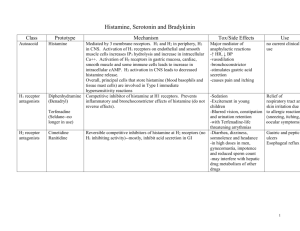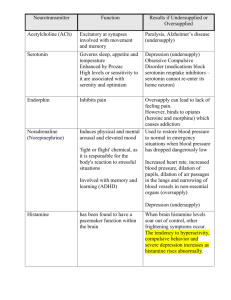
Histamine Potentiation of Nerve- and Drug-Induced Responses
of a Rabbit Cerebral Artery
By John A. Bevan, Sue Piper Duckies, and Tony J-F. Lee
Downloaded from http://circres.ahajournals.org/ by guest on October 2, 2016
ABSTRACT
Rabbit basilar artery rings are normally relatively unresponsive to transmural
stimulation of their sympathetic nerve supply. However, in the presence of histamine
(0.55 /iM)> contractile responses to nerve stimulation were markedly increased. Norepinephrine and serotonin concentrations that produce 50% of a maximum contractile
response (ED50) were considerably decreased in the presence of histamine; maximum
responses to both norepinephrine and serotonin were increased. Although a prejunctional
effect of histamine has not been eliminated, potentiation of responses to transmural nerve
stimulation is probably due to an increase in smooth muscle responsiveness to norepinephrine. In rabbit saphenous artery rings, histamine produced a qualitatively similar
potentiation of responses to nerve stimulation, norepinephrine, and serotonin except that
maximum responses were not increased. Serotonin (0.084 JJM) did not potentiate
contractile responses of the basilar artery to transmural nerve stimulation or norepinephrine. Since histamine and serotonin are released from rabbit platelets in response to tissue
injury, the synergistic effect of these agents on vascular smooth muscle contraction might
be advantageous in minimizing hemorrhage. But such a response could also be deleterious
if the effects of these vasoconstrictors were prolonged.
KEY WORDS
norepinephrine
serotonin
cerebral vasospasm
hemorrhage
nervous control of cerebral circulation
• Numerous studies of the physiology and pharmacology of the cerebral vasculature have been
reported, yet controversy over the control of brain
blood flow continues (1). This is especially true of
nervous control of the cerebral circulation. Although an adequate sympathetic nerve supply to
the larger cerebral arteries has been demonstrated
by morphological techniques (2-4), its functional
significance is still in doubt. Carefully designed
studies in dogs have shown a decrease in cerebral
blood flow with adrenergic nerve stimulation, although the high rates of stimulation which are
necessary to elicit measurable changes cast some
doubt on the relevance of this finding (5).
Cerebral arteries have a low sensitivity to lnorepinephrine in comparison with that of other
vessels (6, 7). Although cerebral arteries contract in
vitro when their intramural nerves are stimulated
electrically, this response is small in comparison
From the Department of Pharmacology and the Brain Research Institute, Center for Health Sciences, School of Medicine,
UCLA, Los Angeles, California 90024.
This investigation was supported by U. S. Public Health
Service Grant HL15805 from the National Heart and Lung
Institute and by Grant 408 IG from the Los Angeles County
Heart Association. Dr. Duckies is a Los Angeles County Heart
Association postdoctoral fellow, and Dr. Lee is a U. S. Public
Health Service postdoctoral fellow supported by Grant NS 2040.
Received October, 15, 1974. Accepted for publication February 27, 1975.
Circulation Research, Vol. 36, May 1975
saphenous artery
basilar artery
with that of other vessels (8); moreover, some
workers have been unable to demonstrate a response in some species (7).
Because of the uncertainty about nervous control
of the cerebral circulation, we were interested to
find that histamine, in concentrations that do not
change vascular tone, dramatically increases the
contractile response of the rabbit basilar artery to
transmural nerve stimulation (9). Thus, in the
presence of histamine, nerve stimulation of cerebral arteries elicits a response more similar in
magnitude to that of peripheral arteries. This
finding suggests that the adrenergic innervation of
cerebral vessels is potentially capable of playing a
significant role in the regulation of cerebral blood
flow in vivo.
In the present paper, the potentiation by histamine of the response of the rabbit basilar artery to
transmural nerve stimulation is described. Evidence is presented that histamine also potentiates
responses to exogenous /-norepinephrine and serotonin and that histamine potentiation, although
not unique to the cerebral circulation, may be
quantitatively greater there than it is in systemic
vessels.
Methods
Ring segments (4 mm long) of the basilar artery and of
the dorsal branch of the saphenous artery were prepared
647
648
Downloaded from http://circres.ahajournals.org/ by guest on October 2, 2016
from adult white rabbits (2-3 kg) of either sex. The
rabbits were killed by a blow on the front of the head and
exsanguinated. The entire brain with blood vessels
attached and the saphenous artery were rapidly removed
and placed in Krebs-bicarbonate solution equilibrated
with a 95% O2-5% CO2 gas mixture at room temperature.
The millimolar composition of the Krebs solution was:
Na + 144.2, K+ 4.9, Ca2+ 1.3, Mg2+ 1.2, Cl~ 126.7, HCO,"
25.0, SO42~ 1.19, glucose 11.1, and calcium disodium
ethylenediaminetetraacetate (EDTA) 0.023. Vessels
were dissected and cleaned of surrounding tissue under a
dissecting microscope.
Ring segments were cannulated with a stainless steel
rod of hemispherical section and a short piece of platinum wire, mounted in an isolated tissue bath in the
Krebs solution, and maintained at 37°C. The platinum
wire was bent into a U shape and anchored to a plastic
gate that could be moved up and down by a fine control
micrometer. The steel rod was connected to a Statham
strain gauge (GlOb 0.150Z) for isometric recording of
changes in force on a Sargent strip chart recorder or a
Grass model 5D polygraph. Resting tension was maintained at 500 mg, which was optimum for the development of responses, and 1 hour was allowed for equilibration.
BEVAN. DUCKLES, LEE
tissue samples were dissected in Krebs solution and then
fixed in 3% glutaraldehyde at 4°C for 2 hours. They were
further fixed in osmium, dehydrated, and embedded in
epoxy resin. Sections 1-2^ thick were stained with 0.14%
toluidine blue in 1% sodium borate and examined under
a light microscope. The thickness of the muscle wall was
determined with the aid of a Beuler micrometer. The
number of layers of muscle cells and the wall thickness
were estimated in four positions in each of three sections
taken from each specimen. The average number of
smooth muscle cells and the average measurement of
medial thickness of each specimen were used to determine mean values.
STATISTICAL METHODS
The data were statistically evaluated by Student's
t-test for paired or unpaired samples as appropriate. The
0.05 level of probability was accepted as significant.
DRUGS USED
The following drugs were used: histamine dihydrochloride (Pfamstiehl Chemical Co.), /-norepinephrine
bitartrate (Calbiochem), serotonin creatinine sulfate
(Calbiochem), pyrilamine maleate (Merck), phenoxybenzamine hydrochloride (Smith, Kline and French),
and tetrodotoxin (Sankyo-Tokyo).
FREQUENCY-RESPONSE CURVES
Contractions to transmural nerve stimulation were
elicited using platinum field electrodes. Trains of 200
biphasic square-wave pulses, 0.3 msec in duration at
supramaximal voltage, were applied at 1, 4, 8, and 16
Hz in random sequence. Stimulus trains were spaced at
8-minute intervals. To study the influence of histamine
or serotonin on the contractile response to transmural
nerve stimulation, data for two frequency-response
curves were obtained from each tissue; the data for the
second curve were obtained 20 minutes after the addition
of either drug. The maximum contractile response to
transmural nerve stimulation was obtained by stimulating the tissue at 32 Hz until a contraction plateau was
reached. At the end of each experiment, the contractile
response to a maximum dose of/-norepinephrine (3 min)
was elicited.
DOSE-RESPONSE CURVES
Dose-response relationships for various agonists were
obtained by a noncumulative technique, i.e., by adding
single doses of drug to the tissue bath. Tissues were
washed for 15 minutes between each drug addition. The
dose sequence was randomized. When the influence of
histamine or serotonin on the dose-response curves for
various agonists was studied, a pair of arterial segments
was used. One dose-response curve was obtained from
each preparation. One segment of each pair was studied
in the presence of histamine or serotonin, and the other
segment acted as the control. ED50 values (the dose that
produces 50% of the maximum response) were determined for each arterial ring studied using regression
analysis of probit values. From these values the geometric mean ED50 with 95% confidence intervals was calculated (10).
MORPHOLOGICAL MEASUREMENTS
To estimate the number of layers of muscle cells in
and the thickness of the media of the blood vessel wall,
Results
HISTAMINE POTENTIATION OF NEUROGENIC CONTRACTILE
RESPONSES OF THE BASILAR ARTERY
Contractile responses of the basilar artery to
transmural nerve stimulation were potentiated by
histamine (0.55 and 1.5 fiM) (Figs. 1A and 2A).
Even arteries that were completely unresponsive to
nerve stimulation at 16 Hz responded to nerve
stimulation in the presence of histamine. Histamine in the concentrations used produced transient
phasic contractions. Although peak contractions
were 20 and 50%, respectively, of the maximum
histamine response, the developed force was often
not increased above resting levels at the time when
the neurogenic responses were elicited. Thus, the
effect of histamine cannot be related to a change in
resting tension. The maximum response to nerve
stimulation was also increased by histamine (Table
1). When histamine was washed out of the bath,
responses returned to control levels (Fig. 1A).
Histamine in lower concentrations that produced
no contractile effects also potentiated responses to
nerve stimulation.
Tetrodotoxin (6 x 10' 6 M) completely blocked
the neurogenic response in seven basilar artery
rings regardless of whether histamine was present
or absent. In four basilar rings, pyrilamine (8.5 x
10" 6M) blocked potentiation by histamine of the
responses to nerve stimulation in doses that had no
effect on the responses to serotonin, norepinephrine, or nerve stimulation itself.
Circulation Research, Vol. 36, May 1975
HISTAMINE POTENTIATION
649
0.3-i A.
Downloaded from http://circres.ahajournals.org/ by guest on October 2, 2016
Histamine
FIGURE 1
1
Effect of histamine (5.5 x 10' M) on contractile responses of basilar artery rings to transmural nerve
stimulation (A), l-norepinephrine (NE) (10-' M) (B), and serotonin (5HT) (7.4 x 10-° M) (C). W =
wash.
HISTAMINE POTENTIATION OF CONTRACTILE RESPONSES OF THE
BASILAR ARTERY TO NOREPINEPHRINE AND SEROTONIN
In the presence of histamine, the response to
/-norepinephrine was greatly potentiated: for example, subthreshold i-norepinephrine concentrations produced responses as great as the maximum
response in the absence of histamine (Figs. IB and
2B). Histamine (1.5 /*M) caused a mean increase in
sensitivity to i-norepinephrine of almost two orders
of magnitude and approximately doubled the force
developed in response to the maximum concentration used (Table 1).
Histamine also influenced the serotonin dose-
response curves (Figs. 1C and 2C). In the presence
of histamine, the serotonin ED50 was decreased and
the maximum response was increased. However,
the effects of the two concentrations of histamine
used were similar (Table 1).
HISTAMINE POTENTIATION OF CONTRACTILE RESPONSES OF THE
SAPHENOUS ARTERY
To determine whether histamine potentiation
was common to all vessels or a unique property of
cerebral arteries, a peripheral artery, the dorsal
branch of the saphenous artery, was studied. This
vessel is comparable to the basilar artery in diameter and neural density and distribution (unpub-
it'13
1 4
8
16
STIMULATION FREQUENCY (Hz)
7
6
5
4
3
2
-LOG /-NOREPINEPHRINE CONCENTRATION(M)
9
8
7
6
5
4
-LOG SEROTONIN CONCENTRATION (M)
FIGURE 2
Effect of histamine on contractile responses of the basilar artery. A: Mean frequency-response curves
obtained by transmural nerve stimulation, 200 pulses. B: l-Norepinephrine dose-response curve. C:
Serotonin dose-response curve. Vertical bars represent the SE.
Circulation Research, Vol. 36, May 1975
650
BEVAN. DUCKLES. LEE
TABLE 1
Effect of Histamine and Serotonin on Responses to Nerve Stimulation, Norepinephrine, and Serotonin
Maximum
response to
nerve
stimulation*
Response to serotonin
Response to norepinephrine
(g)
ED5Of
Maximum:):
ED 5O t
Maximum
(M)
(g)
(M)
(g)
0.4 ± 0.07 (8)
0.9 ± 0.2|| (5)
Basilar Artery
6.2(3.7-10.5) x 10-" (18)
1.1 ±0.2 (18)
1.8(0.5-6.3) x 10-' 11(8)
1.6 ± 0 . 3 (8)
Histamine
0.8 ± 0.2|| (5)
1.2(0.01-104) x 10-1'II (5)
2.0 ± 0.2|| (5)
(1.5 MM)
Serotonin
0.3 ± 0.09§ (6)
2.3(0.72-7.4) x 10-11 (5)
1.36 ± 0.2 (5)
Control
Histamine
3.2 ± 0.2 (6)
2.6 ± 0.4 (5)
Control
Histamine
1.6(0.88-2.9) x 10-' (11)
3.5(0.83-14.6) x 10-''(5)
0.3 ±0.06 (11)
1.5 ±0.3 (5)
( 0 . 5 5 MM)
2.3(1.08-5.1) x 10-"'(6)
1.3 ± 0.3 (6)
(0.084 MM)
Downloaded from http://circres.ahajournals.org/ by guest on October 2, 2016
Saphenous Artery Dorsal Branch
7.9(3.7-16) x 10-' (7)
4.2 ±0.4 (7)
2.2(0.95-5.4) x 10"'(5)
4.3 ±0.2 (7)
3.2(1.1-9.4) x 10-''§(5)
3.1(2.2-4.5) x 10-7 §(7)
3.6 ± 0.2 (5)
3.8 ± 0.2 (5)
( 0 . 5 5 MM)
Histamine
(1.5 MM)
3.1 ±0.3 (5)
The final numbers in parentheses in each column are the number of experiments. Student's t-test for unpaired values was used for
responses to nerve stimulation; the t-test for paired values was used for responses to norepinephrine and serotonin. Control values given
are pooled values from all experiments.
* Stimulation was at 32 Hz; values are means ± SE.
t Geometric mean ED50 with the 95% confidence interval given in parentheses.
% Due to the rapid oxidation of norepinephrine in Krebs solution at 37°C, these values are the maximum responses to the highest
technically feasible concentration.
|| P < 0.025 compared with control.
§ P < 0.05 compared with control.
lished observations). Responses of this vessel to
submaximal transmural nerve stimulation were
potentiated by histamine (Fig. 3A). However, maximum responses to nerve stimulation were not
increased in the presence of histamine (Table 1).
The i-norepinephrine ED60 for the saphenous
artery was decreased by histamine (Table 1, Fig.
3B). Responses to low concentrations of lnorepinephrine were most markedly potentiated.
However, in contrast to the basilar artery, the
o Control
»'Histominel0.5jiM)
&'Hisfamine II.5JIMI
A.
maximum response to /-norepinephrine was not
increased when histamine was in the bath (Table
1).
Serotonin-induced contractions of the saphenous
artery were also markedly potentiated by histamine (Fig. 3C). The serotonin ED 50 was significantly decreased (Table 1). Again, responses to low
concentrations of serotonin were potentiated the
most. The maximum response to serotonin was
unchanged in the presence of histamine (Table 1).
B.
C.
}n-5
4-
n--5
1-
3210
8
16
STIMULATION FREQUENCY (Hz)
8
8
-LOG /-NOREPINEPHRINE CONCENTRATION (M)
-LOG SEROTONIN CONCENTRATION (M)
FIGURE 3
Effect of histamine on contractile responses of the saphenous artery. A: Mean frequency-response
curves obtained by transmural nerve stimulation, 200 pulses. B: l-Norepinephrine dose-response
curve. C: Serotonin dose-response curve. Vertical bars represent the SE.
Circulation Research, Vol. 36, May 1975
651
HISTAMINE POTENTIATION
TABLE 2
Measurements of the Media of Rabbit Basilar and Saphenous
Arteries
Number of layers
of muscle cells
Thickness of media
Artery
Basilar
Saphenous
4.8 ±0.1
9.8 ± 0.5
29.2 ± 2.1
90.3 ± 4.8
(/im)
Values are means ± SE for three rabbits.
COMPARISON OF RESPONSES OF THE BASILAR AND SAPHENOUS
ARTERIES
Downloaded from http://circres.ahajournals.org/ by guest on October 2, 2016
To compare the tensions developed by the basilar and saphenous arteries, the force developed by
arterial ring segments of similar length should be
corrected by the cross-sectional area of the tunica
media or alternatively by the number of layers of
smooth muscle cells in the wall. Measurement of
these parameters (Table 2) indicates that the
saphenous artery should develop tension approximately two to three times greater than that of the
basilar artery, all other factors being equal. The
ratio of control responses to nerve stimulation is
considerably greater than this, indicating that the
saphenous artery is much more responsive to nerve
stimulation. However, if the control responses of
the saphenous artery are compared with the responses of the basilar artery in the presence of
histamine, the ratio of these responses approaches
the theoretical range of two to three.
EFFECT OF SEROTONIN ON CONTRACTILE RESPONSES OF THE
BASILAR ARTERY
It has been reported that low concentrations of
serotonin potentiate responses of the rabbit ear
artery to nerve stimulation, Z-norepinephrine, and
other agonists (11, 12). Because of the findings with
0.2 _, A.
° Control
•+Serotonin(0.084pM)
n
?^ D.
n--/8
UJ
Q_
1-
0.1-
o
n-S
1 4
8
16
STIMULATION FREQUENCY (Hz)
-LOG/-NOREPINEPHRINE
CONCENTRATION (M)
FIGURE 4
Effect of serotonin on contractile responses of the basilar artery.
A: Mean frequency-response curves obtained by transmural
nerve stimulation, 200 pulses. B: l-Norepinephrine dose-response curves. Vertical bars represent the SE.
Circulation Research, Vol. 36, May 1975
histamine, the possible action of serotonin on the
basilar artery was investigated. Serotonin (0.084
/*M) produced a contraction that was 20% of the
maximum serotonin-induced response. Submaximum responses to nerve stimulation were often
decreased in the presence of serotonin (Fig. 4A),
and the maximum response to nerve stimulation
was significantly decreased. The Z-norepinephrine
ED50 and the maximum response to Z-norepinephrine were unchanged (Fig. 4B).
Discussion
Synergistic actions of histamine, serotonin, and
Z-norepinephrine have been briefly mentioned by
other authors (13, 14). A more comprehensive
study (11) has shown that serotonin, but not
histamine, potentiates responses of the rabbit ear
artery to nerve stimulation and Z-norepinephrine:
this finding is in contrast to those of the present
study. In the rabbit basilar artery, histamine
potentiated responses to nerve stimulation, Znorepinephrine, and serotonin, but serotonin did
not potentiate adrenergic responses. The potentiating effect of histamine was not unique to cerebral
vessels; responses of the saphenous artery to various agonists were also increased in the presence of
histamine. Although the characteristics of the
potentiation were not identical, these findings
suggest that even in a single species histamine and
serotonin exert at least quantitatively different
potentiating effects on various blood vessels.
Potentiation by histamine was not the result of a
change in resting tension. In the first place, the
resting tension employed was optimum. Secondly,
a concentration of histamine that did not produce
maintained contraction still increased responses to
nerve stimulation. Finally, serotonin, in doses that
did increase maintained tension, did not increase
responses to other agonists.
The potentiating effect of histamine appears to
be mediated by specific histamine receptors. A
dose of pyrilamine that blocked the contractile
effect of histamine also prevented the increase in
response to other agonists. The response to nerve
stimulation in the presence of histamine was
blocked by tetrodotoxin. Since tetrodotoxin specifically blocks nerve conduction (15), the potentiating
effect of histamine is not due to a change in
sensitivity to electrical stimulation of the smooth
muscle itself; likewise, it is probably not due to an
increased neuronal response to stimulation, since a
supramaximal voltage was employed. Responses to
exogenous Z-norepinephrine and serotonin were
increased by histamine. Although a prejunctional
652
Downloaded from http://circres.ahajournals.org/ by guest on October 2, 2016
effect of histamine cannot be ruled out, it is likely
that the potentiation of responses to nerve stimulation can largely be accounted for by changes in the
response of the smooth muscle cells to /norepinephrine, as reflected in the changed /norepinephrine dose-response curve.
The observation that the basilar artery gives
consistent responses to nerve stimulation in the
presence of low concentrations of histamine indicates that this vessel is able to respond well to
sympathetic nerve stimulation. In fact, in the
presence of histamine, the basilar artery becomes
almost as sensitive to nerve stimulation as are
peripheral arteries when the responses are corrected for different amounts of smooth muscle.
When responsiveness to nerve stimulation is increased, sensitivity to /-norepinephrine is also
greater. This fact lends support to the concept
that cerebral arteries are normally relatively unresponsive to nerve stimulation because of the relative insensitivity of their postsynaptic receptors
to /-norepinephrine.
/-Norepinephrine and serotonin ED 50 values for
both the basilar and the saphenous artery were
decreased in the presence of histamine. The slopes
of the /-norepinephrine dose-response curves were
smaller when histamine was in the bath: contractions induced by lower concentrations of either
agonist were potentiated more than those resulting from higher concentrations. Thus, a comparison of ED5o values underestimates the change in
sensitivity of smaller responses in the presence of
histamine. Maximum contractile responses of the
basilar but not of the saphenous artery to drug or
nerve stimulation were increased with histamine in
the bath.
Histamine could potentiate responses by blocking neuronal uptake of adrenergic transmitter, but
there is no published evidence that histamine has
such an effect. Desmethylimipramine (10~ 6 M),
which blocks the neuronal uptake of /-norepinephrine, does not affect the response of the basilar
artery to /-norepinephrine (Bevan, unpublished
observation). Blockade of neuronal uptake by cocaine in the rabbit ear artery shifts the doseresponse curve to /-norepinephrine by a factor of 10
at the most (16); the increase in sensitivity to
/-norepinephrine produced by histamine in the
basilar artery is 100 times. Histamine is not known
to modify extraneuronal disposition pathways of
/-norepinephrine. Blockade of these pathways produces only a relatively small change in sensitivity
to norepinephrine in the aorta (17).
Nonspecific postjunctional supersensitivity observed in nonvascular smooth muscle following
BEVAN. DUCKLES. LEE
decentralization or denervation (18, 19) has a slow
onset as do the associated changes in resting
membrane potential (20) and nexus density (21).
The effects of histamine in this study, on the other
hand, were detected within minutes of exposure of
the tissue.
The results suggest that histamine is causing
potentiation by altering the general sensitivity of
smooth muscle cells to a variety of agonists.
Histamine could influence the sensitivity of the
specific receptors themselves, but such an action
would imply that histamine has a similar effect on
at least two distinct receptor systems. Of course,
potentiation of responses to /-norepinephrine and
serotonin is not necessarily produced by the same
mechanism. Alternatively, histamine could be acting on some mechanism common to both receptor
systems. Although responses of vascular smooth
muscle to vasoconstrictors can be coupled by
nonelectrical mechanisms (22), an effect of histamine through the membrane potential cannot be
ruled out. Potentiation of responses of the rabbit
ear artery is abolished in the presence of potassium
depolarizing solutions (11).
Drugs may influence the contractile activity of
smooth muscle to various agonists by modifying
the utilization of extracellular or intracellularly
bound calcium (23-25). The magnitude of these
drug effects, however, is much less than those seen
in this study.
Release of serotonin and histamine from rabbit
platelets forms part of the response to tissue injury
(26). These vasoconstrictors might work together to
induce a strong contraction of vascular smooth
muscle that minimizes hemorrhage. This normal
and useful vascular reaction to tissue injury might
become a deleterious response of cerebral vessels in
cases of subarachnoid hemorrhage. When a cerebral aneurysm ruptures, contraction of the vessel
will help to minimize further hemorrhage into the
subarachnoid space. However, if circulation of the
cerebrospinal fluid is impaired, histamine, serotonin, norepinephrine, and other vasoconstrictors
released from nerves, blood, or the brain parenchyma may collect in the cerebrospinal fluid (27).
These substances working together could produce
an especially strong and persistent contraction of
cerebral vessels. Such a mechanism may contribute to the production of persistent vasospasm in
some patients after subarachnoid hemorrhage.
References
1. PURVES MJ: Physiology of the Cerebral Circulation. London,
Cambridge University Press, 1972
2. NELSON E, RENNELS M: Innervation of intracranial arteries.
Brain 93:475-490, 1970
Circulation Research, Vol. 36, May 1975
653
HISTAMINE POTENTIATION
3. IWAYAMA T, FURNESS JB, BURNSTOCK G: Dual adrenergic and
cholinergic innervation of the cerebral arteries of the rat.
Circ Res 26:635-646, 1970
4. DAHL E: Innervation of the cerebral arteries. J Anat
115:53-63, 1973
5. D'ALECY LG, FEIGL EO: Sympathetic control of cerebral
blood flow in dogs. Circ Res 31:267-283, 1972
6. UCHIDA E, BOHR DF, HOOBLER SW: Method for studying
isolated resistance vessels from rabbit mesentery and
brain and their responses to drugs. Circ Res 21:525-536,
1967
7. TODA N, FUJITA Y: Responsiveness of isolated cerebral and
peripheral arteries to serotonin, norepinephrine, and
transmural electrical stimulation. Circ Res 33:98-104,
1973
8. BEVAN JA, BEVAN RD: Localized neurogenic vasoconstriction of the basilar artery. Stroke 4:760-763, 1973
sympathetic innervation on vascular sensitivity to noradrenaline. Br J Pharmacol 31:82-93, 1967
17. KALSNER S: Steroid potentiation of responses to sympathomimetic amines in aortic strips. Br J Pharmacol
36:582-593, 1969
18. TRENDELENBURG U: Mechanisms of supersensitivity and
subsensitivity to sympathomimetic amines. Pharmacol
Rev 18:629-640, 1966
19. FLEMING WW, MCPHILLIPS JJ, WESTFALL DP: Postjunctional
supersensitivity and subsensitivity of excitable tissues to
drugs. Ergeb Physiol 68:55-119, 1973
20. FLEMING WW: Altered membrane potential (RMP) of supersensitive smooth muscle cells (abstr). 5th Int Congr
Pharmacol, no. 70, 1972
21. LEE TJ, WESTFALL DP, SMITH DJ, FLEMING WW: Morpholog-
9. DUCKLES SP, LEE TJ-F, BEVAN JA: Histamine potentiation
of contractile responses of rabbit basilar artery. Proc West
Pharmacol Soc 17:118-121, 1974
22.
Downloaded from http://circres.ahajournals.org/ by guest on October 2, 2016
L0. FLEMING WW, WESTFALL DP, DE LA LANDE IS, JELLETT LB:
Log-normal distribution of equieffective doses of norepinephrine and acetylcholine in several tissues. J Pharmacol Exp Ther 181:339-345, 1972
LI. DE LA LANDE IS, CANNELL VC, WATERSON JG: Interaction of
L2.
L3.
14.
L5.
serotonin and noradrenaline on the perfused artery. Br J
Pharmacol 28:255-272, 1966
SCROOP GC, WALSH JA: Interactions between angiotensin,
noradrenaline and serotonin on the peripheral blood
vessels in man. Aust J Exp Biol Med Sci 46:573-580, 1968
FURCHGOTT RF: Pharmacology of vascular smooth muscle.
Pharmacol Rev 7:183-265, 1955
WURZEL M, ZWEIFACH BW: Contracting principles of arterial
smooth muscle in rabbit and dog plasma. Arch Int
Pharmacodyn Ther 162:1-19, 1966
GERSHON MP: Effect of tetrodotoxin on innervated smooth
muscle preparations. Br J Pharmacol 29:259-279, 1967
16. DE LA LANDE IS, FREWIN D, WATERSON JG: Influence of
Circulation Research, Vol. 36, May 1975
23.
24.
25.
ical evidence for an increase in cell contacts (nexus)
between smooth muscle cells of the rat vas deferens after
postganglionic denervation (abstr). Pharmacologist
15:172, 1973
SOMLYO AP, SOMLYO AV: Vascular smooth muscle: I.
• Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev 20:197-272, 1968
BEVAN JA, VERITY MA: Sympathetic nerve-free vascular
muscle. J Pharmacol Exp Ther 157:117-124, 1967
CARRIER O JR, JUREVICS HA: Role of calcium in "nonspecific" supersensitivity of vascular muscle. J Pharmacol
Exp Ther 184:81-94, 1973
KALSNER S: Mechanism of potentiation of vascular response
by tetraethylammonium: A novel form of sensitization.
Can J Physiol Pharmacol 51:451-457, 1973
26. SOLATUNTURI E, PAASONEN MK: Intracellular distribution of
monoamine oxidase, 5-hydroxytryptamine and histamine
in blood platelets of rabbit. Ann Med Exp Fenn
44:427-430, 1966
27. WURTMAN RJ, ZERVAS NT: Monoamine neurotransmitters
and the pathophysiology of stroke and central nervous
system trauma. J Neurosurg 40:34-36, 1974
Histamine potentiation of nerve- and drug-induced responses of a rabbit cerebral artery.
J A Bevan, S P Duckles and T J Lee
Downloaded from http://circres.ahajournals.org/ by guest on October 2, 2016
Circ Res. 1975;36:647-653
doi: 10.1161/01.RES.36.5.647
Circulation Research is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231
Copyright © 1975 American Heart Association, Inc. All rights reserved.
Print ISSN: 0009-7330. Online ISSN: 1524-4571
The online version of this article, along with updated information and services, is located on the
World Wide Web at:
http://circres.ahajournals.org/content/36/5/647
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in
Circulation Research can be obtained via RightsLink, a service of the Copyright Clearance Center, not the
Editorial Office. Once the online version of the published article for which permission is being requested is
located, click Request Permissions in the middle column of the Web page under Services. Further information
about this process is available in the Permissions and Rights Question and Answer document.
Reprints: Information about reprints can be found online at:
http://www.lww.com/reprints
Subscriptions: Information about subscribing to Circulation Research is online at:
http://circres.ahajournals.org//subscriptions/