible population, it does not matter which model is used – both leads to the same expression of vectorial capacity. However, different models will affect the susceptible and infected population dynamics, which leads to differences in the length and sustainability of epidemic. It affects directly the susceptible population and will in turn affect the effective reproduction number Re. Thus, SIR model used for one type of virus runs out of susceptible quickly and the epidemic dies out if no large birth or migration of people into the infected area to increase the susceptible population. On the other hand, if the dengue infection involves more than one type of viruses at the same time, SIS model allows resupply of the susceptible and makes possible a sustained epidemic or slow decrease of the susceptible population and correspondingly a slow decrease of the epidemic. Limitations of this study and suggestions The calculation of V/m over DTR – diurnal temperature range, depends on five vector parameters. At T= 26 0C, the DTR dependent vector data were found for four out of five parameters: bH , bM, n & µM, and no data on how a depends on DTR. At T= 14 0C, the data were found for only two out of the five parameters: bH and bM, and no data on the dependence of a, n and µM on DTR. Thus, V/m dependent on DTR is not perfect. In addition, at lower mean temperature, the daily biting rate a was extrapolated based on fitting to measured data on higher temperatures. Thus the results presented here can be improved if there is more data on parameters available. Therefore, this study suggests that a systematic review of dengue vector parameters is in great need to support modeling studies. The daily temperature fluctuation modeling used a thermodynamic model from theoretical biology. Due to time limit of this thesis, I could not manage to learn this model to incorporate the DTR dependence of parameters on all of the temperatures. If the DTR dependent values of all the vector parameters were available, it would be possible to produce a 3-D map for V/m as a function of T and DTR. All the results presented here are for mosquito Aedes aegypti. As mentioned earlier, the type that is threatening Europe is Aedes albopictus whose capacity in transmitting dengue is more important to be modeled. Since it is not the dominant mosquito in tropical and subtropical regions for dengue, there are less studies about it and less data available. Even though the few papers that I have found including Albopictus mosquito data showed that the mortality rate is very similar between these two types of mosquitoes, the overall effect of all the parameters on vectorial capacity is not necessarily the same between the two types of mosquitoes. Studies have shown that Aedes aegypti is more likely to be found in dry and warm places while Aedes albopictus are more likely to be found in cool and wet places (Juliano et al., 2002; Alto and Juliano, 2001). Furthermore, in transmission of viruses, Aedes aegypti mainly transmits through horizontal channel – human hosts, while Aedes albopictus does appear to transmit the dengue virus through both horizontal and vertical transmission. - 36 - Given the differences between the two dengue vectors, further study is needed to model more specifically dengue suitability of vector Aedes albopictus. Humidity and rainfall are important climate factors that have not been considered in all the mathematical models that I have encountered. The reason is that it is local effect and is hard to incorporate. Small places holding water such as old tires and waste containers are important breeding places for dengue vectors. Not like temperature, there is not general data on the local humidity and the relationship between the parameters and rainfall and humidity. As mentioned earlier, the vector parameters depend on virus types (Gubler et al., 1979) and dosage (Watts, 1987) and many geographic strains of dengue viruses exists. They can cause different reaction in people. For example, the South American dengue virus strain does not cause bleeding as the Asian virus strain does. It demands more understanding of the interaction between vector and virus, and complete data input in order to set up better mathematical models. Mosquito population depends on temperature as well and on local humidity and rainfall. Due to lack of data and analytical relation between the mosquito population and weather factors, it is not considered explicitly in the model. Regarding to the model itself, the seasonality is built in the temperature variations – both mean value T and DTR – of vector parameters. It can, in principle, be incorporated as a temperature dependent vector parameter in all the parameters in the expression of V, as it has been shown in the results section for two mean temperatures, T. Since it is the adult female mosquitoes that infect human, the effect of egg hatching is to account for the mosquito population changes: increase in the summer and decrease in the winter. Thus, seasonality can also be incorporated in the vector to human population ratio, m (= NM/NH), as a sinusoidal function of time in the basic reproduction number or in vectorial capacity, as part of the transmission rate as shown in Sec. 2.3.1. Thus, it is possible to use the simple expression of V as shown in Eq. (2-12) to calculate dengue vectorial capacity dependence on temperature without the need for a separate term to take into account of seasonality, at least in the dengue risk assessment of a fully susceptible population. This study has focused on part of the physical environment – two climate factors on dengue – mean temperature and diurnal temperature range. However, study has shown that dengue burden is not only the result of environmental (climate) factor but also socioeconomic and demographic factors. The fact that similar climate but across two country borders (Texas, USA and Mexico) has different dengue incidences indicates that other determinants are important also and need to be considered in modeling (Reiter et al., 2003). The social and political environment affect both the human’s cope ability through recovery rate to dengue and mosquito’s population through breading ground availability and human action on adult mosquito control. These factors, in principle, can be taken into account in both the recovery rate, γH, and the mosquito to human population ratio, m, for a specific population in the calculation of the basic reproduction number but not in the relative vectorial capacity. - 37 - Conclusion This thesis has reviewed the basic and some sophisticated theoretical frameworks in infectious disease modeling with focus on dengue mathematical modeling. Temperature effect on dengue transmission was explored from two different models – seasonal and daily variation. Dengue risk based on temperature is calculated through the quantity – relative vectorial capacity (vectorial capacity relative to vector to human population ratio, V/m), as a function of mean temperature and of diurnal temperature range at two different mean temperatures: 14 and 26 0C. This may represent two different seasons in one place or two different places in the same season. The result showed that 28-30 0C is the optimal mean temperature for dengue transmission. Daily temperature variation is a very important climate factor in dengue transmission. Increasing the daily temperature fluctuation reduces greatly the gap between cooler and warmer regions in dengue transmission. As global warming continues with increased temperature and extreme temperature variation, this result is important in considering dengue risk assessment – the spread and establishment in current non-transmission areas. From the Methods section, we have seen that different theoretical frameworks exist with different complexity and approximations. It is a challenge to incorporate temperature effects in dengue studies to give a meaningful prediction of epidemic threshold without losing insight in the jungle of complex mathematics. From the Results section, it is clear that knowing and choosing the right vector parameters are important, but challenging aspect of mathematical modeling. Here empirical studies from biology and zoology are essential. The generalizability of the model depends on the parameters used and assumptions made. From sensitivity analysis, the vector mortality rate or the daily survival probability is the most important vector parameter in affecting the value of vectorial capacity. Thus, its value needs to be chosen carefully in any modeling and to be measured accurately in any empirical study. This work has illustrated that a simple model can give important insight into the disease spreading. A systematic review of the vector parameters is in great need in modeling to increase its usefulness and generalizability in understanding, predicting and guiding disease control. To set up a good model for infectious disease in the face of climate change is quite a challenge. As an individual, it requires broad knowledge from mathematics, epidemiology, computer science, biology, meteorology to sociology. As a field, the challenges of mathematical modeling are in communicating changing assessments of risk in face of great uncertainty. Population is more mobile so that diseases spread faster and wider. This requires faster responses and understanding of potential transmission risk areas (Van Kerkhove et al., 2010). For dengue study specifically, the challenges lie on 1) better natural history and transmission model, 2) data on vector transmission and virus interaction, and 3) maintaining balance between simplicity that gives clear insight and complexity that captures the effect of climate change. - 38 - References Introduction 1. WHO (2003). Climate change and human health - risks and responses. Summary, ISBN 9241590815. http://www.who.int/globalchange/summary/en/index.html (Accessed 2012-03-27). Also see Intergovernmental Panel on Climate Change (IPCC). Climate Change 2001: Third Assessment Report (Volume I). Cambridge: Cambridge University Press, 2001. 2. WHO (2012). Dengue and severe dengue - Fact sheet N°117, January 2012. http://www.who.int/mediacentre/factsheets/fs117/en/# (Accessed 2012-03-27). 3. Heddini A, Janzon R, Linde A. (2009). Increased number of dengue cases in Swedish travellers to Thailand. Euro Surveill.;14(5):pii=19111. 4. ECDC Technical Report (2009), Development of Aedes albopictus risk maps Stockholm, May 2009. Available online: <http://www.ecdc.europa.eu/en/publications/ Publications/0905_TER_Development_of_Aedes_Albopictus_Risk_Maps.pdf> 5. Gething et al. (2011). Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P. vivax. Parasites & Vectors, 4:92. 6. Burattini et al. (2008). Modelling the control strategies against dengue in Singapore, Epidemiol. Infect. 136, 309–319. 7. Macdonald G. (1952). The analysis of equilibrium in malaria. Tropical Disease Bulletin; 49:813-28. 8. Diekmann O, Heesterbeek JP, Metz JAJ (1990). On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. Journal of Mathematical Biology; 28:365–82. 9. Lambrechts et al. (2011). Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. PNAS, 108 (18)7463. 10. Massad E, Coutinho FA, Lopez LF, da Silva DR. (2011). Modeling the impact of global warming on vector-borne infections, Physics of Life Reviews. Jun;8(2):169-99. 11. Annelies Wilder-Smith et al. (2012). DengueTools: innovative tools and strategies for the surveillance and control of dengue. Glob Health Action 5: 17273 . Methods 1 12. Horton (2011). “Prevention and Control of Chronic Diseases: A major global challenge for researchers, clinicians and policy makers!” public lecture in Umeå University, December 6, 2011. 13. Aron J. (2007) Chapter 6: Mathematical Modeling: the Dynamics of Infection. Infectious disease epidemiology: theory and practice. Nelson, K. and Williams, C. eds. Jones and Bartlett Publishers. 14. Kretzschmar M.and Wallinga J. (2010). Chapter 12 Mathematical Models in Infectious Disease Epidemiology. A. Krämer et al. (eds.), Modern Infectious Disease Epidemiology, 209-, Statistics for Biology and Health, Springer Science+Business Media, LLC. - 39 - 15. Dietz K, Heesterbeek JA (2000) Bernoulli was ahead of modern epidemiology. Nature; 408(6812):513–4 16. Hamer WH (1906). Epidemic disease in England. Lancet i:733–9. 17. Kermack WO. & McKendrick AG. (1927). A contribution to the mathematical theory of epidemics. Proc R Soc Lond A115:700–21. 18. Anderson RM & May RM. 1991. Infectious Diseases of Humans. Dynamics and Control. Oxford University Press, Oxford. 19. Wallinga J, Teunis P (2004) Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am J Epidemiol; 160(6):509–16. 20. Keeling M.J. (2005). Review - Models of foot-and-mouth disease. Proc. R. Soc. B 272, 1195–1202. 21. Choisy M., Guégan J.-F. and Rohani P. (2007). Chapter 22 Mathematical Modeling of Infectious Diseases Dynamics. Encyclopedia of Infectious Diseases: Modern Methodologies, by Tibayrenc M., John Wiley & Sons, Inc. P379-411. 22. Burnham KP, Anderson DR. (2002). Model Selection and Multimodel Inference: a Practical Information-theoretic Approach. Springer-Verlag, Berlin. 23. CDC (2012). History and Epidemiology of Global Smallpox Eradication. From the training course titled "Smallpox: Disease, Prevention, and Intervention", slides 16 & 17. www.bt.cdc.gov/agent/smallpox/training/overview. (Accessed 2012-04-26, 14:22). 24. Macdonald G. (1952). The analysis of equilibrium in malaria. Tropical Disease Bulletin 49:813–28. 25. Cintron-Arias A., Castillo-Chavez C., Bettencourt L., Lloyd A.L. and Banks H. T. (2009) The Estimation of The Effective Reproductive Number From Disease Outbreak Data. Mathematical Biosciences and Engineering; 6 (2), pp. 261–282. 26. Iannelli M. (2005). The Mathematical Modeling of Epidemics. Summer School on the Mathematical Models in Life Science: Theory and Simulation. Dobbiaco, Italy, July 1-5. 27. D´ebarre, Florence (2012). SIR models of epidemics, Level 1 module in Modelling course in population and evolutionary biology. (Accessed 2012-04-30, 15:14). http://www.tb.ethz.ch/education/model/SIR/sir.pdf Methods 2 28. Shroyer DA.(1990). Vertical maintenance of dengue-1 virus in sequential generations of Aedes albopictus. J Am Mosq Control Assoc. 6(2):312-4. 29. Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, et al. (2012). Ross, Macdonald, and a Theory for the Dynamics and Control of Mosquito-Transmitted Pathogens. PLoS Pathog 8(4): e1002588. 30. Garrett-Jones C & Grab B. (1964). The Assessment of Insecticidal Impact on the Malaria Mosquito's Vectorial Capacity, From Data on the Proportion of Parous Females. Bull World Health Organ. 31:71-86. 31. Coutinho FAB, Burattini MN, Lopez LF & Massad E (2006). Threshold conditions for an non-autonomous epidemic system describing the population dynamics of dengue. Bulletin of Mathematical Biology 68, 2263–2282. 32. Gubler DJ (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11: 480–496. - 40 - Results 33. Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A (1987). Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg 36:143–152. 34. Focks DA, Daniels E, Haile DG, Keesling JE (1995). A simulation model of the epidemiology of urban dengue fever: Literature analysis, model development, preliminary validation, and samples of simulation results. Am J Trop Med Hyg 53:489– 506. 35. Scott TW, et al. (2000) Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Blood feeding frequency. J Med Entomol 37:89–101. 36. Yang H.M., Macoris MLG., Galvani KC, Andrighetti M.T.M., and Wanderley D.M.V. (2009). Assessing the effects of temperature on the population of Aedes aegypti, the vector of dengue. Epidemiol. Infect. 137, 1188–1202. Discussion 37. Hii, Yien Ling (2012). Climate-based dengue early warning system, Middle term seminar at Dept. of Public Health and Internal Medicine, Epidemiology and global health, Umeå University, May 8. 38. Juliano SA, O’Meara GF, Morrill JR, and Cutwa MM (2002). Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes Oecologia. 130(3): 458– 469. 39. Alto, B.W. and Juliano S.A. (2001). J Precipitation and Temperature Effects on Populations of Aedes albopictus (Diptera: Culicidae): Implications for Range Expansion. Med Entomol. 38(5): 646–656. 40. Gubler D.J., Nalim S., Tan R., Saipan H., And Sulianti Saroso J. (1979). Variation In Susceptibility to Oral Infection with Dengue Viruses Among Geographic Strains of Aedes aegypti. Am. J. Trop. Med. Hyg. 28(6): l045 - l052. 41. Van Kerkhove MD, Asikainen T, Becker NG, Bjorge S, Desenclos J-C, et al. (2010) Studies Needed to Address Public Health Challenges of the 2009 H1N1 Influenza Pandemic: Insights from Modeling. PLoS Med 7(6): e1000275. Available online: <http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.10002 75> 42. Reiter P. et al. (2003). Texas Lifestyle Limits Transmission of Dengue Virus. Emerging Infectious Diseases, 9:86-89. - 41 - - 42 -