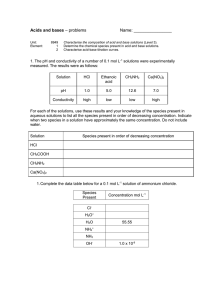
People credited with this unit standard are able to predict the
advertisement

US 8950 version 3 Chemistry student help sheet 2008 Predict the formation of precipitates of sparingly soluble substances. People credited with this unit standard are able to predict the formation of precipitates of sparingly soluble substances Elements and Performance Criteria element 1 Predict the formation of precipitates of sparingly soluble substances. performance criteria Solubility data for sparingly soluble salts is used to determine Ks values and vice versa. Range: calculations are limited to AB, A2B and AB2 type salts. 1.1 Examples all answers must be calculated to 3 sf. All working must be shown 1. The solubility of lead(II) hydroxide at 25oC is 6.64 x 10-4 mol L-1. Calculate its solubility product. Pb(OH)2(s) + aq Pb2+(aq) + 2OH-(aq) Ks = [Pb2+(aq)] [OH-(aq)] 2 Since s = 6.64 x 10-4 mol L-1 ; [Pb2+(aq)] = 6.64 x 10-4 mol L-1.; [OH-(aq)] = 2 x 6.64 x 10-4 mol L-1. Ks = 6.64 x 10-4 x (2 x 6.64 x 10-4) 2 ANSWER: KS = Or just use Ks= 4s3 for an AB2 type of compound 2. Given that the solubility product of lead(II) sulphate, PbSO4, at 25oC is 1.60 x 10-8 mol L-1, calculate the solubility at this temperature in mol L-1 and g L-1. M(PbSO4) = 303 g mol-1 PbSO4(s) + aq [Pb2+(aq)] + [SO42-(aq)] Solubility of lead(II) sulphate = concentration of PbSO4 in solution [Pb2+(aq)] = [SO42-(aq)] Ks = s2 Just go straight to the formula if you prefer s= Ks s = .............................. mol L-1 Convert mol L-1 into gL-1 by multiplying by molar mass, M s = ............................ g L-1 NOTE: solubility, s, as worked out from Ks expression is always in mol L-1 since concentration [x] is always expressed in mol L-1. If you are asked for concentration in g L-1 instead, multiply answer by MOLAR MASS, (M). (n = m/M, therefore m = n x M) WANGANUI HIGH SCHOOL US 8950 version 3 Chemistry student help sheet 2008 Predict the formation of precipitates of sparingly soluble substances. Elements and Performance Criteria 1.2 Precipitation is predicted using information on amounts, volume and Ks values. Examples 3. Ks (BaSO4) = 1.1 x 10 -10. Will a precipitate form when barium chloride solution and sodium sulphate solution are mixed so that at the moment of mixing the concentration of Ba2+(aq) = 0.00500 mol L -1 and the concentration of SO4 2-(aq) = 0.0500 mol L -1 ? Ionic product = [Ba2+(aq)] [SO4 2-(aq)] = 0.00500 x 0.0500 Since I.P. is ______________.. Ks a precipitate WILL / WILL NOT form. 4 You work it out! Will a precipitate form when 50 mL of 4.00 x 10-3 mol L-1 NaCl solution and 50 mL of 6.00 x 10-5 mol L-1 AgNO3 solution are mixed? Ks(AgCl) = 1.80 x 10-10. Answer: Calculate the concentration of the relevant ions in the mixed solutions. If the final vol is Volume of mixed solutions = 100 mL doubled (ie 50 mL + 50 mL then the [ions] is halved [Ag+] = 6.0 x 10-5 x 50/100 = 3.0 x 10-5 mol L-1 [Cl-] = = 4.0 x 10-3 x 50/100 2.0 x 10-3 mol L-1 Then calculate the ionic product when the solutions are mixed… Q = = = [Ag+][Cl-] 3.0 x 10-5 x 2.0 x 10-3 6.0 x 10-8 Don’t forget to write this as part of your answer Since Q > Ks, there will be a precipitate. Exercises: 1. Will a precipitate of BaF2 form if 150 mL of 0.100 mol L-1 Ba(NO3)2(aq) is mixed with 50 mL of 0.0500 mol L-1 KF(aq)? Ks (Ba F2) = 1.70 x 10-6 2. Which of the following pairs of solutions (all of concentration 0.0100 mol L-1) will form a precipitate when equal volumes are mixed at 25oC? Show your calculations a) b) c) d) silver nitrate and sodium chloride lead (II) nitrate and sodium bromide silver nitrate and potassium bromate magnesium sulfate and sodium hydroxide. Solubility data at 25oC Ks (AgCl) = 2.00 x 10-10 Ks (lead II bromide) = 3.90 x 10-5 Ks (silver bromate V) = 6.00 x 10-5 Ks (magnesium hydroxide) = 2.00 x 10-11 WANGANUI HIGH SCHOOL US 8950 version 3 Chemistry student help sheet 2008 Predict the formation of precipitates of sparingly soluble substances. Answers 1. Will a precipitate of BaF2 form if 150 mL of 0.100 mol L-1 Ba(NO3)2(aq) is mixed with 50 mL of 0.0500 mol L-1 KF(aq)? Ks (Ba F2) = 1.70 x 10-6 You need to Calculate the [ions] involved at the time of mixing calculate the new [ ] after mixing [Ba2+] = 0.100 x (150mL/200mL) = 0.0750 mol L‐1 [F‐] = 0.0500 x (50mL/200mL) = 0.0125 mol L‐1 Now calculate the ionic product Ks = [Ba2+] [F‐]2 IP = [Ba2+] [F‐]2 Don’t forget to write = 0.0750 x (0.0125)2 this as part of your = 0.0750 x 1.5625 x 10‐4 answer = 1.17 x 10‐5 Since IP (Q) > Ks (BaF2) a precipitate will form ___________________________________________________________________ 2 Which of the following pairs of solutions (all of concentration 0.0100 mol L-1) will form a precipitate when equal volumes are mixed at 25oC? Show your calculations (a) the ppt will be AgCl Mixing equal volumes halves the [ion ] after At time of mixing [Ag+] = 0.0100 /2 = 5 x 10‐3 mol L-1 ‐3 -1 mixing! [Cl‐] = 0.0100/2 = 5 x 10 mol L IP = (5 x 10‐3)2 = 2.5 x 10‐5 which is greater than Ks (AgCl) = 2.00 x 10‐10 so a precipitate of AgCl(s) will form (b) the ppt will be PbBr2 At time of mixing [Pb2+] = 0.0100 /2 = 5 x 10‐3 mol L-1 [Cl‐] = 0.0100/2 = 5 x 10‐3 mol L-1 IP = [Pb2+] [Cl‐]2 = 5 x 10‐3 x (5 x 10‐3 )2 = 1.25 x 10‐7 which is less than Ks (lead II bromide) = 3.90 x 10‐5 so a precipitate will not form (c) ppt = silver bromate IP = IP = (5 x 10‐3)2 = 2.5 x 10‐5 which is less than Ks (AgBrO3) so a precipitate of AgBrO3(s) will not form (d) ppt = Mg(OH)2 Ionic product = 5 x 10‐3 x (5 x 10‐3 )2 = = 1.25 x 10‐7 which is greater than Ks(Mg(OH)2 so a precipitate will form WANGANUI HIGH SCHOOL