PC-3 ML - Cancer Research
advertisement

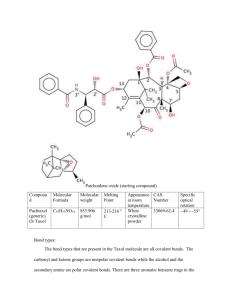
[CANCER RESEARCH 52, 3776-3781. July 1, 1992] Advances in Brief Taxol Blocks Processes Essential for Prostate Tumor Cell (PC-3 ML) Invasion and Métastases1 Mark E. Stearns2 and Min Wang Department of Pathology, Medical College of Pennsylvania, Philadelphia, Pennsylvania I9I29 Abstract We have examined the antimetastatic effects of taxol on a PC-3 human prostatic tumor variant (PC-3 ML) which metastasizes to the lumbar vertebrae in severe combined immunodeficiency-carrying (SCID) mice. Immunofluorescence labeling indicated that taxol (0.5 to 1.0 JUMfor 6 h) produced an abnormal bundling of microtubules in a dosage-dependent manner. Slot blotting and gelatinase assays revealed that taxol inhibited secretion of the M, 72,000 and M, 92,000 type IV collagenases plus a V/, 57,000 gelatinase. Radioimmunoprecipitation measurements con firmed that the drug inhibited both the secretion and the synthesis of the M, 72,000 collagenase. Taxol also blocked total protein secretion but did not influence total protein synthesis or turnover. Boyden chamber chemotactic studies further showed that taxol (0.5 to 1.0 JIM)inhibited invasion of Matrigel. More importantly, studies in SCID mice demonstrated that taxol (50 to 250 mg/mVday) blocked the establishment, growth, and long-term survival of PC-3 ML cells. Introduction Taxol, an alkaloid from Japanese and Pacific yew, is a potent drug with tremendous potential in the therapeutic treatment of cancer. Horwitz et al. (1) and others (2) reported that the principle effect of taxol was to stabilize microtubule structures. One striking effect of taxol was that it induced an abnormal bundling of microtubules in cells, blocking cell division (2). Basically, taxol binds tubulin to form abnormally stable taxol microtubules (3-5). In the past few years, taxol has been successfully tested on patients with cancer (6-12), specifically in the treatment of malignant melanoma (7) and ovarian carcinoma (8, 9). The ovarian cancer studies indicated that taxol was active at dosages of 110 to 175 mg/m3 (8). The drug had significant activity against melanoma with some non-life-threatening side effects (i.e., nausea, vomiting) (7). Holmes et al. (2) have recently shown that the drug can be used to treat metastatic breast cancer in phase I and II trials. Taxol, therefore, may be of value in the treatment of a wide range of cancers, including colon and prostate cancer. Future trials need to assess drug treatment protocols, optimum dosages, and dose-limiting toxic effects. In addition, the utility of taxol in treating multidrug-resistant, hormone-resistant and metastatic tumor lesions should be addressed. In attempts to study prostate cancer métastases,we have developed a SCID2 mouse model for investigating métastases to the bone. A variant (PC-3 ML) of human prostate PC-3 Received 1/15/92; accepted 5/11/92. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. 1This study was supported by NIH-NCI Grant CA53518 to M. E. S. 2To whom requests for reprints should be addressed, at Department of Pa thology, Medical College of Pennsylvania, 3300 Henry Avenue, Philadelphia, PA 19129. 3The abbreviations used are: SCID, severe combined immunodeficiency; CM, conditioned medium. tumor cells has been isolated which preferentially metastasizes to the lumbar vertebrae with >80% efficiency following i.v. injection via the tail vein in SCID mice (13). In this paper, we have utilized the SCID mouse model to test whether taxol might have therapeutic utility in the prevention or treatment of metastatic bone cancer. We describe novel observations on the antitumorigenic effects of taxol on biological processes (i.e., protease secretion) impor tant for tumor cell invasion in vitro. More importantly, we show that taxol is effective with marginal toxicity in the treatment of multidrug-resistant, metastatic bone tumors formed by the PC3 ML variant in SCIDs. Materials and Methods The isolation and culturing of human PC-3 cells and DU 145 cells have been detailed in previous studies (13, 14). The cells were plated on Matrigel (2 mg/ml) unless indicated differently. We have described the methods for preparation and use of PC-3 ML CM (15), the Boyden chamber chemotactic assays, and the preparation and use of Matrigel (14, 15). The slot blotting and immunofluorescence techniques for labeling cells also have been developed previously (14, 15). As described in the table legends, we labeled the cells with [3H]methionine using standard procedures (13). For the mouse studies, the cells were injected i.v. and s.c. in SCID mice using published methods (13). The resulting tumor tissue was examined by light microscopy following histológica! preparation of tissue (13). Cryosections were prepared for antibody labeling and im munofluorescence studies according to the methods described by Brandtzaeq (16). The monoclonal /î-tubulin antibodies and polyclonal M, 72,000 type IV collagenase antibodies used in these studies have been characterized previously (14, 15). Western blots and slot blots with the type IV collagenase antibodies used at a dilution of 1:200 (~\ Mg/ml) showed that the antibodies detected as little as 0.10 ng/m\ protease and showed a linear increase in labeling up to about 10 Mg/m' of purified protease. The levels measured in this paper were within this linear range. Gelatinase zymography assays were carried out according to the procedures of Heussen and Dowdle (17). All protein measurements were with a Bio-Rad kit using their procedures. All the reagents were from Sigma Chemical Co. unless stated otherwise. Taxol (Sigma Chemical Co., St. Louis, MO) was solubili/ed in castor oil and aliquots (i.e., 5-10 ¿il) were added to solutions. Similar amounts of castor oil were added in control experiments. In general, care was taken to ensure animal welfare on a daily basis. Toxicity end points were determined by exposing mice to taxol at increased dosages (25, 50, 100, 200, 250, 300, 350 mg/m2/day) for 5 days. Three mice were tested per dosage. Lethargy, sickness, and hyperventilation were observed at dosages greater than 250 mg/m2/day and the animals sacrificed by cervical dislocation. No histopathological criterion was used to assess toxicity. Results and Discussion Micromolar levels of taxol had a dramatic effect on the microtubule networks of PC-3 ML cells cultured on Matrigel. 3776 ANTIMETASTAT1C EFFECTS OF TAXOL ON PROSTATE TUMOR Following immunofluorescence labeling with /3-tubulin antibod ies, Fig. 1 compares the normal microtubule arrays emanating from a central microtubule-organizing center in untreated cells (Fig. IA); and the microtubule bundles observed in the taxoltreated cells (Fig. 15), where the cells were exposed to 0.5 UM taxol for 6 h at 37°C.Note the discontinuous nature of the microtubules located in the peripheral margins of the taxolirouted cells. The dose-dependent effects of taxol on microtubule bundling were summarized in Table 1. The amount of bundling observed by immunofluorescence labeling was minimal or nonexistent at low levels of taxol tested (<0.05 n\t for 1 to 6 h). In contrast, at higher dosages (>0.5 /¿M for periods of 6 h) 100% of the cells exhibited microtubule bundling. A similar response to taxol was observed in the other PC-3 variants (i.e., 3 x noninvasive) and DU 145 cells (data not shown). Moreover, these results Fig. 1. Immunofluorescence images of PC-3 ML cells labeled with /3-tubulin antibodies and secondar)' antibody conjugated to fluorescein isothiocyanate. A. untreated; B, 0.5 I/M taxol for 6 h showing microtubule bundles (arrows) in the cell periphery. A, xlOOO; B, xlSOO. Aliquots (5 M') of castor oil were added to the untreated cells. Table 1 Immunofluorescence microscopy studies of microtubule bundling in taxol-treated PC-3 ML cells Values show the number of cells (of 100) exhibiting abnormal microtubule assays in each experiment. Immunolabeling with antibodies for the pi 70 multidrug resistance glycoprotein (antibodies courtesy of Dr. Vic Ling, University of Toronto) indicated the PC-3 ML variants uniformly expressed this phenotype. Treatment time Taxol (fM)0.01 0.05 0.10 0.50 1.001 h0/100 0/100 5/50 1/100 20/100 33/50 45/100 30/100 50/50 100/100 25/1003h0/5050/506h0/100 100/100 M Fig. 2. Slot blots with the M, 72,000 type IV collagenase antibodies (1:200 dilution) showing the whole cell extracts (£)and the medium (M) from the same cells. The cells were (Lane 1) untreated and exposed to taxol for 6 h at (Lane 2) 0.5 fiM and (Lane 3) 1.0 fiM levels. The PC-3 ML cells (passage 5) were seeded at 2 x 10' cells/ml in 60-mm dishes overnight on Matrigel (2 mg/ml), washed 3 times with Dulbecco's modified Eagle's medium, exposed to drug in Dulbecco's modified Eagle's medium for 6 h, washed 4 times with Dulbecco's modified Eagle's medium, and exposed to fresh PC-3 ML CM (10 mg/ml) for 6 h at 37°C in a 5% CO2 incubator. The medium (3 ml) and whole cell extract (3 ml) from each dish was collected, concentrated to 0.3 ml by dialysis against PEG 20,000 for 3 to 4 h at 4'C, and blotted (0.3 ml/well) according to published procedures (15). 23456 Fig. 3. Gelatinase assays (7% gel) demonstrating the relative collagenase levels (72, =A/r 72,000; 57, M, 57,000) in the medium of PC-3 ML cells untreated (Lanes 1, 2) or exposed to increased amounts of taxol at (Lanes 3, 4) 0.1 p M or (Lanes S, 6) 0.5 JIMfor 6 h. The lanes were each loaded with (Lanes 1, 3, 5) 10 fig and (Lanes 2, 4, 6) 20 fig protein, and gels were electrophoresed overnight (=18 h) at 40 amp/gel. The cells were plated at 2 x IO7 for 3 h. exposed to taxol for 6 h, washed with serum-free medium, and incubated in serum-free medium containing 10 mg/ml PC-3 ML CM for 6 h. This medium was harvested and concentrated 10-fold by dialysis against dry polyethelene glycol powder (M, 20,000) for the zymogram assays. agree closely with that originally reported for other cells (1, 2). Therefore, we believe that the limiting dosage-dependent parameter in these studies was the time for diffusion of taxol across the cell membranes. Although radiolabeled drug uptake studies were not done, drug uptake in the cells appeared to be relatively slow and largely irreversible. For example, microtu bule bundling was observed following exposure to 0.5 ¿tM taxol for 6 h and removal of the drug from the medium for 48 h. Effects of Taxol on Protease Secretion. Previously, we and others (13, 15, 17, 18) have reported that type IV collagenase was secreted by human prostatic tumor cells. Plating these cells on type IV collagen (14), in the presence of their own CM (13), significantly elevated the levels of type IV collagenase secreted. In this paper, the cells were plated on Matrigel (2 mg/ml) which stimulated the cells to secrete collagenase (i.e., it had a similar effect as type IV collagen). The inhibitory influence of taxol on type IV collagenase secretion was then examined by slot blotting of the culture medium (Fig. 2). Slot blots with antibodies raised against a A/r 72,000 type IV collagenase indicated that the protease was normally secreted by PC-3 ML cells in response to the PC-3 ML CM (10 mg/ml for 6 h). If the cells were pretreated with taxol (0.5 and 1.0 MMfor 6 h) prior to stimulation of secretion with conditioned medium, the drug blocked the secretion of the M, 72,000 type IV collagenase. Slot blots of whole cell extracts from the same cells confirmed that type IV collagenase was in fact present in the taxol-treated PC-3 ML cells (Fig. 2). A standard curve assay with affinitypurified collagenase showed a linear antibody (1:200 dilution) detection range from 0.01 to 30 ng, with saturation at 30 fig. Control experiments showed that when l ¿IM taxol was added to PC-3 ML CM (10 mg/ml) prior to (for 3 h) and during the experiment, the drug did not block the ability of CMs to stimulate protease release (data not shown). We interpret this to mean that taxol probably does not bind or interfere with processes activated by factors in the CM perse (14). Gelatinase assays confirmed that taxol (0.5 pM for 6 h) inhibited the secretion of two high molecular weight gelatinases, including a faint band at A/r 92,000 and a pronounced band at M, 72,000 (Fig. 3). Secretion of lower molecular weight gelati- 3777 ANTIMETASTATIC EFFECTS OF TAXOL ON PROSTATE TUMOR Table 2 Pulse labeling experiments measuring effects oflaxol on synthesis (cpm x 1000 ±SD) The PC-3 ML cells were seeded at 2 x 10" cells/ml in 10 ml and were exposed to 0.5 /AI taxol for 0, 6, and 9 h, then pulse labeled with (rani-labeled | 'I l| methionine (200 ¿iCi/ml;NEN) for 6 h in methionine-free medium, washed 3 limes with SFM," and exposed to SFM containing 10 mg/ml PC-3 ML CM for 6_h. Taxol treatment Protein synthesis Collagenase synthesis 30 Collagenase turnover 28 Collagenase turnover" •¿* 25 Collagenase secretion" 'Oh350 0.1 ±5.1 ±4.0 ±1.5 ±0.3 0.6 ±0.1 0.4 ±0.05 ±0.2 25 ±0.4 24 ±0.3 ±0.4 ±0.3 16 ±0.5 17 ±0.3 ±0.13h15 12 ±0.46h330 13 ±0.29h295 11 ±0.5 " SFM, serum-free medium. h The cells were pulse labeled as in the above studies and exposed to SFM containing 1.0 JIM taxol plus 10 mg/ml PC-3 ML CM for 0, 3, 6, and 9 h. ['H|collagenase was immunoprecipitated from whole cell extracts' and the med ium'' of the same cells. Table 3 Effects of taxol on total protein and collagenase secretion (cpm x 7000 ±SD) The cells (2 x 106/ml in 10 ml) were labeled (200 ^Ci/ml |'H]methionine for 6 h in methionine-free medium), washed 6 times, exposed to 0.5 MMtaxol for 6 and 9 h. and transferred to serum-free medium containing 10 mg/ml PC-3 ML conditioned medium for 6 h. Scintillation counting of the medium from each 60mm dish was in triplicate ±SD. Trypan blue (0.1 %) dye exclusion assays indicated that there was little or no cell death in the presence of taxol. Exposure time(h)0 6 9Total protein secreted360 ±0.20 0.3 ±0.20 0.2 ±0.09Total showed that the amount of 'H-type IV collagenase [i.e., M, 72,000] immunoprecipitated from the whole cell extracts of taxol-treated cells was drastically reduced from that found in untreated cells [=30,000 ±300 (SD) cpm]. By comparison, the immunoprecipitates from treated cells contained 600 ±100 cpm after 6 h and 400 ±50 cpm after 9 h exposure. The amount of total labeled protein in whole cell extracts was 350,000 ± 5,100 cpm in controls, 330,000 ±4,000 cpm after 6 h exposure, and 295,000 ±1,500 cpm after 9 h exposure to taxol. To test further whether taxol influenced type IV collagenase turnover rates, the cells were first labeled (200 nCi/m\ ['H] methionine for 6 h) and then exposed to 1.0 n\i taxol for 0, 6, and 9 h. Immunoprecipitation measurements revealed cytoplasmic counts (cpm) of 28,000 ±220 (at 0 h), 25,000 ±420 (after 6 h), and 24,000 ±330 (after 9 h) incubation in the presence of taxol. The counts were somewhat lower in the experiments compared to the controls, but overall the data indicated that there was very little reduction of the type IV collagenase levels over a 9-h interval. As an alternative ap proach, following pulse labeling for 6 h, we measured collagen ase turnover rates in cells exposed to serum-free medium con taining 10 mg/ml PC-3 ML CM and 1.0 /¿M taxol (Table 2). The data showed that the cytoplasmic levels of ['H]collagenase dropped from about 25,000 ±400 cpm to 15,000 ±300 cpm during the first 3 h. Conversely, the ['H]collagenase levels in collagenase secreted40 the medium rose to about 12,000 ±cpm as a result of secretion in the first 3 h. The levels of ['H]collagenase in the cytoplasm ±0.40 0.05 ±0.01 0.06 ±0.02 and medium remained fairly constant at levels comparable to that measured at 3 h after 6- and 9-h intervals. We interpret Table 4 Effects of taxol on PC-3 ML cell invasion the data to mean that collagenase secretion probably occurs for The cells were prelabeled with 200 >iCi/ml ['HJmethionine for 6 h, seeded at the first 3 h of exposure to taxol but was then inhibited by 6 1x10' cells/well for 3 h, and then exposed to taxol (5-jil aliquots) for 1 to 9 h. and 9 h as a result of the diffusion of taxol in the cells. We Next, PC-3 M L conditioned medium ( 10 mg/ml protein) was added to the bottom compartment for 6 h. Cells were harvested to determine the extent of invasion by conclude that taxol probably has no direct effects on stability scintillation counting (i.e., the percentage invasion is equal to the percentage of and turnover of cytoplasmic collagenase. Also, the PC-3 ML radioactivity in the bottom compartment versus the total radioactivity added per CM does not appear to influence the stability of collagenase in well). The data were averaged from triplicate experiments ±SD. the taxol-treated cells. timeTaxol(CM)0.00"0.010.050.100.501.001 Treatment It was important to compare if total protein secretion or type h8.4 h8.6 h8.0 IV collagenase secretion were inhibited by taxol in radiolabeled ± 0.27.9 ± 0.47.8 ± ±0.26.3 0.45.5 0.38.3 ± cells. Table 3 shows that the total labeled protein secreted in ±0.13.1 0.22.0 ± ±0.16.2 0.36.0 ± 0.23.0 ± ±0.1000 response to PC-3 ML CM (10 mg/ml) was about 300 ±200 0.43.1 ± 0.30.5 ± ±0.30009h°8.3 0.23.1 ± ±0.1006 ±0.1009h8.1 ±0.10.4 cpm after 6 h and 200 ±95 cpm following 9 h preexposure of ±0.10.1 the cells to taxol, indicating inhibition of total secretion of ±0.23 protein. Control cells which were not treated with taxol secreted " Taxol was removed from the medium for 18 h prior to beginning the invasion total protein counts of ~360,000 ±200 cpm. Immunoprecipi assay. * Aliquots (5 ul) of castor oil were added. tation measurements of the amounts of 'H-labeled type IV nases (i.e., at M, 57,000) was also inhibited. The cells failed to recover an ability to secrete collagenase following washes and incubation in fresh medium in the absence of taxol for 48 h (data not shown). Since the inhibition of secretion was coinci dental with the disruption of the microtubules, we interpret the data (Figs. 1-3) to mean that taxol induced rearrangements or changes in the microtubules might interfere with their func tional ability to mediate protease vesicle transport and secretion of the gelatinases. Effects of Taxol on Protein Synthesis, Turnover, and Secretion. Collagenase secretion is thought to occur immediately following synthesis (15). Therefore, we tested to see if taxol might indi rectly block secretion by altering the half-life of type IV colla genase or by inhibiting either total protein synthesis or type IV collagenase synthesis. Table 2 summarizes the data from these studies. After pulse labeling for 6 h, immunoprecipitation studies collagenase secreted revealed that the medium contained 50 ± 10 cpm (after 6 h) and 60 ±20 cpm (after 9 h) exposure to taxol, indicating an inhibition of protease secretion. By com parison, the medium obtained from control cells contained 40,000 ±400 cpm. In sum, the data showed that taxol inhibited both the synthesis and secretion of the M, 72,000 type IV collagenase. We suggest that when type IV secretion is blocked, translation is somehow inhibited, perhaps by mechanisms de pendent on the cytoplasmic processing and packaging of the protease. Interestingly, total protein secretion was also blocked perhaps as a direct result of the disruption of the microtubule distribution. Boyden Chamber Invasion Studies. Boyden chemotactic as says have been developed previously for studying the ability of tumor cells to invade the basement membrane material (13, 15, 20). Utilizing this approach, we have quantitatively measured the influence of taxol on the ability of the PC-3 ML cells to penetrate Matrigel (Table 4). The measurable levels of invasion 3778 ANTIMETASTATIC EFFECTS OF TAXOL ON PROSTATE TUMOR observed in untreated cells (i.e., the ability to penetrate through the Matrigel) was about 8 percent. Similar amounts of invasion (about 7.9%) were observed in cells exposed to low taxol levels (<0.01 MMfor 3 h) Invasion was partially inhibited at slightly higher taxol levels (0.05 to 0.10 MM taxol for 1 to 6 h). In comparison, the invasive response was reduced to zero in the cells exposed to 0.1 to 1.0 MMtaxol for 3 to 9 h. The inhibitory influence of taxol was not reversible in cells exposed to 0.1 to 1.0 MMtaxol for 9 h when taxol was subsequently removed from the medium for 18 h. Taxol blockage of the secretion of collagenases would play a significant role in preventing pene tration of the Matrigel (14, 17). Cell Attachment and Motility Studies. During invasion, the cells must execute a series of complex maneuvers involving partial detachment from the substrate, cell elongation and movement through the matrix. We tested if taxol might indi rectly block cell detachment/attachment to substrate, and/or interfere with cell motility. Relatively low levels of taxol (i.e., 0.5 MMfor 3 h) were found to completely inhibit attachment of the PC-3 ML subline (at 1 x IO6 cells/ml in suspension) to Matrigel (2 mg/ml), type IV collagen (2 mg/ml) substrates in vitro. Light microscopic studies of stained filters (American Scientific Products, New NJ) showed that 30-40 cells/cm- normally attached and plastic Diffi-QuikBrunswick, after about 3 h, regardless of which substrate was used. Following exposure to taxol (0.1 MMfor 3 h) only 1, 3 and 0 cells/cm2 attached to the above substrates, respectively. Taxol also blocked cell migration through membrane pores (8 MM)in Boyden chemotactic chambers. Here, the membranes were lightly coated with Matrigel (0.1 mg/ml) (so as not to block the pores), in order to promote maximal cell attachment and migration (15). PC-3 ML cells were then seeded at 1 x 106/ml in the top compartment, allowed to attach for 3 h, and the unattached cells removed (i.e., «100 cells/ml) prior to starting the experiment. Hemacytometer counts of cells col lected from the bottom chamber with trypsin-EDTA showed about 20% (i.e., 0.2 x IO6cells) normally migrated through the pores by 3 h in response to PC-3 ML CM (10 mg/ml) added to the bottom compartment. If the attached cells were exposed to 0.5 MMtaxol for 3 h, washed and then stimulated to migrate, a very small number of the cells (about 5 to 6 cells per well) migrated to the bottom compartment by 3 and 6 h. Light microscopy confirmed that the cells remained attached to the membranes in the top compartment, indicating taxol interfered with cell detachment. Northern blots showed that type IV collagenase transcription was up regulated in response to the PC-3 ML CM. Taxol (1.0 MM)alone did not turn on transcrip tion and taxol pretreatment (0.1 to 1.0 MMfor 6 h) prior to activation with PC-3 ML CM failed to inhibit upregulation of transcription. We suggest that taxol's effects on microtubules might prevent detachment and indirectly block cell motility, as has been previously shown for fibroblasts (20). The combined inhibitory activity of taxol on protease secretion, cell attach ment/detachment and motility, appear to completely block invasion. Activity of Taxol in Vivo: SCID Mouse Studies. We investi gated if taxol inhibited the invasive metastatic activity of PC-3 ML cells in SCIDs. The PC-3 ML cells were exposed to taxol (0.1 to 1.0 MM)for 0, 3 and 6 h, washed 3 x with DMEM and injected i.v. in SCID mice (Table 5) according to methods described previously (13). With untreated cells, the mice exhib ited tumors in the lumbar vertebrae (greater than 80%) after 20 days following i.v. injection of 2 x IO5 cells/ml in 0.2 ml (13). Table 5 Effects of taxol on PC-3 ML métastasesto the lumbar vertebrae in SCIDs Cells in suspension were exposed to taxol immediately prior to injection i.v. in SCIDs. The cells were washed 3 times with Dulbecco's modified Eagle's medium to remove unincorporated taxol, and 0.2 ml at 2 x 10' cells/ml as injected per mouse. Mice were left for 20 days and the presence of tumors in the lumbar vertebrae was assessed. Taxol was solubilized at 1 mg/ml in 50% polyoxyethylated castor oil (Cremophor EL) and 50% ethanol (USP), and aliquots were added to Dulbecco's modified Eagle's medium. Due to toxicity, taxol and/or the Cremophor vehicle were administered to mice in 0.05-ml dosages over 4-h periods (0.2 ml total volume/mouse/day). To reduce acute hypersensitivity, mice received i.v. injections of 1 mg dexamethasone at 2-h intervals, 3 times over 6 h prior to administering the drug. One h prior to drug treatment, mice were given 2 mg diphenhydramine and 10 mg Cimetidine i.v. in 0.05-ml volumes of doubledistilled H2O. Control mice were given the Cremophor vehicle. Mice were carefully monitored and given oxygen if they exhibited hypersensitivity. Mice were sacrificed by cervical dislocation if toxicity was apparent. time3h4/20 Taxol(MM)0.1 0.5 1.0Oh18/20 17/20 18/20Treatment 0/20 0/206h1/20 0/20 0/20 When the cells were pre-exposed to taxol at 0.1 MMfor 3 h and 6 h, a substantially reduced number of the mice (4/20 and 1/20, respectively) exhibited métastasesto the vertebrae. Tu mors were not present in other tissues (i.e., lung, liver, kidney, spleen, brain) as determined by gross dissections and histology. Fig. 4, is an H & E image of a tumor in the lumbar vertebrae of a mouse injected with PC-3 ML cells previously exposed to reduced levels of taxol, 0.1 MMtaxol for 3 h. The tumor size and appearance (Fig. 4A) was not unlike that observed in mice injected with untreated cells. Moreover, no obvious microtubule bundling was apparent in the mitotic spindles following immunofluorescence labeling with beta tubulin antibodies (Fig. 4B). Therefore, we believe that taxol was not taken up by these cells in significant amounts. Preexposure of the cells to 0.5 or 1.0 MMtaxol for 3 to 6 h prior to injection i.v. completely blocked métastases.Gross dissections and histology revealed that none of the mice had tumor tissue in the vertebrae or other tissues. We believe, that this may result from poor survival, and/or an inability of the cells to invade tissue and/or to grow tumors. When 10 mice were injected with excess untreated cells (2 x IO7 cells/ml in 0.2 ml) numerous lung nodules formed by 20 days (13). In mice injected with taxol treated cells (0.5 MMfor 6 h), absolutely no tumors were found after 20 days, 30 days and 60 days, indicating cell number and time were probably not limiting factors. Effect of Taxol on Tumor Growth in Vivo. Previous experience has shown that bone tumors are usually evident after 5 days following the injection of PC-3 ML cells at 2 x 10* cells per ml (13). To determine if taxol prevented tumor growth in vivo, SCIDs were injected i.v. with PC-3 ML cells (2 x 10s cells/ml in 0.2 ml) and left 5 days. On day 6, the mice were injected i.v. via the tail vein with taxol (50 mg/rrr/day and 250 mg/m2/day in 0.2 mis). Ten mice were treated with each taxol dosage tested and five control mice received equivalent amounts of polyoxyethylated castor oil, the vehicle in which taxol was formulated. After 15 days of treatment, the mice were sacrificed and ex amined for tumors by dissection and histology. Gross dissection revealed that tumors grew specifically in the lumbar vertebrae (i.e., filling the bone marrow) in all the control mice. The bone was usually destroyed in several areas and the tumors had metastasized into the peritoneal cavity. Gross dissection of the taxol treated mice showed that none of the 20 mice exhibited noticeable tumors in any tissues examined (lungs, liver, colon, 3779 ANTIMETASTATIC EFFECTS OF TAXOL ON PROSTATE TUMOR Immunofluorescence labeling with beta-tubulin antibodies revealed abnormal mitotic spindles (=15 pM in length) in tumor cells of the taxol treated mice (Fig. 5). Also, the number of mitotic cells detected per field of view were about 5 fold higher than in the controls. Note that it was not technically possible to resolve individual interphase microtubules (or bundles of microtubules) as discrete structures, but the tubulin rich cyto plasm fluoresced as a result of antibody binding to tubulin. The control sections labeled with 2°ab-FITC alone failed to fluoresce. Interestingly, antibody labeling showed that the cells were positive for the p 170 multi-drug resistance glycoprotein (data not shown). To determine if any tumor growth occurred months after the taxol treatment was discontinued, mice were injected i.v. with 2 x 10s cells/ml (0.2 ml), left 5 days and exposed to taxol from day 5 to 15 (i.e., 50 and 250 mg/nr/day). Treatment was discontinued on day 15 and the mice sacrificed 3 months later. Gross dissection and histology revealed that only 40 to 60% of the mice contained tumors; 6/10 (50 mg/nr/day taxol) and 4/10 (250 mg/irr/day taxol). In all cases, the tumors observed were small and delimited to the marrow cavity of the lumbar vertebrae. Immunolabeling of cryosections with tubulin anti bodies revealed that the spindles were about 1/3 shorter in length (x5 /¿m)than normal spindle (=7 /¿m)found in control cells. Taxol treatment may, therefore, have long-term inhibitory effects on cell division and tumor growth. The apparent total eradication of the vertebrae tumors, in at least 20 to 40 percent of the mice tested, is noteworthy and may inadvertently arise from cell death. Note that these latter values were corrected (i.e., the raw data reduced 20%) to account for a potential 20% failure by the PC-3 ML cells to initiate tumors (13). The dosages tested here were somewhat low in comparison to the levels which have been used in clinical trials (i.e., up to 350 mg/nr/day; 7-11). Unfortunately, if these higher dosages were administered in mice, death or extreme hypersensitivity (vomiting, droopiness) usually occurred in the animals. In conclusion, the data demonstrates that taxol is of signifi cant importance in the treatment of metastatic bone tumors, including tumors exhibiting the multi-drug resistance phenotype (see Table I). More specifically, we believe that taxol has therapeutic utility in inhibiting molecular processes activated during invasion of the basement membrane and, therefore, may be used to prevent micrometastases in malignant tissue during surgery, radiation or chemotreatment. The future use of taxol Fig. 4. A, transverse section of the lumbar vertebrae showing PC-3 ML cells in the bone marrow. H & E, X1200. B, immunofluorescently ß-tubulin-labeled PC-3 ML tumor tissue. Cells were exposed to 0.1 »JM taxol for 6 h followed by i.v. injection into mice; the mice were sacrificed at 20 days postinjection. x400. testicles, muscle, brain, vertebrae). Histológica! studies revealed that at day 20 there was tumor tissue but only in the bone marrow of the lumbar vertebrae of the taxol treated mice. The tumor burden in mice exposed to 50 mg/m2/day or 250 mg/ m:/day taxol was minimal and about the same as that observed in untreated mice sacrificed at day 5. Fig. 5. The tumor was established for 5 days and mice were exposed to taxol (25 mg/m2/day) for 15 days. Shown are the normal spindle structures labeled with /3-tubulin antibody. x600. 3780 ANTIMETASTATIC EFFECTS OF TAXOL ON PROSTATE TUMOR as an anti-cancer drug may be enhanced greatly with the devel opment of non-toxic derivatives, or of receptor or antibody mediated taxol delivery mechanisms to improve tumor uptake and to reduce toxicity. Higher, more effective dosages could then be used without generating severe long-term reactions by patients. References 1. Horwilz, S. B.. Parness, J., Schiff, P. B.. and Manfredi, J. J. Taxol: a new probe for studying the structure and function of microtubules. In: Cold Spring Harbor Symp. Quant. Biol., 46: 219-226, 1982. 2. De Brabander, M.. Geuens, G., Nuydens, R., Willebrords, R.. and De May, J. Microtubule stability and assembly in living cells: the influence of metabolic inhibitors. Taxol and pH. Cold Spring Harbor Symp. Quant. Biol., 46: 227240, 1982. 3. Shiff, P. B., Fant, J., and Horwitz. S. B. Promotion of microtubule assembly in vitro by taxol. Nature (Lond.), 277: 665-667, 1979. 4. Vallee, R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule associated proteins (MAPs). J. Cell Biol., 92: 435-441, 1982. 5. Collins, C. A. Reversible assembly pu ritirai ion of taxol-treated microtubules. Methods Enzymol., 196: 246-251, 1991. 6. Blume, E. Investigators seek to increase taxol supply. J. Nati. Cancer Inst.. 81: 1122-1123, 1989. 7. Einzig, A. I. A phase II study of taxol in patients with malignant melanoma. Invest. New Drugs, 9: 59-64, 1991. 8. Rowinsky, E. K., Cazenave, L. A., and Donehower, R. C. Taxol: a novel investigational antimicrotubule agent. J. Nati. Cancer Inst.. 82: 1247-1259, 1990. 3781 9. Weiss, R. B. Hypersensitivity reactions from taxol. J. Clin. Oncol., *: 12631268. 1990. 10. Grem, J. L. Phase I study of taxol administered as a short IV infusion daily for 5 days. Cancer Treat. Rep., 71: 1179-1189, 1987. 11. Donehower, R. C., Rowinsky, E. K., Cerochow, L. B., Longnecker. S. M., and Ettinger. D. S. Phase I trial of laxol in patients with advanced cancer. Cancer Treat. Rep., 71: 1171-1177. 1987. 12. Holmes, F. A., Walters, R. S., Theriault. R. L.. Forman, A. D.. Newton, L. K., Raber, M. N.. Buzdar, A. U., Frye, D. K., and Hortobagyi. G. N. Phase II trial of taxol. an active drug in the treatment of metastatic breast cancer. J. Nati. Cancer Inst., S3: 1797-1805, 1991. 13. Wang, M., and Stearns. M. E. Isolation and characterization of 3 PC-3 human prostatic tumor sublines which preferentially metastasize to select organs in s.c.i.d. mice. Differentiation, 48: 115-125, 1991. 14. Wang, M., and Stearns, M. E. Blocking of collagenase secretion by estramustine during in vitro tumor cell invasion. Cancer Res.. 48: 6262-6271, 1988. 15. Stearns, M. E., and Wang. M. Regulation of kinesin expression and type IV collagenase secretion in invasive human prostate PC-3 tumor sublines. Can cer Res., SI: 5866-5875. 1991. 16. Brandtzaeq, P. Tissue preparation methods for immunohistochemistry. In G. R. Bullock and P. Petrusz (eds.), Techniques in Immunocytochemistry, Vol. I, pp. 1-76. New York: Academic Press, 1982. 17. Heussen, C., and Dowdle. E. B. Elcctrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem., 102: 196-202. 1980. 18. Albini, A., Iwamoto. Y., Kleinman, H. K., Martin, G. R., Aaronson, S. A., Kozlowski, J. M., and McEwan, R. N. A rapid in ritro assay for quantitating the invasive potential of tumor cells. Cancer Res.. 47:3239-3245. 1987. 19. Stearns, M. E., Wang, M.. and Sousa. O. Evidence that Estramusline binds MAP IA to inhibit type IV collagenase secretion. J. Ceil Sci., 98: 55-63. 1991. 20. Schiff, P. B.. and Horwitz, S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Nati. Acad. Sci. USA, 77: 1561. 1980.