texas children`s hospital evidence-based clinical decision support
advertisement

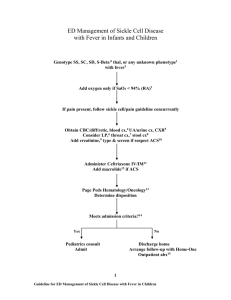
TEXAS CHILDREN'S HOSPITAL EVIDENCE-BASED CLINICAL DECISION SUPPORT DATE 1/2008 ACUTE CHEST SYNDROME (ACS) GUIDELINE Definition: Acute illness associated with lower respiratory symptoms, new hypoxemia, or new infiltrate on Chest Xray. It is a leading cause of hospitalization and death in (1,2) children [and adults] with sickle cell disease. It is (3) estimated to account for 25% of SCD related deaths. Pathophysiology: About half of the patients diagnosed with ACS develop the respiratory complication during hospitalization for another diagnosis such as pain crisis. Opioid analgesics, post-operative atelectasis, and asthma (1) may promote the development of an ACS episode. Although the etiology is undetermined in many cases, pulmonary infarction, fat embolism, and infection are now recognized as important causes of ACS. Chlamydia pneumoniae, Mycoplasma pneumoniae, Streptococcous pneumoniae, Staphylococcus aureus and parvovirus are (2) some of the pathogens commonly isolated. Diagnosis: Clinical history, physical examination and chest X-Ray are used to diagnose ACS. History: Assess for Lower respiratory symptoms (cough, wheezing, tachypnea, chest pain) Fever Previous history of ACS episode Physical Examination Assess respiratory rate (RR), use of accessory muscles, color. Auscultate lungs Obtain pulse ox. Detect new onset hypoxemia Rate severity of ACS according to Clinical Respiratory Score Table 1. ACS Clinical Respiratory Score (21) Assess A RR B Auscultation C Use of Accessory Muscles D Mental Status E Room Air SpO2 Color F Score 0 Score 1 Score 2 1-5 years <30 > 5 years <20 Good air movement, expiratory scattered wheezing or loose rales/crackles 1-5 years 30-40 > 5 years 20-30 Depressed air movement, inspiratory and expiratory wheezes or rales/crackles Mild to no use of accessory muscles. Mild to no retractions or nasal flaring on inspiration. Normal to mildly irritable > 95% Moderate intercostals retractions, mild to moderate use of accessory muscles, nasal flaring. 1-5 years >40 > 5 years >30 Diminished or absent breath sounds, severe wheezing or rales/crackles or marked prolonged expiration. Severe intercostals and substernal retractions, nasal flaring Normal Irritable, agitated, restless Lethargic 90-95% <90% Pale to normal Cyanotic, dusky Add score from A to F to obtain the total CRS score. (see Table 1) Guideline Eligibility Criteria Age 1-21 years Patient with Sickle Cell Disease Principles of Clinical Management (see Table 2) Guideline Exclusion Criteria Patients without sickle cell disease Critical Points of Evidence Evidence Supports Use of incentive spirometry in patients with vaso-occlusive (13,22) crisis (VOC) decreases development of ACS Evidence Lacking/Inconclusive Use of IV steroids for pain crisis (rapid recovery vs (5,6,12) rebound effect) Early use of Packed Red Blood Cells (PRBC) in patients (5,23) with vaso-occlusive crisis (VOC) can prevent ACS Serum phospholipase A2 (sPLA2) could be used as a (23) screening tool 1 Table 2. Principles of Clinical Management RECOMMEND MONITORING DIAGNOSTICS Vital signs (blood pressure, heart rate, respiratory rate) every 4 hours Pulse oximetry every 12 hours or continuous (encouraging ambulation) Weigh daily Strict charting of fluid intake / output Pain severity rating minimum every 4 hours Monitor Clinical Respiratory Scale (CRS) at least every 4 hours (see table1) if CRS 2 follow ACS Respiratory Support management flowchart CBC, differential, platelet count and reticulocyte count (initially & daily until improving—compare with patient´s baseline values) CXR if cough, chest pain, hypoxemia or any respiratory symptoms present or develop after admission CONSIDER FLUIDS, NUTRITION, GENERAL CARE Total fluid intake [intravenous plus oral] at 1x maintenance. Encourage ambulation, activity IF patient 5 yo use Incentive Spirometry (IS) as follows: Supervised Incentive spirometry [IS] - 10 breaths every 2 hours between 8 AM and 10 PM. Alternate the use of positive expiratory pressure (PEP). IS should be documented alternately by respiratory therapist and nursing services. OXYGEN PAIN MANAGEMENT ANTIBIOTICS Maintain pulse ox > 94% Notify care providers of increased FiO2 requirement or increase in CRS score Acetaminophen Per Protocol PRN T>100.4F (MAX: 75 mg/kg/day, not to exceed 4,000 mg/day) Morphine 0.1-0.2 mg/kg/dose IV every 2 hours OR PRN pain OR PCA (MAX: 15mg/dose) Ibuprofen: (if no contraindications) 10 mg/kg/dose PO every 6-8 hours (MAX: 800 mg/dose, not to exceed 40mg/kg/day or 2,400 mg/day) – Limit frequent dosing to 72 hour duration Ketorolac (if no contraindications, administer either ketorolac OR ibuprofen) 0.5 mg/kg/dose IV every 6 hours for 5 days (MAX: 30 mg/dose) – Do not exceed 5 days/month Discontinue prophylactic penicillin while on wide spectrum antibiotics IF history of recent fever CONSIDER Blood culture IF severe illness or Hb >1g/dL below baseline Hb strongly CONSIDER Type & crossmatch IF severe illness CONSIDER BUN, creatinine, ALT, AST, bilirubin, IF severe abdominal pain CONSIDER abdominal ultrasound IF patient is dehydrated or insensible losses are increased as with persistent fever ADMINISTER more fluids. IF patient < 5 yo: CONSIDER introducing the use or IS and PEP depending on cooperation. CONSIDER making the patient blow soap bubbles IF febrile, Cefuroxime 50 mg/kg/dose IV every 8 hours (MAX: 1,500 mg/dose) IF patient is PENICILLIN/CEPHALOSPORIN ALLERGIC, Substitute Clindamycin 10 mg/kg/dose IV every 6-8 hours (MAX: 900 mg/dose) IF severe illness CONSIDER addition of Vancomycin; for children <70 kg: 15 mg/kg/dose IV every 8 hours; for children ≥70 kg: 1,000 mg IV every 12 hours (MAX: 1,000 mg/dose) 2 ACS RESPIRATORY SUPPORT MANAGEMENT Patient meets ACS criteria Acute illness associated with lower respiratory symptoms, new hypoxemia, or new infiltrate on Chest X-ray FOLLOW Flowchart for SCD patients with pain and fever with the following modifications: Encourage ambulation (may come off pulse oximeter to ambulate) ADD Azithromycin: 10 mg/kg PO on day 1 [MAX: 500 mg/dose], then 5 mg /kg PO once daily [MAX: 250 mg/dose] for 4 days. (Erythromycin may be substituted) CXR initially, repeat for clinical deterioration Continue Incentive spirometry in patients 5years as follows: 10 breaths IS and 10 breaths PEP alternating every 2 hours between 8 AM and 10 PM In patients < 5 years old, PEP and/or IS can be introduced, but lack of cooperation should lead to earlier consideration of use of vest therapy. CONSIDER adding Vancomycin CONSIDER Furosemide 0.5 mg/kg IV x 1dose. IF signs of fluid overload (MAX: 40 mg/dose) Assess patient according to Clinical Respiratory Scale CRS 2-3 Vital signs every 4 hours Contiuous pulse oximeter. Goal SpO2 ≥ 94% Transfuse to Hb goal of 9-10 g/dL Continue IS + PEP every 2 hours IF Patient < 5 years Vest TID if 10kg IF Patient not wheezing: ≥ 5 years Vest or IPV TID (vest preferred) IF Wheezing or rales/crackles present Vest TID Inhaled bronchodilator per beta2 agonist protocol Methylprednisolone 1mg/kg/dose IV every 12 hours (MAX: 80mg/day) Ranitidine for GI prophylaxis Measure Peak Expiratory Flow (PEF) BID CRS 4 Vital signs every 2 hours Continuous pulse oximeter. Goal SpO2 ≥ 94% Transfuse to Hb goal of 9-10 g/dL Transfer to PCU IPV or Vest therapy to every 4 hours . Inhaled bronchodilator per beta2 agonist protocol Consider BiPAP IF Wheezing or rales/crackles present Vest every 4 hours Methylprednisolone 1mg/kg/dose IV every 6 hours (MAX: 80mg/day) Ranitidine for GI prophylaxis Measure PEF BID CRS ≥6 CRS 5 Vital signs every 2 hours Continuous pulse oximeter. Goal SpO2 ≥ 94% Transfuse to Hb goal of 9-10 g/dL. Consider exchange transfusion to 10 g/dl and Hb S 25% or Hb S+C 25% Transfer to PCU or PICU. Consider management in PICU if history of previous ACS episode with exchange transfusion. CCM consult upon admission and Pulmonary Service Consult at least prior to discharge BiPAP + IPV every 4 hours Inhaled bronchodilator per beta2 agonist protocol IF Wheezing or rales/crackles present Vest every 4 hours Methylprednisolone 1mg/kg/dose IV every 6 hours (MAX: 80mg/day) Ranitidine for GI prophylaxis Measure PEF BID Vital signs every 2 hours Continuous pulse oximeter. Goal SpO2 ≥ 94% Perform exchange transfusion Remove Venous Catheters as soon as possible after exchange transfusion Manage in PICU BiPAP + IPV Inhaled bronchodilator per beta2 agonist protocol IF Wheezing or rales/crackles present Vest every 4 hours Methylprednisolone 1mg/kg/dose IV every 6 hours (MAX: 80mg/day) Ranitidine for GI prophylaxis Measure PEF BID 3 Discharge Criteria (1) Improved pulmonary symptoms and documentation of adequate oxygenation on room air Afebrile 24h and negative cultures for 24-48 h if applicable Stable hemoglobin/hematocrit. Taking adequate oral fluids and able to take PO medications if applicable Adequate pain relief, if needed, with oral analgesics Slow wean from steroids (over 7-10 days) Parent understands discharge care, including steroid wean when Hematology Clinic and Pulmonary SCD Clinic follow-ups scheduled Home care agencies notified as needed Follow-Up Follow-up plans coordinated with hematology service to consider disease modifying therapy (Hydroxyurea, chronic transfusions,stem cell transplantation) Follow up in Pulmonary Sickle Cell Clinic if CRS 3 any time during hospital admission, should have formal Pulmonary Function Tests performed on the same day 4 References 1. Lane P, Buchanan G, Hutter J, Austin R, Britton H, Rogers Z, et al. Sickle cell disease in children and adolescents: Diagnosis, guidelines for comprehensive care, and care paths and protocols for management of acute and chronic complications. 2001 2. Vichinsky E, Neumayr L, Earles A, Williams R, Lennette E, Dean D, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. NEJM. 2000; 342: 1855-65 3. Castro O, Brambilla D, Thorington B, Reindorf C, Scott R et al. The Acute Chest Syndrome in Sickle Cell Disease: Incidence and Risk Factors. Blood 1994; 2: 643-648 4. Ohene-Frempong, Weiner S, Sleeper L, Miller S, Embury S, Moohr J et al. Cerebrovascular Accidents in Sickle Cell Disease: Rates and Risk Factors. Blood 1998; 91:288-294 5. Ballas S. Sickle Cell Anaemia: Progress in Pathogenesis and Treatment. Drugs 2002; 62(8):11431172 6. Knight J, Murphy T and Browning I. The Lung in Sickle Cell Disease. Pediatric Pulmonology.1999. 28:205-216. 7. Dreyer Z. Chest Infections and Syndromes in Sickle Cell Disease of Childhood. Seminars in Respiratory Infections.1996; 11(3):163-172 8. Stuart M and Setty Y. Acute chest syndrome of sickle cell disease:new light on an old problem. Curr Opin in Hematol.2001:111-120 9. Needleman J,Benjamin L,Sykes J and Aldrich T. Breathing patterns during vaso-occlusive crisis of sickle cell disease. Chest.2002; 122(1):43-46 10. Swerdlow P. Red Cell Exchange in Sickle Cell Disease. Hematology 2006 11. Lawson S,, Oakley S, Smith N, Bareford D. Red cell exchange in sickle cell disease. Clin Lab Haem 1999; 21:99-102. 12. Bernini JC, Rogers Z, Sandler E, Reisch J, Quinn C, Buchanan G. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood 1998; 92: 3082-89 13. Bellet P, Kalinyak K, Shukla R, Gelfand M and Rucknagel D. Incentive spirometry to prevent acute pulmonary complications in sickle cell diseases. NEJM. 1995; 333:699-703 14. Kress JP,Pohlman AS, Hall JB. Determination of Hemoglobin Saturation in Patients with Acute Sickle Chest Syndrome: A comparison of arterial blood gases and pulse oximetry. Chest 1999; 115:1316-1320. 15. Needleman JP, Setty BN, Valotta L, Dampler C and Allen J. Measurement of Hemoglobin Saturation by Oxygen in children and Adolescents With Sickle Cell Disease. Pediatric Pulmonology 1999; 28: 423-428. 16. Langenderfer B. Alternatives to percussion and postural drainage: A review of mucus clearance therapies: Percussion and postural drainage, autogenic drainage, positive expiratory pressure, flutter valve, intrapulmonary percussive ventilation, and high frequency chest compression with the ThAIRapy vest. Journal Of Cardiopulmonary Rehabilitation. 1998; 18:283-289. 17. McCool FD, Rosen MJ. Nonpharmacologic Airway Clearance Therapies: ACCP Evidence-Based Clinical Practice Guidelines. Chest. 2006; 129:250-259. 18. Elkins MR, Jones A, Van der Schans C. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. 2006. Cochrane Database. 19. Boyd J, Macklin E, Strunk R and DeBaun M. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108 :2923-2927 20. Padman R and Henry M. The use of bilevel positive airway pressure for the treatment of acute chest syndrome of sickle cell disease. Abstract. Del Med J.2004.76: 199-203 21. Myers J The Complete Respiratory Assessment Score accurately predicts outcomes in children with acute RAD exacerbations. * Power Point presentation (Unpublished) 22. Graham L. Sickle Cell Disease: Pulmonary Management Options. Pediatric Pulmonology, Supplement 26:191-193. 2004 23. Styles, L. A., M. Abboud, et al. (2007). Transfusion prevents acute chest syndrome predicted by elevated secretory phospholipase A2.Br J Haematol 136(2): 343-4. 5 Guideline Preparation This guideline was prepared by the Evidence-Based (EB) Clinical Decision Support Team in collaboration with content experts at Texas Children’s Hospital and the Texas Children’s Pediatric Associates. Development of this guideline supports the TCH Quality and Patient Safety Program initiative to promote clinical guidelines and outcomes that build a culture of quality and safety within the organization. ACS Content Expert Team Gladstone Airewele MD, Pediatric Hematology/Oncology Michele Mariscalco MD, Critical Care Julie Katkin MD, Pulmonary Medicine Joseph Allen MD, Emergency Medicine Lee Evey, RT Respiratory Therapy Suzanne Iniguez RT, Respiratory Therapy Joy Hesselgrave RN, Nursing Elizabeth Fredeboelling, RN Tangula Taylor, RN Shelley Oh, Pharm D. EB Clinical Decision Support Team Marilyn Hockenberry PhD, RN, PNP-CS, FAAN Co-Chair Virginia Moyer MD, MPH, Co-Chair Research Specialist Mireya Paulina Velasquez MD Development Process This guideline was developed using the process outlined in the EB Clinical Decision Support Manual (2007). The review summary documents the following steps: 1. Review Preparation -PICO questions established -Evidence search terms confirmed with content experts 2. Review of Existing Internal and External Guidelines - One published guideline from the Sickle Cell Disease Care Consortium - One published guideline from the ACCP on Nonpharmacologic Airway Clearance Therapies Search for Relevant Evidence -Searched: Medline, EmBase, Cochrane, BIREME, Google Scholar 3. 4. Critically Analyze the Evidence -Three systematic reviews from the Cochrane Database, 5 RCTs , 29 non-randomized studies, 16 review articles 5. Summarize the Evidence by preparing the guideline, order sets and interdisciplinary plan of care -Materials used in the development of the guidelines, review summaries and content expert team meeting minutes are maintained in an Acute Ches Syndrome EB review manual within the Center for Quality. Study/systematic review with minor limitations - specifically lacking in one of the above criteria Study/systematic review with major limitations - specifically lacking in several of the above criteria. Published clinical guidelines evaluated for this review using the AGREE criteria. The summary of these guidelines are found at the end of this document. AGREE criteria uses a 1-4 point likert scale to evaluate 23 questions evaluating: Guideline Scope and Purpose, Stakeholder Involvement, Rigor of Development, Clarity and Presentation, Applicability, and Editorial Independence. The higher the score the more comprehensive the guideline. This guideline specifically summarizes the evidence in support of or against specific interventions and identifies where evidence is lacking. The following categories describe how research findings provide support for treatment interventions. “Evidence that supports” the guideline (p.1) provides clear evidence from more than one well-done randomized controlled trial (RCT) (based on CASP criteria) that the benefits of the intervention exceed harm. “Evidence against” (p.1) provides clear evidence from more than one well-done RCT (based on CASP criteria) that the intervention is likely to be ineffective or that it is harmful. “Evidence lacking” (p.1) indicates there is currently insufficient data or inadequate data to recommend for or against specific intervention. Recommendations Recommendations for the guidelines were developed by a consensus process directed by the existing evidence, content experts and patient and family preference when possible. The Content Expert Team and EB Clinical Decision Support Team remain aware of the controversies in the management acute chest syndrome in children with sickle cell disease. When evidence is lacking, options in care are provided in the guideline and the order sets that accompany the guideline. Approval Process Guidelines are reviewed and approved by the Content Expert Team, EB Clinical Decision Support Team, EB Executive Steering Team, Pharmacy and Therapeutics Committee and other appropriate hospital committees as deemed appropriate for the guideline’s intended use. Guidelines are reviewed and updated as necessary every 2 years within the EB Clinical Decision Support Team at Texas Children’s Hospital. Content Expert Teams will be involved with every review and update. Disclaimer Guideline recommendations are made from the best evidence, expert opinions and consideration for the patients and families cared for within TCH/TCPA. The guideline is NOT intended to impose standards of care preventing selective variation in practice that are necessary to meet the unique needs of individual patients. The physician must consider each patient’s circumstance to make the ultimate judgment regarding best care. Evaluating the Quality of the Evidence The Critical Appraisal Skills Program (CASP) criteria were used to evaluate the quality of articles reviewed. Application of the CASP criteria are completed by rating each reviewed study or review as: Strong study/systematic review - well designed, well conducted, adequate sample size, reliable measures, valid results, appropriate analysis, and clinically applicable/relevant. ___________________________________________________ © Evidence-Based Decision Support Team, Quality and Outcomes Center, Texas Children’s Hospital; October 2007 6