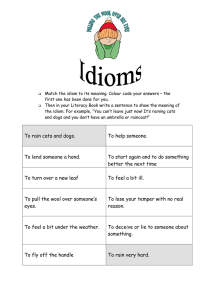
Zoonotic diseases in companion animals
advertisement

Editorial Zoonotic diseases in companion animals Companion animals have been important to humans since the first dog joined man, and the positive aspects of human-animal interaction are well known and have been thoroughly documented. However, there is a concern relating to this close contact between people and their pets: companion animals carry the risk of transmitting diseases to humans. This group of diseases is termed zoonoses. Zoonotic diseases are diseases being common to, shared by or transmitted between human beings and other vertebrate animals. How do we handle the challenge of zoonotic diseases while maintaining the positive sides of sharing household with companion animals? Not by diminishing the human-animal interaction, but by increasing the knowledge on the actual diseases. This includes knowledge on disease recognition and management as well as prophylactic measures. Veterinarians need to be able to recognise zoonotic diseases and the effect of such diseases in animals primarily. But companion animal veterinarians also need knowledge on the effect of these diseases on humans, thus being able to work in cooperation with human doctors on disease control. The second online issue of EJCAP, the official journal of FECAVA, is dedicated to the topic of zoonotic diseases. You will find a collection of articles written by outstanding European experts in their field. It is our goal that the information gained through these articles will help you in identifying and handle zoonoses in the best way possible. Ellen Bjerkås Scientific Editor of EJCAP 212 ZOONOSES Companion animals as reservoir for zoonotic diseases J. Grøndalen, B. Sævik, H. Sørum SUMMARY Companion animals live in close contact with the human population, and the risk of transmitting zoonotic diseases to humans is therefore significant if the animal itself has been infected. Increased travel activity increases the possibility of transfer of infection between animal populations, and increased risk for contact with new infectious agents. An increasing number of people suffer from immunodeficiencies. Environmental- and climatic conditions cause a change in distribution of vectors in need of special climatic conditions to establish. Exotic species are also to an increasing extent introduced as family pets, which may contribute to a wider panorama of infections. However, the traditional zoonotic diseases are still the most important. Vaccination, proper hygiene measurements and knowledge on preventive measures restrict the risk of transmittance of infections from companion animals. The most significant risk of companion animals in Norway are mostly related to dog and cat bites or other physical injuries. In total the benefit and pleasure of this type of animal husbandry is more important than the fear of zoonotic diseases. may be transferred indirectly from companion animals. Climatic changes cause a change in distribution of invertebrates that may act as vectors in transmitting diseases between species. One example is the cat flea (Ctenocephalides felis) that at present is slowly spreading from Denmark through Southern Sweden to Norway. This paper was first published in the Norwegian Veterinary Journal, 11/2004. Introduction Companion animals are of great significance in today’s society. 37% of Norwegian households own a family pet [1]. Of these, 44% include dogs and 50% cats. Stray dogs are a nonexisting problem in Norway. Cats are to a major extent kept as companions, although in the cities there may be colonies and stray cats. The use of medicines and increased prevalence of diseases causing immunodeficiency result in humans being more susceptible to infections, and they may develop more serious clinical symptoms if diseased. Increased knowledge on epidemiology and infections reveal zoonotic significance in more infectious diseases than earlier anticipated. Thus, we must be open to the increased risk of transmittance between species. In Norway, with a population of about 4.5 million inhabitants, there are about 42,000 horses, 216,000 rabbits, 135,000 captive birds and 11,000 reptiles kept as pets [1]. These animals normally live in close contact with humans, and, conditions permitting, there may be risk of transmittance of zoonotic agents. This paper describes the most relevant zoonotic diseases that may be transferred from companion animals. Table 1 presents an overview of the zoonoses that should be known to veterinarians. Relevant references may also be found in earlier issues of the Norwegian Veterinary Journal [2-9]. The regulations for import of dogs and cats from the EU and EFTAcountries were subject to change in 1994, thus facilitating easier transport of animals between the European countries. Through this, the risk of animals attracting “new” infectious diseases and further transmittance to humans is increased. Infections may be transmitted from animals that suffer from illness, or the animals can be asymptomatic carriers. Agents dependent on a vector Dogs and cats Diseases mainly transferred by saliva through bites and scratches Many bite wounds caused by dogs or cats develop into (1) The Norwegian School of Veterinary Science. 213 Companion animals as reservoir for zoonotic diseases - J. Grøndalen through saliva, and there is highest risk of transmittance from young adult cats [10]. The most common clinical symptoms in humans are non-pruritic swelling on the inoculation site and lymphadenopathy. In some cases fever and general malaise occur. In rare cases, acute encephalopathy, liver- and spleen abscesses and pneumopathies may develop [14]. Most infected cats are asymptomatic carriers, but debilitated individuals may develop generalised infection. Dogs are believed also to develop and transfer disease caused by Bartonella spp. In the period 1998-99 samples were taken from 100 cats in Mid-Norway. None of the cats were seropositive. This finding corresponds with the fact that the disease has not been reported in humans in Norway [15]. Rabies Rabies is an acute, progressive non-treatable virus encephalitis [2, 3]. Carnivores and bats are the most important reservoirs. Transmittance occurs through saliva, most often through bites from infected animals. The virus follows the nerve pathways to the central nervous system. After virus replication virus is excreted through saliva. The incubation time is from one to three months, however, development of clinical symptoms have occurred days to years after transmittance of infection. The dog represents the most significant reservoir for disease in humans. About 35,000 humans are infected every year as a consequence of dog bites [16]. The infection is maintained through infected fox herds in arctic, tempered and tropical areas, these representing the most important reservoir for infection in Eastern Europe. Hares, rabbits, rodents and birds are considered to have little significance in transmittance of infection. After 1980 more rabies cases have been reported in cats than in dogs in the US [17]. However, cats still not represent the most important reservoir for infections to humans. Because of the long incubation time and increased global travel activity, rabies may be imported to areas formerly free of the disease. Clinical symptoms in humans include fever, anorexia, ataxia, anxiety, altered mental state, paralysis, coma and death. The patient usually dies at latest ten days after appearance of clinical symptoms. Rabies has not been diagnosed in mainland Norway since the beginning of the nineteenth century, but has been diagnosed sporadically in polar fox, reindeer and seal in Svalbard, the most recent cases in 1999 [2, 18]. Vaccine delivered in meat dropped form aeroplanes as feed for foxes has reduced the rabies problem in Europe significantly the last 25 years. The disease is at present more common in Eastern Europe. Turtles are asymptomatic carriers of salmonella. It has been reported that reptiles can have a carrier frequency of more than 90%. All reptiles must be considered potential carriers. Here an information leaflet about zoonoses from the Institute of Protection from Infections in Sweden. infections. Cat bites are usually associated with a higher risk of wound infection than dog bites. Potential pathogen bacteria can be cultivated from about 90 % of bite wounds caused by a cat or a dog, and in most cases more than one agent is diagnosed. The most common isolates include species of Pasteurella, Streptococcus, Staphylococcus, Neisseria and Moraxella. Other agents are sporadically isolated [10]. Capnocytophaga canimorsus (former Bacteroides ochraceus, DF-2) is a gram negative rod considered to be part of the normal flora of the oral cavity in dogs and cats. The bacteria is rarely causing disease in humans, however, and if so, a predisposing factor is usually present, including former splenectomy or other cause of immunodeficiency [11], caused by an infection after a dog bite. Fatal outcome has also been reported from Norway [12]. Pasteurella multocida is also part of the normal flora in most vertebrates, including the dog and cat. Pasterurella infections are especially seen after cat bites [10, 13]. Clinical signs may include cellulitis, lymphangitis and lymphadenopaty, in some cases in combination with arthritis [10]. Enteric diseases with mainly faecal-oral transmittance Salmonellosis The most common sero-variant affecting animals in mainland Norway are S.typhimurium. Rodents and other wild animals, including birds, serve as a reservoir for infection [4]. “Cat scratch disease” The disease is caused by Bartonella henselae (former Rochalimaea henselae) that is a small, bent gram negative rod. Other Bartonella spp. may also cause disease in humans. Most humans that develop “cat scratch disease” are children or young adults, and the disease is considered to be the most common cause of chronic lymphadenopathy in this age group [11]. In some geographic areaes where the cat flea (C.felis) is endemic, up to 40 % of the healthy cats may suffer from Bartonella bacteriaemia. Cats with chronic bacteriaemia are contagious Norwegians are most commonly infected when travelling to other countries, in this cases the sero-variant S.enteritidis is the most common agent isolated, mainly after consumption of contaminated food. The most common sources of infection both for animals and humans in Norway are contaminated food and direct faecal-oral transmittance. Dogs have a relatively low 214 EJCAP - Vol. 18 - Issue 3 December 2008 susceptibility for infection with salmonella bacteria compared to other animals, like horse and cattle, however, salmonellosis has recently been diagnosed in dogs infected through contaminated chewing bones made from slaughter offals. So-called inverse infection, where animals are being infected through owners suffering from salmonellosis in not uncommon. and unkempt fur. Clinical signs may be constantly present or occur periodically. Giardia spp. as cause of diarrhoea in puppies and young dogs are occasionally diagnosed. The prevalence of Giardia spp. and cryptosporidiae seems relatively high among about 600 dogs included in a study in Norway. The prevalence in cats in Norway has not been studied (I.S.Hamnes, personal information, 2004). Clinical signs in animals vary dependent on number of bacteria and immune status in the host animal. Gastroenteritis is the most common manifestation of disease. Bacteriaemia and enterotoxaemia are usually subclinical signs in the gastrointestinal manifestation in dogs and cats, however, serious depression, hypothermia and shock may also be seen with or without gastrointestinal signs. Animals recovering from infection may shed bacteria up till six weeks post infection. Toxoplasmosis is caused by Toxoplasma gondii with cat as definite host. The cat only plays a minor role for direct transfer of the protozoan. However, cats shed oocysts through faeces. These oocysts sporulate within one to five days causing the cat to act as a source of infection. Thus, new faeces does not represent a source of infection, but sporulated oocysts may survive for months to years. Infection to humans through contaminated sand and soil is considered rare compared to ingestion of uncooked meat. Infected cats only shed oocysts for a short period in life. Routine screening of cats for antibodies against T.gondii is of limited value, but pregnant women are recommended not to touch cat faeces because of the risk of infection, which may cause abortion or congenital disease in the child [18]. Infected cats are most often asymptomatic, but signs of generalised infection may occur in young or debilitated cats [17]. Camphylobacteriosis Camphylobacteriosis may be caused by different species of the camphylobacter- bacteria in dog and cats. The most common sub species to cause diarrhoea in humans is Camphylobacter jejuni sub species jejuni. C.jejuni can cause enteric disease in the dog. The dog is also considered to be the most important carrier of C.upsaliensis. In a Norwegian survey from 2000-2001, 24 % of 595 dogs and 18 % of 332 cats tested positive for Camphylobacter spp. Most of the isolates from both dogs and cats were C.upsaliensis. The frequency was equal in animals with and without diarrhoea [19]. This bacteria may also cause diarrhoea in humans. Echinococcosis Echinococcus granulosus and E.multilocularis are the tapeworms of dogs and foxes, respectively. The parasite has a life cycle that includes two hosts, and it is the larval stage of the parasite that causes disease. In predators the tapeworm develops to maturation and produces eggs that are spread to the environment. When eggs are ingested by grazing animals, rodents or humans, the larvae are activated and migrate to different organs where cysts develop. These cysts can be large and destroy the surrounding tissue. The cysts can also develop microcysts that spread further. Dogs and cats are invaded when ingesting either prey with tapeworm, in earlier years also through slaughter offal from contaminated reindeer. Today antiparasitic treatment of reindeer is routinely performed and E.granulosus is almost extinct in Mainland Norway. However, of ten examined mice (Microtus epiroticus) in Svalbard in 2002, two were found positive for E.multilocularis [18]. Both types of tapeworm are found in areas in Europe, however, thus antiparasitic treatment of dogs immediately before and after import to Norway is mandatory. An increased risk for camphylobacteriosis has been found in humans that keep dogs or cats as pets [20]. Toxocarosis Toxocara canis and T.cati are the nematodes of the dogs and cats, respectively, and the most important parasite forming migrating larvae (larvae migrans) in humans. Adult dogs and cats usually present with only asymptomatic (“dormant”) infections, while untreated puppies and kittens may be heavily infested. In connection with pregnancy in the dam the larvae are reactivated. This may cause transplacental transmittance to the foetuses. If the dam and puppies do not undergo antiparasitic treatment during the first two weeks after birth, eggs are shed through faeces. In humans ingesting eggs, the eggs will develop in the intestine. Since humans are not natural hosts, the larvae will migrate and may cause serious damage in many organs, including the retina. Luckily, such cases are rare in Norway. Cats carry a lower significance than dogs for transmitting the parasite to humans [21]. Antiparasitic treatment with regular intervals, plus removal of faeces in the environment reduces the risk of transmittance of the parasite. Diseases mainly transmitted through direct or indirect contact with skin or skin lesions Ringworm Ringworm (dermatophytosis) is caused by closely related fungi (dermatophytes) that are only able to infect keratinised tissues in nails and claws, hair and stratum corneum. Protozoans In giardiosis and cryptosporidiosis acute and chronic small intestinal diarrhoea may develop both in humans and animals. Wild animals serve as potential reservoirs for dogs and cats, which are infected through contaminated feed or drinking water. Most infected dogs and cats do not show signs of disease, however, some animals do develop diarrhoea, anorexia The dermatophytes are classified as anthropophilic, zoophilic and geophilic and have humans, animals and soil as their main reservoirs. All dermatophytes able to cause ringworm in animals can also cause disease in humans [9]. The significance of dermatophytes as cause of skin lesions in dogs and cats is not known, but there are indications that the 215 Companion animals as reservoir for zoonotic diseases - J. Grøndalen papules and/or erythematous maculae, often in groups in the contact area(s); arms, chest and abdomen. Gradually, vesiculae and pustules develop [25]. disease is over diagnosed clinically, especially in dogs. Cats may be an asymptomatic carrier. However, this is considered rare in dogs. In a Norwegian study of skin and hair samples from animals suspected of suffering from ringworm 5% of 780 samples from dogs and 30,8% of 279 samples from cats were found positive [22]. Ear mites The ear mite, Otodectes cynotis, can invade the aural canal in cats, dogs and ferrets and cause otitis externa. Kittens and puppies are most susceptible for invasion. Transfer to humans with development of pruritic, papular lesions have been reported [26,27]. The cat is considered the most important reservoir for infection to humans. Transfer of the fungus occurs either through direct or indirect contact. In Norway, as in other countries, Microsporum canis is the dominant agent in ringworm infections in the cat [22, 23]. Stenwig [22] reported that M.canis represented 83 of the samples in 86 positive cats. Likewise, E. Christensen (personal information 2003) reported that 33 of 36 positive samples from cats were diagnosed as M.canis. Affected cats may show clinical signs, they may be subclinically infected or they may serve as vectors. About 50% of humans exposed to M.canis positive cats will show skin lesions. In households with infected cats at least one person will be infected in about 70% of the households. Typical skin lesions in humans are circular, slightly prominent, crustaceous and erythematous [10]. Cowpox Cowpox virus causes sporadic infection in cats, and cowpoxinfection has also been reported in cats in Norway [28]. The natural reservoir is wild rodents. Cats are usually infected through hunting. Generalised disease transmitted through direct or indirect contact Leptospirosis Leptospirosis is caused by the bacteria Leptospira interrogans, a spirochaet that is further subdivided into many serologic variants (serovars), of which many are able to cause disease [7]. Scabies The mite Sarcoptes scabei var. canis is most often diagnosed in dogs. However, it is not species specific and the mite has been diagnosed in many other animal species [24]. About 24 hours after contact with an affected dog, humans may develop papulous severely pruritic lesions on the contact area of the skin, most often the arms and body. Spontaneous remission occurs after 12-14 days. It has been shown that the mite can survive on a human being up till six days and may produce eggs in this time period [25]. In Norway the serovariants Ichterohaemorrhagiae and Canicola have been connected with disease. The disease has not been reported in humans in Norway for more than 20 years, Thus, infection from endemic areas in other countries is considered the most important risk [6]. Up to the 1950ies leptospirosis was relatively common in dogs in Norway. In 1947 Strande found that 6.5% of dogs hospitalised for internal medicine disease suffered from leptospirosis [29]. During the last years, there have been single cases of leptospirosis in dogs unrelated to import [6]. In other countries in Europe and in USA the disease is still stationary, and increased prevalence may be seen in years with humid weather. Leptospirosis is probably the most widespread zoonosis in the world [30]. Fur mites The fur mites, Cheyletiella yasguri, C.blakei, C.parasitivorax, cause varying severity of pruritus and dandruff in dogs, cats and rabbits, however, cats and dogs may also be asymptomatic carriers. In humans, the mites show a “hit-and-run” behaviour, so that repeated direct contact with an infested animal is necessary in order for skin lesions to develop. Typical lesions in humans are Clinical signs of infection in dogs include generalised infection that may range from per acute to more chronic development. Petechiae and bleeding tendency may be seen, as well as icterus, which is more common in the subacute form. Hepatitis may lead to intrahepatic cholestasis with grey faeces as one manifestation. Rabbits are popular and common pets. Rabbits can be affected by fur mites and transfer mites to humans through direct contact. Rabbits can also carry ringworm and can spread the infection to humans [14]. Tularemia can spread to rabbits through infection via ticks and fleas from wild rabbits and hares. Photo: Yngvild Wasteson Horse Salmonellosis Salmonellosis in horses presents with enteritis and septicaemia, with foals and stressed adults being especially susceptible. S.typhimurium is the most common serovar isolated from horses in Norway [4]. Clinical signs of salmonellosis in horse may be dramatic, especially in acute enteritis and may even result in the horse dying. Large amounts of contagious, soft stool, often gaseous and excreted under strong pressure represent a significant risk for transfer of bacteria to other animals and to humans. Isolation of infected horses and restricted access for humans taking care of the horses is of utmost importance, and caretakers must be 216 EJCAP - Vol. 18 - Issue 3 December 2008 of the examined animals. Even if many of these salmonella types belong to subgroups that are not pathogenic to humans, the risk of salmonella infection represents a significant problem as all reptiles must be considered potential carriers of infection [34]. The bacteria are shed periodically, thus single samples for diagnosis are of restricted value. The risk of infection is lowest in terrestrial species, snakes and lizards. These animals are rarely exposed to their own faeces, which in addition is hard and rarely excreted due to the slow metabolism. Aquatic turtles and terrapins represent a greater risk, as the water is contaminated by faeces. Children are especially exposed to infection. The animals should not be treated with antibacterial agents, as this may result in resistant bacteria. Reptiles are not recommended as pets for children (M.Heggelund, personal communication). instructed about risk of infection and necessary steps to avoid transfer of bacteria. Tuberculosis Horses are relatively resistant to tuberculosis, but may occasionally be infected with M.bovis, M.tuberculosis or M.avium [5]. Clinical signs are unspecific, and may include diarrhoea and respiratory signs. A case of avian tuberculosis was described in a horse in the nineties [31]. Ringworm Ringworm in horses in Norway is most often caused by Tricophyton equinum or Microsporum equinum [9, 22]. Cases of ringworm may be diagnosed in riding horses and trotters. Control of ringworm outbreaks in stables with sports horses and thus transport of animals between competitions and horse shows may be a challenge. In stables where horses are in frequent contact with humans, many of which are young people, the zoonotic aspect of the disease should be emphasized in the information given. Vector borne diseases Many infections causing disease in animals and humans are spread through an invertebrate vector. In Norway, the tick Ixodes ricinus is the most important vector transferring disease between species [8]. Birds Borreliosis (Lyme disease) Borreliosis is caused by the bacteria Borrelia burgdorferi and is probably the most widespread vector borne zoonotic disease in Northern Europe [35]. In humans, the disease leads to general infection, with skin, joint, nervous tissue and heart most often affected. Among animals, dogs and horses are especially susceptible. Serology is frequently used for establishing a diagnosis. A Swedish/Norwegian study of dogs from areas where I.ricinus is endemic, showed 4% and 27% respectively, of tested healthy animals to be seropositive [36,37]. Most seropositive animals do not develop clinical illness, but both acute and chronic disease with inflammatory signs may develop. The prevalence among cats has not been studied. In addition to salmonellosis, psittacosis is the most important zoonotic disease related to infection from wild or captive birds. The agent is found in secretion form eyes, nose and from faeces and transmittance is aerogenic or through direct contact. Birds with psittacosis are most often asymptomatic, however, generalised signs of infection may be seen in immune compromised birds. Respiratory symptoms and headache are the most common symptoms in humans [32]. Rabbits, rodents and ferrets Rabbits can be affected by fur mites (Cheyletiella spp.) and transmit invasion through direct contact – see description for dogs and cats. Rabbits are also susceptible to ringworm (Tricophyton mentagrophytes, M.canis and Microsporum gypseum) and may transfer infection to humans [14]. Tularaemia can be transferred to rabbits from wild rodents through ticks and fleas. Rodents can also suffer from ringworm (T.mentagrophytes). Horses may also suffer from borreliosis. Clinical signs are similar to what is found in dogs. The diagnosis is most often established in animals with disease, known exposure to ticks and positive serology. A Swedish study showed a seroprevalence for B.burgdorferi of 17%. No difference in seroprevalence was found between healthy hoses and horses with signs of disease [36]. Salmonellosis can be transferred from rodents to humans. Guinea pigs will most often die if they develop salmonellosis, while rats and mice may have subclinical infection. The most important serovariants are Typhimurium and Enteritidis. Anaplasmosis Anaplasma phagocytophilum is the present name of bacteria formerly termed Ehrlichia phagocytophila, E.equi and “Human granulocytic ehrlichial agent” (HGE). The intracellular bacteria affect granulocytes in the blood in particular and may be diagnosed through direct microscopy. “Granulocytic ehrlichiosis” in dogs was described from Sweden in 1989. Later studies showed that the dogs had been infected with A.phagocytophilum, the same type causing disease in humans and horses [38]. Clinical signs in affected dogs include fever, depression, lameness plus gastrointestinal and nervous symptoms. Haematology signs include thrombocytopenia, leukopaenia and anaemia. Asymptomatic cases are common. Studies have shown a seroprevalence in Swedish dogs to be 17% and 23% in dogs from Southern Norway. The significance of anaplasmosis in cats is not known, however, natural infection has been described. Leptospirosis can be transferred from rats, usually wild, and these are also considered a cause of infection in humans, through contaminated water. Ferrets may suffer from salmonellosis, camphylobacteriosis and ringworm. Reptiles Turtles are asymptomatic carriers of salmonella. A carrier frequency of more than 90% has been reported. In a Swedish study on snakes from 1988, salmonella was diagnosed in 41% 217 Companion animals as reservoir for zoonotic diseases - J. Grøndalen “Equine granulocytic ehrlichiosis” was described in USA in 1969. In Sweden the first cases were described in 1990. DNA analysis showed the bacteria to be closely related to E.phagocytophila and identical with agents causing human granulocytic ehrlichiosis [38]. Clinical signs in horses are similar to what is described in dogs, however, the infection can also be asymptomatic. The seroprevalence in a Swedish study was 17 %, antibody findings in healthy and diseased animals being equal [38]. Resistance to antibiotics Bacterial resistance to antibiotics is an increasing and challenging problem both in veterinary as well as in human medicine. Antibiotic resistance can develop through genetic adaptation in the bacteria. This occurs to a significant degree through genes causing antibiotic resistance frequently being transferred between bacteria as mobile genetic elements. Mobile resistance genes can be used by many bacteria, not necessarily closely related. This means that widespread use of antibiotics and increase in resistance genes in veterinary medicine can be of significance for the increase in bacteria being resistant to antibiotics and causing disease in humans. These bacteria pick up resistance genes directly from the animal pathogenic bacteria, or resistance genes are delivered through the normal flora in animals or humans as a type of zoonotic problem. Similarly, resistance genes included in animal pathogenic bacteria may be of human origin. Tick borne encephalitis (TBE) TBE is caused by flavivirus and is relatively widespread in Eastern Europe and the Baltic states. The disease has been known in humans in Sweden from 1954 with 40-80 new cases diagnosed annually in endemic areas [39]. The disease was diagnosed for the first time in humans in Norway in 1997. 5 cases are described from 1998–2001, all from Trom island in Southern Norway. Based on this, as serologic study of 317 healthy dogs from this area was carried out. Antibodies against TBE virus were found in 16.4% of the dogs [40]. Based on this, it can be stated that the disease is present in Norway and that dogs may serve as potential carriers. Clinical signs in diseased dogs include fever, and progressing CNS signs. In a study of 545 dogs from Austria, 131 had antibodies against TBE virus and about half of these showed clinical signs of disease [41]. Discussion and conclusion Companion animals are of benefit to humans. One study showed that people owning pets visited the doctor less frequently and had lower blood pressure and blood cholesterol than nonowners [43]. The highest risk in companion animal keeping is connected to the risk of bites and the risk of transfer of diseases from animals to humans. There has also been a focus on the risk of sensibilisation and development of allergy, especially to cats, but this is controversial. More recent studies have shown that children in households without a cat or a dog are at greater risk of developing allergies against these animal species [44]. Leishmaniasis Leishmaniasis is caused by an intracellular protozoan. In the Mediterranean area, the dog represents the main reservoir for infection to humans, the parasite being transmitted through a sand fly [3]. The disease has a cutaneous and a visceral form, the latter being caused by Leishmani donovania infantum and being endemic in areas in the Mediterranean area. The disease may progress slowly, and it may take years before development of clinical symptoms, although individual differences exist. In both humans and animals the visceral form leads to generalised symptoms of infection, emaciation, muscular atrophy, anaemia, liver- and kidney failure plus failure of other organs. The disease is diagnosed in Norway in animals imported from endemic areas in Italy and Spain. The sand fly does not survive the Norwegian winters, however, thus there is no risk for the disease to become endemic. The parasite may also be transferred to humans through skin contact with infected organs. The risk of transmitting disease from companion animals to humans is related to certain factors, including young animals, dense animal population and poor hygiene, insufficient prophylactic measures, free-roaming animas eating cadavers, contact with stray animals and increased travel activity. Small children, pregnant women and immunodeficient persons form the group at risk, but also veterinarians, nurses and other staff working with animals with infections are exposed. Even if the risk of transferring infection from companion animals is relatively low, the risk of infection can be reduced further. Owners, and especially those in the group at risk must be informed about the potential risk and possible routes of infection, including the life cycles of vectors and agents. This is a task for both veterinarians and doctors. Heartworm (Dirofilariasis) Dirofilaria immitis is the dog heartworm transferred through a mosquito that demands certain temperature and environmental conditions for survival. The mosquito has spread north in USA, but routine antiparasitic treatment of dogs during the summer months has prevented spreading of the parasite. Single cases of infection causing abortion have been described in humans. In Europe, Dirofilaria repens is diagnosed in humans in an increasing number. Noduse develop in skin, subcutis and internal organs. This parasite is also transferred through a mosquito. Studies of dogs from endemic areas in Italy have shown an increase in number of seropositive dogs. It therefore seems reasonable to consider a role from the dog as reservoir and in transfer of the parasite between species [42]. Dogs rarely develop skin nodules, but the parasite migrates through subcutaneous tissues. In short, the risk of transfer of infection can be reduced through - Good hygiene, in particular hand hygiene - Removal of faeces in outdoor areas, and regular cleaning of litter boxes - Antiparasitic treatment of the animals - Not letting children play in contaminated areas - Cover sand boxes when not being used - Avoid scratches and bites - Not bringing animals into areas with increased risk of infection/ invasion unless proper prophylactic measures have been taken However, in total the benefit of having a pet is higher than the fear of zoonotic disease. 218 Poxviridae Genus Orthopoxvirus Cowpox and other pox virus Brucella canis Campylobacter jejuni C. upsaliensis Bartonella henselae Bartonella spp. Leptospira interrogans serovar Ichterohemorrhagiae and Canicola Bacillus anthracis Chlamydophila psittaci Chlamydophila felis Salmonella spp. Capnocytophaga canimorsus (DF-2) Pasteurella multocida Mycobacterium spp. Brucellosis Campylobacteriosis “Cat Scratch Disease” Leptospirosis Anthrax Psittacosis, ornithosis Salmonellosis Capnocytophaga infection (wound infection, septicaemia) Pasteurellosis (wound infection, septicaemia) Tuberculosis Bacterioses Rhabdoviridae Genus Lyssavirus Agent Rabies Viroses Disease 219 Uncommon in Western countries Occurs, serious in humans with immunodeficiency Occurs, serious in humans with immunodeficiency Rel. common Occurs Global Dog, cat Dog, cat Dog, cat, most mammals, birds, reptiles Caged birds, wild birds Cat (zoonotic aspect uncertain) Horse Dog, rat, rarely cat and other mammals Cat, dog Dog, cat, other mammals, birds Most often (aerogenic) Dog, cat, rodents, infection from humans primates, horse, fish to animals (”inverse zoonosis”) Bites Bites Global Global Faecal-oral transmittance Aerogenic, faecal-oral transmittance Aerogenie, alimentary, contact infected organs Global Global Global Contact, urine and urine contaminated water, bite Global Not uncommon in Mid- and Southern Europe and other parts of the world Uncommon in the Western world Cat bites, cat scratches, through fleas and ticks Faecal-oral transmittance Dog Cat, dog, small rodents Contact with infected skin Infected organs, amniotic fluid, urine All mammals Affected species Saliva, usually through bites Transmittance Global, Global Not in Norway Global, endemic among small rodents in Norway Global, except Australia, Iceland, Sweden, Mainland Norway, UK Geographic distribution Rel. common in certain areas with fleas and ticks Rel. common Uncommon Uncommon Uncommon in Western world Human aspects Asymptomatic, granulomas in lungs, gen. infection, ulcerative dermatitis, meningitis Normal bacterium in the oral cavity Normal bacterium in the oral cavity Asymptomatic, gastroenteritis, septicaemia Asymptomatic, gen. infection. Conjunctivitis, upper respiratory tract signs (cat) More protracted development in horse than in cattle Fever, haemorrhage, icterus Asymptomatic, fever, uveitis, gingivitis, CNS signs. Dogs: endocarditis, hepatitis Asymptomatic, gastroenteritis Asymptomatic, abortion, lymphadenopathy, orchitis, bacteriaemia, discospondylitis Skin lesions on head, neck, front legs. Asymptomatic in small rodents Progressive CNS signs and death Clinical signs in animals EJCAP - Vol. 18 - Issue 3 December 2008 Tab. 1 Zoonoses that can be transferred from companion animals to humans. Under ”affected species” only companion animals are included. 220 Toxoplasma gondii Toxoplasmosis Echinococcus multilocularis Echinococcosis (Fox tapeworm) Toxocara canis Toxocara cati Toxocariasis (human: larvae migrans) Fur mite (cheyletiellosis) Cheyletiella spp. Parasittoses – mites and insects Ancylostoma spp. Uncinaria stenocephala Hookworm Parasittoses - nematodes Echinococcus granulosus Echinococcosis (Dog tapeworm) Parasittoses - tapeworm Giardia spp. Cryptosporidium spp. Giardiosis Cryptosporidiosis Parasittoses - protozooans Sporotrichosis ”rose Sporothrix schenkii growers disease” Ringworm (dermatophytoses) Hunters esp. susceptible Human aspects Occurs Uncommon Uncommon Uncommon in the Western world Uncommon in the Western world Uncommon Rel. common Rel. common Uncommon Rel. common Microsporum canis, Tricophyton mentagrophytes, Microsporum equinum, Tricophyton equinum Francisella tularensis Tularaemia Mycoses Agent Disease Global Global Global Global Global Global Global Global Most common in warm environment Global Global Geographic distribution Contact Faecal-oral transmittance Faecal-oral transmittance Faecal-oral transmittance Faecal-oral transmittance Faecal-oral transmittance Faecal-oral infection through contaminated water Faecal-oral transmittance Contact skin through scratches and bites, environmental pollutant Contact skin, directly or indirectly Direct contact with infected organs and bites from infected animals Transmittance Dog, cat, rabbit Dog, cat Dog, cat Dog, cat, rodents Dog Cat Asymptomatic, dandruff, pruritus Asymptomatic, depression, gastrointestinal and respiratory signs Asymptomatic, anaemia, diarrhoea, respiratory signs, linear skin lesions, pododermatitis Asymptomatic, organ changes due to pressure from cysts and granulomas Asymptomatic, organ changes due to pressure from cysts and granulomas Asymptomatic, gen. infection and organ failure in debilitated humans (rare) Dog, cat, bird, reptiles Asymptomatic, diarrhoea Asymptomatic, diarrhoea Topical cutaneous form, lymphocutaneous form and multifocal disseminated form Cat Cat, transfer from dog not documented Skin lesions with varying morphology, also asymptomatic (mainly cat) Ulcerativ dermatitis, lymphadenopathy, fever Clinical signs in animals Cat, dog, rabbits, rodents, horse Hare and other wild rodents, dog, cat, other animals Affected species Companion animals as reservoir for zoonotic diseases - J. Grøndalen Global Ctenocephalides felis, Occurs Ctenocephalides canis Fleas 221 Borrelia burgdorferi (bacterium) Anaplasma phagocytophilum m.fl (bacterium) Leishmania spp. (protozooan) Dirofilaria repens Borreliosis Anaplasmosis (ehrlichiosis) Leishmaniosis Dirofilariosis (heartworm) Global Areas where the vector is present Southern Europa Mediterranean, Africa, Asia, South- America Rel. common in areas Mediterranean, with sandflies Africa, Asia, South- America Global Mid-Eastern Europe, Nordic countries, Baltic states Areas where the vector is present Occurs, rel. common in the Baltic states Dog, cat Cat, dog and ferret Dog (cat and other mammals) Affected species Mosquito bites Bites from sandflies, probably also direct transmittance of infection Tick bites Ixodes ricinus, Rhipicephalus sanguineus m.fl. Dog Dog, cat Dog, horse Tick bites Ixodes ricinus Dog, horse m.fl. Tick bites Ixodes ricinus Dog Contact, direct and indirect Contact Contact Transmittance Asymptomatic, skin and subcutaneous nodules, heart symptoms Cutane form, visceral form, gen. signs of infection Asymptomatic, gen. Signs of infections, polyarthritis Asymptomatic, fever, polyarthritis Asymptomatic Asymptomatic, skin lesions, pruritus Asymptomatic, otitis externa, pruritus Papules, pruritus Clinical signs in animals The diseases listed below are uncertain with regard to the zoonotic aspect and if they can be transmitted from companion animals to humans. • Helicobacteriosis, enteric disease (Helicobacter spp.) may possibly be transferred through contact with infected organs via cats • Anaerobiospirillosis, enteric disease (Anaerobiospirillum spp.) may be transferred from infected cats • Yersiniosis, (Yersinia pseudotuberculosis) septicaemic form and classical syndrome with organ nodules in guinea pigs and other rodents, rabbits and wild and captive birds may possibly be transferred via dog and cat. Y. enterocolitica causes enteric form • Q-fever, infectious syndrome (Coxiella burnetti) may possibly be transferred through contact with infected organs via cat • Babesiosis, (Babesia spp.) may possibly be transferred via ticks and through bites and scratches from dogs • ”Rocky Mountain spotted fever”, infectious syndrome(Rickettsia rickettsiae) may possibly be transferred from cats to humans through flea bites Flaviviridae TBE virus TBE (tick borne encephalitis) Vector borne zoonoses Global Occurs Otodectes cynotis Global Ear mite (otodectosis) Occurs Sarcoptes scabiei var. canis Geographic distribution Scabies (sarcoptosis) Human aspects Agent Disease EJCAP - Vol. 18 - Issue 3 December 2008 Companion animals as reservoir for zoonotic diseases - J. Grøndalen References [24] CURTIS (C.) - Current trends in the treatment of Sarcoptes, Cheyletiella and Otodectes mite infestations in dogs and cats. Vet Dermatol, 2004,15: 108-14. [25] SCOTT (D.W.), MILLER (W.H.), GRIFFIN (C.E.) - Muller and Kirk’s small animal dermatology. 6th ed. Philadelphia : WB Saunders, 2001. [26] HERWICK (R.P.) - Lesions caused by canine ear mites. Arch Dermatol, 1978,114:130. [27] LOPEZ, (R.A.) - Of mites and man. J Am Vet Med Assoc, 1993,203: 606-7. [28] TRYLAND (M.), SANDVIK (T.), HOLTET (L.), HAUKENES (G.), OLSVIK (Ø.), TRAAVIK (T.) - Cowpoxvirus-infeksjon påvist hos katt i Norge. Nor Vet Tidsskr, 1996,108: 871-6. [29] STRANDE (A.) - Leptospirose. Nor Vet Tidsskr, 1947,59: 13-23. [30] LEWITT (P.) - Leptospirosis. Clin Microbiol, Rev 2001,14: 296326. [31] GUNNES (G.), NORD (K.), VATN (S.), SAXEGAARD (F.) - A case of generalized avian tuberculosis in a horse. Vet Rec, 1994,135: 565-6. [32] BRUU (A.L.) - Psittakose/ornitose. Zoonoser. Oslo: Alpharma, 1996: 30-2. [33] AHL (I.) - Salmonella ock reptilimport. Sven Vet Tidn, 1995,47: 613 –7. [34] TORFOSS (D.), ABRAHAMSEN (T.G.) - Salmonellasmitte fra skilpadder. Tidsskr Nor Lægeforen, 2000,120: 3670-1. [35] BERGLUND (J.) - Lyme borreliosis in Northern Europe, a general overview. Kristiansand: Den norske veterinærforenings etterutdanningskurs, 2003. [36] Engvall EO Borreliainfections in horses, dogs and cat. Kristiansand: Den norske veterinærforenings etterutdanningskurs, 2003. [37] ÅKERSTEDT (J.), BLAKSTAD (E.), ARTURSSON (K.) - Seroprevalens av Borrelia burgdorferi senso lato og Ehrlichia sp. hos hund fra et kystområde i Aust-Agder. Nor Vet Tidsskr, 1996,108: 537- 42. [38] EGENVALL (A.), FRANZÈN (P.), GUNNARSSON (A.), ENGVALL (E.O.), VÅGSHOLM (I.), WIKSTRÖM (U.B.) et al - Cross-sectional study of the seroprevalence to Borrelia burgdorferi sensu lato and granulocytic Ehrlichia spp. and demographic, clinical and tick-exposure factors in Swedish horses. Prev Vet Med, 2001,49: 191-208. [39] BRUU (A.L.) - Flåttbåren encephalitt (TBE). Zoonoser. Oslo: Alpharma, 1996: 45-7. [40] CSÁNGÓ (P.A.), BLAKSTAD (E.), KIRTZ (G.C.), PEDERSEN (J.E.), CZETTEL (B.) - Tick-borne encephalitis in Southern Norway. Emerg Infect Dis, 2004,10: 533-4. [41] KIRTZ (G.) - Tick-borne-encephalitis in an Austrian dog population. Kristiansand: Den norske veterinærforenings etterutdanningskurs, 2003. [42] PAMPIGLIONE (S.), RIVASI (F.), ANGELI (G.), BOLDORINI (R.), INCENSATI (R.M.), PASTORMERLO (M.) et al - Dirofilariasis due to Dirofilaria repens in Italy, an emergent zoonosis; report of 60 new cases. Histopathology, 2001,38: 344- 54. [43] HEADEY (B.), KRAUSE (P.) - Health benefits and potential budget savings due to pet. Australian and German survey results. Aust Social Mont, 1999,2: 4-6. [44] RÖNMARK (E.), PERZANOWSKI (M.), PLATTS-MILLS (T.), LUNDBÄCK (B.) - Four-year incidence of allergic sensitization among schoolchildren in a community where allergy to cat and dog dominates sensitization: Report from the obstructive lung disease in Northern Sweden group. J Allergy Clin Immunol, 2003,112: 747-54. [1] LANDBRUKSDEPARTEMENTET. Om dyrehold og dyrevelferd. Oslo 2002. St. meld. Nr 12 (2002 – 2003). [2] GUDDING (R.), HYLLSETH (B.), GRØNDALEN (J.) - Rabies. Nor Vet Tidsskr, 1996,108: 625-33. [3] HOLSTAD (G.), RIMSTAD (E.), THARALDSEN (J.), ULSTEIN (T.L.), ÅKERSTEDT (J.) - Infeksjoner som kan overføres fra hund til menneske (zoonoser) sett i relasjon til økt innførsel og tilbakeførsel av hund fra utlandet. Nor Vet Tidsskr, 1998,110: 337-43. [4] FOSSUM (K.), GRØNSTØL (H.), KAPPERUD (G.), SCHALLER (G.), SKJERVE (E.) - Salmonellainfeksjoner hos dyr og mennesker. Nor Vet Tidsskr, 1996,108: 639-45. [5] SAXEGAARD (F.), GRØNSTØL (H.), HOLSTAD (G.) - Tuberkulose. Nor Vet Tidsskr, 1996,108: 647-51. [6] SUNDE (M.), HEIENE (H.), FONNUM (K.J.), WOLD (A.) Leptospirose - en infeksjon med ny aktualitet? Nor Vet Tidsskr, 2003,115: 563-70. [7] SAXEGAARD (F.), ØDEGAARD (Ø.) - Leptospirose. Nor Vet Tidsskr, 1996,108: 667-71. [8] LARSEN (H.J.S.), HOLSTAD (G.), GRØNDALEN (J.) Infeksjonsssjukdommer hos hund som overføres med flått. Nor Vet Tidsskr, 1996,108: 751-3. [9] LUND (A.), GUDDING (R.), BAUSTAD (B.), ULSTEIN (T.L.) - Ringorm hos dyr. Nor Vet Tidsskr, 1996,108: 659-65. [10] GUAY (D.R.P.) - Pet-assisted therapy in the nursing home setting: potential for zoonosis. Am J Infect Control, 2001,29: 178-86. [11] ODOM (R.B.), JAMES (W.D.), BERGER (T.G.) - Andrews’ diseases of the skin. Clinical dermatology. 9th ed. Philadelphia, Saunders, 2000. [12] HOLTER (J.), GUNDERSEN (R.O.), NATÅS (O.), HAAVIK (P.E.), HOEL (T.) - Dødelig infeksjon etter hundebitt. Tidsskr Nor Lægeforen, 1989,109: 693- 4. [13] DAHL (E.) - Dyrebitt ved Oslo Kommunale Legevakt. Tidsskr Nor Lægeforen, 1998,118: 2614- 7. [14] LITWIN (C.M.) - Pet-transmitted infections: diagnosis by microbiologic and immunologic methods. Pediatr Infect Dis J, 2003,22: 768- 77. [15] BERGH (K.), BEVANGER (L.), HANSSEN (I.), LØSETH (K.) - Low prevalence of Bartonella henselae infections in Norwegian domestic and feral cats. APMIS, 2002,110: 309-14. [16] RUPPRECHT (C.E.), HANLON (C.A.), HEMACHUDHA (T.) - Rabies re-examined. Lancet Infect Dis, 2002,2: 327- 43. [17] American Association of Feline Practitioners 2003 Report on Feline Zoonoses. Compend Contin Educ Pract Vet, 2003,25: 93664. [18] HOFSHAGEN (M.), AAVITSLAND (P.), KRUSE (H.) Zoonoserapporten 2002. Oslo: Norsk zoonosesenter, 2003. [19] SANDBERG (M.), BERGSJØ (B.), HOFSHAGEN (M.), SKJERVE (E.), KRUSE (H.) - The occurrence of Campylobacter spp. in Norwegian cats and dogs. Prev Vet Med, 2002,55: 241-53. [20] KAPPERUD (G.) - Risikofaktorer for campylobakterinfeksjon i Norge. Norsk Epidemiologi, 1995,5: 44-9. [21] KRAVETZ (J.D.), FEDERMAN (D.) - Cat- associated zoonoses. Arch Intern Med, 2002,162: 1945- 52. [22] STENWIG (H.) - Isolation of dermatophytes from domestic animals in Norway. Nord Vet Med, 1985,37: 161-9. [23] PEPIN (G.), OXENHAM (M.) - Zoonotic dermatophytosis (ringworm). Vet Rec, 1986,118: 110. 222 EJCAP - Vol. 18 - Issue 3 December 2008 BUSTER Comfort Collar with Soft Rubber Outer Edge Our range of BUSTER collars has been extended with the new BUSTER Comfort Collar. To ensure that you get the real BUSTER Comfort Collar look for this label. Hi there My name is BUSTER. Check out my new Comfort Collar! It has a rubber outer edge which makes it more comfortable for me and less damaging to my surroundings. Comfort Collar Cat. No 273903 Made in Denmark by 15 cm "534%2 WWW.KRUUSE.COM © KRUUSE 10060 223 ZOONOSES Diagnostic tools for the detection of rabies virus L. M. McElhinney(1,3), A. R. Fooks(1,3), A. D. Radford(1,2) SUMMARY Rabies has the highest case-fatality rate of any currently recognized infectious disease with an estimated 55,000 human deaths per year. The disease is routinely diagnosed in animals based on clinical signs and on epidemiological grounds in rabies-endemic countries. The use of conventional diagnostic tests including the fluorescent antibody test (FAT), rabies tissue-culture infection test (RTCIT) and mouse inoculation test (MIT) can now be complemented with molecular diagnostic tools such as reverse transcription polymerase chain reaction (RT-PCR) and in situ hybridisation (ISH). Negative diagnostic test results do not exclude the clinical diagnosis as these tests are entirely dependent on the quality of the sample supplied. Molecular tools and virus typing are becoming more widely used for the rapid detection and strain identification of rabies viruses from ante-mortem clinical samples. The currently available diagnostic methodologies for both post-mortem and ante-mortem detection of rabies virus are reviewed. (Aravan virus, Khujand virus, Irkut virus and West Caucasian Bat Virus), have recently been proposed as new members of the Lyssavirus genus [4-6]. This paper was commissioned by FECAVA for publication in EJCAP. Rabies virus is largely maintained in two ecologically inter-related disease cycles; urban and sylvatic (wildlife). Urban rabies, primarily in dogs and cats, has been eliminated in an increasing number of developed countries via parenteral vaccination programmes. However, sylvatic rabies remains widespread throughout parts of Europe, Africa and North America and is maintained in a variety of species including the red fox, raccoon-dog, raccoon, skunks and bats. Urban canine rabies remains widespread throughout developing countries and human mortality from endemic canine rabies was calculated to be 55,000 deaths per year with 56% of the deaths estimated to occur in Asia and 44% in Africa [7]. The total global cost of rabies prevention is estimated to exceed $1 billion per year. However, the prevalence and cost of rabies is believed to be significantly underestimated due to poor reporting and surveillance [7]. Introduction Animal rabies is endemic throughout all continents with the exception of several islands and peninsulas including Australia and New Zealand, an increasing number of European countries and Antarctica. Having eliminated rabies, countries that are striving to maintain their rabies free status, usually do so at considerable cost and with a continual risk of re-importation [1,2]. Rabies viruses are members of the Lyssavirus genus, within the Rhabdoviridae family. The lyssaviruses comprise seven related virus groups (genotypes 1 - 7). These include, classical rabies virus (RABV, genotype 1), Lagos bat virus (LBV, genotype 2), Mokola virus (MOKV, genotype 3), Duvenhage (DUVV, genotype 4), European bat lyssavirus type 1 (EBLV-1, genotype 5), European bat lyssavirus type 2 (EBLV-2, genotype 6) and Australian bat lyssavirus (ABLV, genotype 7). With one exception, (MOKV), all genotypes have been isolated from bats (Tab. 1) [3]. Four additional rabies-related viruses, isolated from bats in Eurasia In Europe, sylvatic rabies (predominantly fox rabies) has been significantly controlled by the successful implementation of oral rabies vaccination (ORV) campaigns. The majority of the western European countries are now free of Classical rabies (RABV), with reported rabies restricted to relatively rarer bat (1) National Centre for Zoonosis Research, Neston, South Wirral, CH64 7TE, UK (2) Department of Veterinary Clinical Sciences, Leahurst Campus, Neston, South Wirral, CH64 7TE, UK (3) Rabies and Wildlife Zoonoses Group, WHO Collaborating Centre for the Characterisation of Rabies and Rabies-Related Viruses, Veterinary Laboratories Agency (VLA, Weybridge), New Haw, Addlestone, Surrey, KT15 3NB, UK Corresponding author: Dr Lorraine McElhinney. Tel.: +44 (0)151 7956058; Fax: +44 (0)151 7946005. E-mail: l.mcelhinney@vla.defra.gsi.gov.uk 224 Diagnostic tools for the detection of rabies virus - L. M. McElhinney, A. R. Fooks, A.D. Radford Genotype Phylogroup Species Abbrev. (ICTV) Distribution Potential vector(s) 1 I Rabies virus RABV World (except several islands) carnivores (world) bats (Americas) 2 II Lagos bat virus LBV Africa frugivorous bats 3 II Mokola virus MOKV Africa unknown (isolated from shrews) 4 I Duven-hage virus DUVV Africa insectivorous bats 5 I European bat lyssavirus 1 EBLV-1 Europe insectivorous bats (Eptesicus serotinus) 6 I European bat lyssavirus 2 EBLV-2 Europe insectivorous bats (Myotis sp) 7 I Australian bat lyssavirus ABLV Australia frugivorous/insectivorous bats Proposed I Aravan virus ARAV Central Asia Insectivorous bat (isolated from Myotis blythi) Proposed I Khujand virus KHUV Central Asia insectivorous bat (isolated from Myotis mystacinus) Proposed I Irkut virus IRKV East Siberia insectivorous bat (isolated from Murina leucogaster) Proposed II or III West Caucasian bat virus WCBV Caucasus region insectivorous bat (isolated from Miniopterus schreibersi) Tab. 1 Classification of Lyssaviruses. or imported cases. The most recent European country to be declared rabies free was Germany (September 2008). Rabies Bulletin Europe (WHO) reported 9563 cases of rabies in Europe in 2007 (includes figures for Russian Federation, Ukraine and Turkey). These cases were associated with red foxes (4515 cases, 47.2%), cats (1411 cases, 14.8%), dogs (1394 cases, 14.6%), cattle (1288 cases, 13.5%), raccoon-dog (364 cases, 3.8%), bat (26 cases, 0.3%) and humans (9 cases, 0.1%). Although fox rabies is the principal vector in Europe as a whole, the raccoondog plays a significant role in the epidemiology of rabies in the Baltic countries where numbers of infected raccoon-dogs can exceed that of foxes. The cattle and human cases are generally considered to represent dead end host spill-over events. Dog and cat rabies is still prevalent in some Eastern European countries, Belarus, Ukraine, Russia and Turkey. to demand serological testing under an EU derogation in place until June 2010. In addition, treatment for ticks and tapeworm is required for entry into Ireland, Malta and the United Kingdom, while Sweden requires tapeworm treatment and not treatment for ticks. Approximately 546,908 pets have entered the UK between February 2002 and August 2008 (http://defraweb/ animalh/quarantine/pets/procedures/stats.htm). Commercially traded eligible animals are transported without quarantine under the Balai Directive (Council Directive 92/65/EEC of 13 July 1992) as amended (e.g. regulation 998/2003). Rabies cases in Europe are principally attributed to three of the Lyssavirus genotypes, namely genotype 1 (RABV, classical rabies) and to a lesser extent genotypes 5 and 6 (European bat lyssaviruses type-1 and -2). The 26 insectivorous bat cases reported in 2007 were due to European Bat Lyssaviruses (EBLV-1 or -2). The majority (>95%) of the reported EBLV-1 cases in European bats have been identified in one bat species, Eptesicus serotinus (Serotine bat). The rarer EBLV-2 cases (2%) are associated with Myotis species, M.daubentonii (Daubenton’s bat) and M.dascyneme (pond bat). EBLV-1 has been reported in several countries across Europe (not UK) whereas EBLV-2 has only been confirmed in the UK, Netherlands, Switzerland and Germany. In 2000, the UK Government introduced a system whereby eligible dogs and cats from qualifying countries could enter or return to the UK without being subjected to quarantine. The UK Pet Travel Scheme (PETS) comprised of identification, vaccination and serological testing combined with tick and tapeworm treatment prior to entry. The scheme was extended to include further qualifying countries and in 2004 the EU Pet Travel Scheme succeeded it. The EU PETS allowed for the transportation of ferrets, a greater list of qualifying countries but no obligation to confirm seroconversion in the vaccinated animal or administer treatment for the cestode Echinococcus multilocularis (exotic to the UK). The UK, Malta, Sweden and Ireland retain the authority Spill-over of EBLV-1 into sheep has occurred on two separate occasions in Denmark, into a stone marten in Germany, domestic cats in France and one confirmed and possibly other unconfirmed human cases [8]. EBLV-2 has been recorded in two 225 EJCAP - Vol. 18 - Issue 3 December 2008 Human and animal rabies is usually acquired via the transdermal inoculation of infected saliva in the bite of a rabid animal but on rare occasions human rabies can be transmitted via aerosols or corneal and organ transplantation [9] or via routes other than bites [10-13]. In the majority of human cases, the exposure to an infected animal is known and the disease can be prevented by timely post exposure treatment. The successful administration of anti-rabies prophylaxis is enhanced by speedy and accurate diagnosis. early but significant antibody response (6 days after the onset of signs) which encouraged the clinicians to attempt a druginduced coma and a cocktail of anti-viral drugs. No virus or antigen was detected. Her treatment included the induction of coma with the anaesthetic ketamine and antiviral agents ribavirin and amantadine. This appeared to be successful and the patient recovered with only minor residual neurological deficits [17]. Despite a number of attempts to follow the costly procedure (known as the Milwaukee Protocol), this remarkable success has not been repeated. It is not clear if the success was due to the viral strain (assumed to be an American bat strain), development of paralytic rabies (lack of damaging hydrophobic spasms) or the immune status of the patient (rare early neutralising antibody production) [18]. Clinical Signs Clinical Diagnosis The long and variable incubation period is a key feature of rabies. It relates to the time that the neurotropic virus is protected from immune responses within peripheral cells and nerves. The incubation period can vary between 15 days to 6 years (average 2–3 months) depending on the strain, species, viral dose and the proximity of the site of virus entry to the CNS. A short incubation period would be expected for a deep bite near to the head of the victim. Most clinically infected species excrete rabies virus in the saliva. Experimental data suggests that different species exhibit varying times of virus excretion before the onset of clinical signs (cats <24hours, dogs<13 days, foxes <29 days post infection) [14]. Rabies is a notifiable disease in the EU. Clinical signs are not characteristic, especially early in the course of disease. Clearly the history may raise the index of suspicion e.g. travel especially smuggling, contact with infected wildlife including bats, lack of vaccination. When presented with a suspect case, owners and veterinarians must contact the competent authority in their country. human cases (Finland 1985 and Scotland 2002) but no other spill-over event has been reported. There has been no evidence of transmission of EBLV by any other terrestrial species; hence to date all spill-over events have been dead-end. Clinical diagnosis is based on the observation of clinical signs, the first of which usually appear after the variable incubation period and vary depending upon species. Differential diagnosis can be difficult due to common sequelae resulting from various diseases including transmissible spongiform encephalopathies, tetanus, listeriosis, poisoning, Aujeszky’s disease and other viral nonsuppurative encephalitidies. Paralytic rabies is often mistaken for Guillain-Barré Syndrome [19, 20]. In addition, secondary infections can mask the presence of rabies leading to misdiagnosis [21]. A report by Laothamatas et al. [22] demonstrated that Magnetic Resonance Imaging (MRI) could not differentiate the two forms of human rabies (paralytic and furious) as both share a similar MRI pattern. However, such a pattern in a non-comatose patient with suspected encephalitis may prove useful in distinguishing rabies from other viral encephalitidies. Prophylaxis and treatment Vaccination is the mainstay of control in at-risk human and animal populations. In humans, following suspect exposure, prophylaxis including repeated vaccinations with or without immunoglobulin therapy is usually administered. In cats and dogs, there is little evidence to show the effectiveness of such postexposure prophylaxis, and this is not generally recommended. Animals that are bitten by potentially infected animals should be immediately reported to the relevant authority. In the clinical course of rabies, three stages are classically described; prodromal, excitement (furious) and paralytic (dumb) (Tab. 2). However, it is important to note that not all stages are observed in individual cases. The first clinical symptom is usually neuropathic pain at the site of infection (bite wound) due to viral replication in dorsal root ganglia and ganglionitis. This is usually accompanied by non-specific signs typical of a viral encephalitis. Following the non-specific prodromal phase, either or both the excitement and/or paralytic forms of the disease may be observed in a particular species, with disease commonly progressing to the paralytic form. Cats are more likely to develop furious rabies than dogs [23]. In some cases, no clinical signs are observed and rabies virus has been identified as the cause of sudden death. Without intensive care interventions, death occurs within 1–10 days of neurological signs. In cats and dogs, the clinical phase can be relatively rapid at 3-4 days [15,16]. Although the mortality for clinical rabies is believed to be 100%, sporadically cases of survival are reported. At least five humans that had all received either pre- or post-exposure prophylaxis are believed to have recovered after developing clinical signs of rabies [7]. There is some doubt as to whether the clinical signs in some of these cases could be attributable to vaccine-induced encephalitis rather than rabies infection. However, in 2004, an unvaccinated teenager in Wisconsin, USA, was bitten by a bat, developed early signs of disease, yet became the first person to recover from the disease after experimental therapy was initiated. Rabies biologicals (vaccine or rabies immunoglobulin), usually administered as post exposure treatment, were not given to this patient. She developed fever, hypersalivation, parasthesiae of the bitten hand, progressive cranial nerve paralysis and leg weakness. Rabies was diagnosed by the detection of a rare In a recent study, Laothamatas et al. compared furious and paralytic rabies in dogs using neuroimaging (MRI) and RT-PCR (RABV RNA and brain cytokine mRNA) [24]. They concluded that the early stage of furious rabies in dogs is characterized 226 Diagnostic tools for the detection of rabies virus - L. M. McElhinney, A. R. Fooks, A.D. Radford Stage Signs Prodromal Pyrexia, altered personality, increased nervousness, loss of fear, disorientation, loss of appetite, hyperactivity, hypersensitivity to noise or light, rubbing or biting the site of inoculation (wound), tremor, dyspnoea. Excitement (furious) Increased aggression, abnormal vocalisations (high pitch howl in canines, characteristic bellowing in ruminants), ‘fly snapping’ at imaginary targets, wandering long distances, hyper sexuality, head butting (particularly ruminants), staring eyes, partial paralysis of tongue and lower jaw with drooling of saliva, ‘bone in throat’ syndrome. Acute colic with abdominal straining (horses and ruminants). Paralytic (dumb) Hind limb paralysis, generalised paralysis, dysphagia, respiratory arrest, inability to drink or swallow, excessive salivation, hydrophobia (only in man), convulsions, coma, death. Tab. 2 Clinical stages of rabies. Clinical progression is variable and although most end in the paralytic phase, some do not show many signs associated with the excitement phase. Rabies virus antigen detection techniques by severe virus neuroinvasiveness and moderate inflammation whereas paralytic rabies was associated with a delayed viral neuroinvasion and a more intense inflammation. However, during the late stage of infection, little difference was observed in the brains of furious and paralytic rabid dogs. In humans, classical signs of brain involvement include spasms in response to tactile, auditory, visual or olfactory stimuli (e.g. aerophobia and hydrophobia). Such signs occur during the clinical phase in almost all rabid patients in whom excitation (furious rabies) is prominent and are interspersed with periods of lucidity, agitation, and confusion. However, excitation is less evident in paralytic rabies. Atypical rabies or non-classic rabies is increasingly observed and may result in underreporting. In the past decade, a diverse number of methods have been published for the detection and identification of rabies viruses in clinical specimens. However, the most commonly used diagnostic test is the fluorescent antibody test (FAT) which detects virus antigen in the brain using fluorescently labelled anti-rabies antibodies [26] and is recommended by both WHO and OIE. It may be used to confirm the presence of rabies virus nucleocapsid protein in the original sample (brain smear) or sub-passaged material (see virus isolation below). The FAT gives reliable results on fresh specimens within a few hours in 95–99% of cases [25]. However, the sensitivity of the FAT is dependent on the quality of the specimen, conjugate, equipment and the skills of the diagnostic staff. The sensitivity of FAT can be affected by autolysis, lyssavirus species and the accuracy of dissection of the brain and may be lowered in samples from vaccinated animals. For direct rabies diagnosis, smears prepared from hippocampus, cerebellum or medulla oblongata are fixed in high-grade cold acetone and then stained with a drop of a specific antibody conjugated to fluorescein isothiocyanate. In the FAT, the specific aggregates of nucleocapsid are identified by their specific applegreen fluorescence. There are no gross lesions characteristic for rabies visible at autopsy. In most animals, microscopic non-specific lesions suggestive of viral encephalomyelitis with ganglioneuritis may be observed in the nerve centres, as well as histolymphocytic cuffs and gliosis. The most significant lesions are usually in the cervical spinal cord, hypothalamus and pons. The only specific lesions consist of intracytoplasmic eosinophil inclusions (Negri bodies) corresponding to the aggregation of developing rabies virus particles. These oval inclusions, measuring from 4 to 5 µm, are usually located in Ammon’s horn. The enzyme-linked immunosorbent assay (ELISA) and rapid rabies enzyme immunodiagnosis (RREID) methodologies have been shown to be of benefit for large scale surveillance having the potential to be automated [27, 28]. These methods detected the presence of EBLV-1 antigen in a cat in France in 2007 when conventional FAT failed (H. Bourhy, personal communication). Consequently, diagnosis can only be confirmed by laboratory tests preferably conducted post mortem on central nervous system (CNS) tissue removed from the cranium. Diagnostic techniques for rabies in animals have been internationally standardised [25]. The hippocampus, cerebellum and the medulla oblongata are the recommended tissues of choice. The head of the suspect animal is generally submitted for laboratory diagnosis. However, if this is impractical, for example for large numbers of carcasses, the ‘straw technique’ may be considered, whereby brain material is collected by passing a plastic straw through the occipital foramen [25]. Rabies virus isolation The OIE recommends the use of a confirmatory virus isolation test, particularly when FAT results are equivocal and for human exposure cases. 227 EJCAP - Vol. 18 - Issue 3 December 2008 rabies infection was confirmed using FAT on subsequent post mortem brain specimens [32]. All positive PCR products should be sequenced to confirm the origin of the virus and rule out possible contamination. Such studies are increasingly reported in the literature and are giving new insights into the epidemiology and evolution of this most important virus. The mouse inoculation test (MIT) involves the intracerebral inoculation of mice with a clarified supernatant of a homogenate of brain material (cortex, Ammon’s horn, cerebellum, medulla oblongata). Clinical signs (positive result) can be observed as soon as six to eight days for RABV but have been known to take up to 20 days for EBLV-2 [29]. Mice should be observed for a period of 28 days. This in vivo test is time-consuming, expensive and involves the use of animals. The OIE recommends that it should be avoided for routine diagnosis if validated in vitro methods are established within the laboratory. In vitro virus isolation tests such as the Rabies Tissues Culture Inoculation Test (RTCIT) involve the inoculation of the sample into a neuroblastoma cell line. Positive results are commonly obtained within 2-4 days. The FAT is then used to confirm the presence of rabies antigen in both infected mice or cell monolayers. One advantage of the above in vivo and in vitro assays includes the amplification of the virus isolate for future typing and analysis. Several RT-PCR methods have been reported over the past decade for the detection of lyssaviruses. These methods invariably involve multiple transfers of nucleic acids between different tubes and coupled with the high sensitivity of PCR methodologies, any small amount of contamination will undoubtedly produce falsepositive results. Attempts have been made to adapt RT-PCR to reduce manipulations thereby reducing contamination risks. The visualisation of PCR products by gel electrophoresis exposes facilities and operators to large quantities of amplified material and thus many adaptations have been directed at replacing this step. New and improved rapid diagnostic tools for rabies including PCR ELISA [33] and Taqman technology [34-36] have recently been developed for diagnostic purposes. Histopathology Rabies diagnosis based upon the detection of Negri bodies and histological tests such as Sellers’s or Mann’s tests, are no longer routinely performed by the majority of diagnostic laboratories, as they are considered unreliable, particularly for decomposed material. A rapid rabies TaqMan assay that distinguishes between classical rabies virus (genotype 1) and European bat lyssaviruses 1 and 2 (genotypes 5 and 6) has recently been reported [37]. This sensitive and specific assay is performed in a single step in a closed tube system, thereby dramatically decreasing the risk of contamination and allows for the genotyping of unknown isolates. Such real time assays can be applied quantitatively and the use of an internal control (e.g. ß-actin RNA or 18s ribosomal RNA) enables the quality of the isolated template to be assessed, thereby minimising the chance of false negative results associated with poor sample quality. Real time Taqman assays have been successfully employed in the rapid diagnosis and genotype confirmation of EBLV-2 infected Daubenton’s bats in the UK [38]. The assay was more recently used for the intra vitam detection and genotyping of a human rabies case in the UK in July 2005 within five hours of sample receipt [20] and for the detection of a rabies infected puppy in UK quarantine in 2008 [39]. A protocol suitable for the detection of classical rabies virus and European bat lyssaviruses type 1 and 2 has more recently been described [30]. A robust, highly sensitive and specific in situ hybridisation technique, employing digoxigenin labelled riboprobes was used for the detection of lyssavirus RNA in mouse-infected brain tissue. Using this method, both genomic and messenger RNA were detected. The ability to detect messenger RNA is indicative of the presence of replicating virus. Nucleic acid based methods Molecular based tools are becoming more widely acceptable and accessible for the diagnosis of rabies. The use of the reverse transcriptase polymerase chain reaction (RT-PCR), nested or hemi-nested RT-PCR and other PCR based techniques are increasingly reported but are not currently recommended by the WHO for routine post mortem diagnosis of rabies. However, in laboratories with strict quality control procedures in place and demonstrable experience and expertise, these molecular techniques have been successfully applied for confirmatory diagnosis and epidemiological surveys. Reverse transcription (RT)-PCR has been reported to confirm rabies diagnosis intra vitam in suspect human cases, when conventional diagnostic methods have failed and post-mortem material is not available [31]. Rabies virus RNA can be detected in a range of biological fluids and samples (e.g. saliva, cerebrospinal fluid, tears, skin biopsies and urine). Owing to the intermittent shedding of virus, serial samples of fluids such as saliva and urine should be tested but negative results should not be used to exclude a diagnosis of rabies. This was demonstrated in 2001 in the UK when multiple ante mortem saliva specimens taken during the clinical phase of infection in a Nigerian patient failed to yield rabies viral RNA but In 2001 Wacharapluesadee and Hemachudha reported the use of a rapid automated nucleic acid sequence-based amplification (NASBA) technique which was successfully applied to the saliva and CSF of four living patients in Thailand with rabies and detected rabies viral RNA as early as two days after onset of symptoms [40]. This technique differs from RT-PCR in that the viral RNA is directly amplified under isothermal conditions and detected by an automated reader. It is relatively easy to use and the whole process from extraction to detection can take as little as four hours using automated equipment. This technique has also been adapted to investigate rabies virus replication in situ [41]. Serology Post-infective rabies antibodies are rarely detectable before death, allowing antibody detection methods such as the rapid fluorescent focus inhibition test (RFFIT) or the fluorescent antibody virus neutralisation (FAVN) test to determine post- 228 Diagnostic tools for the detection of rabies virus - L. M. McElhinney, A. R. Fooks, A.D. Radford potential benefits of such methodologies in the hands of trained staff in appropriately equipped laboratories, both in terms of diagnosis and molecular epidemiological surveys. As molecular methodologies are adopted and adapted in increasingly more laboratories in both developed and developing countries, global recognition of the importance of quality assurance is essential. In order to ensure confidence in diagnostic test results, the OIE published guidelines for quality assurance in veterinary laboratories (http://www.oie.int/eng/publicat/ouvrages/A_112. htm) based on the requirements of the internationally recognised standard ISO/IEC 17025:1999. vaccinal immunity in vaccinated animals. In previously nonimmunised humans, virus neutralisation assays are sometimes successful in detecting rabies specific antibodies in the CSF or serum and thus confirming exposure to a lyssavirus. Neutralising antibodies, if present in serum tend to appear on average eight days after clinical signs occur whereas rabies antibodies are infrequently found in CSF. Virus neutralisation (VN) assays in cell cultures are more commonly used to determine levels of immunity in vaccinated animals (domestic and wildlife). The FAVN and RFFIT are the OIE prescribed tests for international trade of companion animals. In both cases, a given quantity of the virus is mixed with different dilutions of the serum to be tested and the neutralising limit dilution is determined. Virus escaping neutralisation by the test antisera is identified by fluorescence. The results are expressed in international units (IU) calibrated from standard reference materials available from the OIE Reference Laboratory in AFSSA, Nancy, France (for animal tests) or from the WHO (for human tests). Due to the nature of both tests, dedicated biosecure facilities and highly trained vaccinated staff are required. Enzyme linked immunosorbent assays (ELISAs) suitable for the detection of rabies virus-specific antibodies in serum samples from companion animals and humans have recently been developed [42-45]. The companion animal ELISAs have been accepted by the OIE as a screening tool or alternative to the FAVN but await approval for the EU Pet Travel Scheme. The major advantages of the ELISA test are that it can be completed in four hours, does not require the use of live virus and can be performed without the need for specialised laboratory containment. This contrasts with the four day turnaround using conventional rabies antibody virus neutralisation assays. With respect to serology for international trade, annual proficiency schemes coordinated by the OIE and Community reference laboratory AFFSA, Nancy, Malzeville, France, have been in operation for a number of years. Inter-laboratory ring trials of diagnostic techniques in European rabies laboratories will also be coordinated by AFSSA in the near future. Rabies diagnostic proficiency schemes are in operation in the USA and have highlighted the need for the standardisation of methodologies. The Centers for Disease Control and Prevention (CDC) have published a protocol for the FAT on their website to encourage harmonisation (http://www.cdc.gov/ ncidod/dvrd/rabies/professional/publications/DFA diagnosis/DFA protocol-b.htm). A report by Rudd et al. emphasised the dramatic effects that small modifications to the FAT protocol, such as a change in mountant composition, can have on the specificity or sensitivity of the test [47]. All laboratories should endeavour to validate their procedures using the array of virus strains likely to be locally encountered and not rely on the positive control material (e.g. laboratory adapted strain) for test validation. Test validation exercises must be repeated upon the introduction of any modifications to the test, however minor, and also if the range of lyssaviruses to be detected is expanded to include, for example, newly isolated diverging bat strains. As increasing numbers of laboratories strive to achieve quality assurance standards, the requirement to participate in inter-laboratory proficiency schemes will facilitate a more harmonised approach to rabies diagnosis and a greater confidence in epidemiological data. Recently, a virus neutralisation based assay has been developed utilising lentiviral pseudotypes as antigen [46]. The pseudotype viruses were constructed using G-protein cDNA sequences from rabies Challenge Virus Standard-11 (CVS-11), European bat lyssavirus type-1 or -2 (EBLV-1 and -2), cloned and co-expressed with HIV/MLV gag-pol and GFP/luciferase in human epithelial cells. The pseudotypes have three major advantages over the conventional virus neutralisation assays: they can be handled in biohazard level 2 laboratories, the use of reporter genes such as green fluorescent protein (GFP), luciferase or β-galactosidase allows the assay to be used at low cost in laboratories throughout the world and the pseudotype assay only requires minimal volumes (<10µl) of sera. The applicability of the pseudotype viruses is currently being validated in a collaborative study involving a number of Rabies Reference Laboratories. Conclusion Rabies is one of the best known and most feared of viral infections. It is notifiable and suspect cases must be reported to competent authorities that are responsible for further diagnosis. Diagnostic tests based on antigen detection, virus isolation and histopathology have been the mainstay of diagnosis. However, these are relatively slow and insensitive, and in some cases require the use of live animals. There is now an increasing pressure towards the development and use of modern molecular technologies. These promise great advances with increased sensitivity providing for ante-mortem diagnosis. By allowing sequence analysis, they have also revolutionised our understanding of the evolution and epidemiology of these viruses, challenging concepts of what it is to be “rabies free”. Inter-laboratory collaboration and test standardisation will allow for a better understanding of the global presence of rabies viruses, and is likely to be necessary to facilitate their greater control. Validation of Diagnostic Assays Rabies is a notifiable disease in most developed countries and as such, standardised diagnostic procedures are prescribed by the OIE. Due to the high sensitivity of PCR based methods, there is concern that appropriate procedures aimed at reducing the risk of contamination will not be fully adhered to, thereby generating false positive results. Consequently, the WHO expert committee agreed that molecular methods should not be globally recommended [7]. However, they did recognise the 229 EJCAP - Vol. 18 - Issue 3 December 2008 Acknowledgements [19] HEMACHUDHA (T.), MITRABHAKDI (E.) - Rabies. In: Davis LE, Kennedy PGE, editors. Infectious diseases of the nervous system. Oxford: Butterworth-Heinemann; 2000. p. 401-44. * [20] SOLOMON (T.), MARSTON (D.), MALLEWA (M.), FELTON (T.), SHAW (S.), MCELHINNEY (L.M.), DAS (K.), MANSFIELD (K.), WAINWRIGHT (J.), KWONG (G.N.), FOOKS (A.R.) - Paralytic rabies after a two week holiday in India. Br Med J. 2005:331;1501-3.* [21] MALLEWA (M.), FOOKS (A.R.), BANDA (D.), CHIKUNGWA (P.), MANKHAMBO (L.), MOLYNEUX (E.), MOLYNEUX (M.E.), SOLOMON (T.) - Rabies encephalitis in malaria-endemic area, Malawi, Africa. Emerg Infect Dis. 2007:13(1);136-139.* [22] LAOTHAMATAS (J.), HEMACHUDHA (T.), MITRABHAKDI (E.), WANNAKRAIROT (P.), TULAYADAECHANONT (S.) - MR imaging in human rabies. AJNR Am J Neuroradiol. 2003:24;1102–1109.* [23] FOGELMAN (V.), FISCHMAN (H.R.), HORMAN (J.T.), GRIGOR (J.K.) - Epidemiologic and clinical characteristics of rabies in cats. J Am Vet Med Assoc. 1993:202;1829-1838. * [24] LAOTHAMATAS (J.), WACHARAPLUESADEE (S.), LUMLERTDACHA (B.), AMPAWONG (S.), TEPSUMETHANON (V.), SHUANGSHOTI (S.), PHUMESIN (P.), ASAVAPHATIBOON (S.), WORAPRUEKJARU (L.), AVIHINGSANON (Y.), ISRASENA (N.), LAFON (M.), WILDE (H.), HEMACHUDHA (T.) - Furious and paralytic rabies of canine origin: neuroimaging with virological and cytokine studies. J Neurovirol. 2008:14(2);119-29. * [25] Office international des epizooties. Manual for diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees). 4th ed. Paris (France): 2004;1 p. 328-346. * [26] DEAN (D.J.), ABELSETH (M.K.) - THE FLUORESCENT ANTIBODY TEST. IN: KAPLAN MM, KOWPROWSKI H, editors. Laboratory Techniques in Rabies. Geneva: World Health Organization; 1973. p. 73-84. * [27] BOURHY (H.), ROLLIN (P.E.), VINCENT (J.), SUREAU (P.) Comparative field evaluation of the fluorescent antibody test, virus isolation from tissue culture and enzyme immunodiagnosis for rapid laboratory diagnosis of rabies. J Clin Microbiol. 1989:27;519–23. * [28] PERRIN (P.), SUREAU (P.) - A collaborative study of an experimental kit for rapid rabies enzyme immunodiagnosis (RREID). Bulletin WHO. 1987:65;489– 93.* [29] JOHNSON (N.), SELDEN (D.), PARSONS (G.), HEALY (D.), BROOKES (S.M.), MCELHINNEY (L.M.), HUTSON (A.M.), FOOKS (A.R.) Isolation of a European bat lyssavirus type 2 from a Daubenton’s bat in the United Kingdom. Vet Rec. 2003:152;383-7. * [30] FINNEGAN (C.J.), BROOKES (S.M.), JOHNSON (L.), FOOKS (A.R.) - Detection and strain differentiation of European bat lyssaviruses using in situ hybridisation. J Virol Methods. 2004:121;223-9.* [31] SMITH (J.), MCELHINNEY (L.M.), PARSONS (G.), BRINK (N.), DOHERTY (T.), AGRANOFF (D.), MIRANDA (M.E.), FOOKS (A.R.). - Case report: Rapid antemortem diagnosis of a human case of rabies imported into the UK from the Philippines. J Med Virol. 2003:69;150-155. * [32] JOHNSON (N.), LIPSCOMBE (D.W.), STOTT (R.), GOPAL RAO (G.), MANSFIELD (K.), SMITH (J.), MCELHINNEY (L.M.), FOOKS (A.R.) Investigation of a human case of rabies in the United Kingdom. J Clin Virol. 2002:25;351-356. * [33] BLACK (E.M), MCELHINNEY (L.M.), LOWINGS (J.P.), SMITH (J.), JOHNSTONE (P.), HEATON (P.R.) - Molecular methods to distinguish between classical rabies and the rabies-related European bat lyssaviruses. J Virol Methods. 2000;87:123-131. * [34] BLACK (E.M.), LOWINGS (J.P.), SMITH (J.), HEATON (P.R.), MCELHINNEY (L.M.) - A rapid RT-PCR method to differentiate six established genotypes of rabies and rabies-related viruses using TaqMan (TM) technology. J Virol Methods. 2002;105, 25-35. * This review was financially supported by the Department for Environment, Food and Rural Affairs (Defra), UK (SV3500). References [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] FOOKS (A.R.) - Risk factors from rabies re-emergence in Europe. Proceedings of the 13th ECVIM-CA Congress 2003; Uppsala, Sweden, pp. 68-70. * GALPERINE (T.), NEAU (D.), MOITON (M.P.), ROTIVEL (Y.), RAGNAUD (J.M.) - The risk of rabies in France and the illegal importation of animals from rabid endemic countries. Presse Med. 2004:10(33);791-2. * FOOKS (A.R.) - The challenge of Emerging Lyssaviruses. Expert Rev Vaccines. 2004:3;89-92. * KUZMIN (I.V.), ORCIARI (L.A.), ARAI (Y.T), SMITH JS, HANLON CA, KAMEOKA Y, RUPPRECHT CE. Bat lyssaviruses (Aravan and Khujand) from Central Asia: phylogenetic relationships according to N, P and G gene sequences. Virus Res. 2003:97;65–79. * ARAI (Y.T), KUZMIN (I.V), KAMEOKA (Y.), BOTVINKIN (A.D.) New lyssavirus genotype from the lesser mouse-eared bat (Myotis blythi), Kyrghyzstan. Emerg Infect Dis. 2003;9:333–337.* KUZMIN (I.V.), HUGHES (G.J.), BOTVINKIN (A.D.), ORCIARI (L.A.), RUPPRECHT (C.A.) - Phylogenetic relationships of Irkut and West Caucasian bat viruses within the Lyssavirus genus and suggested quantitative criteria based on the N gene sequence for lyssavirus genotype definition. Virus Res. 2005:111;28–43. * World Health Organization Expert Committee. On rabies. Geneva: 2005. WHO Technical Report Series No.: 931. * MÜLLER (T.), COX (J.), PETER (W.), SCHAFER (R.), JOHNSON (N.), MCELHINNEY (L.M.), GEUE (J.L.), TJORNEHOJ (K.) - Spill-over of European bat lyssavirus type 1 into a stone marten (Martes foina) in Germany. J V Med Series B – Infectious Diseases and Veterinary Public Health 2004:51;49-54. * SUMMER0 (R.), ROSS (R.S.), KEIHL (W.) - Imported case of rabies in Germany from India. Eurosurveillance Weekly 8.www. eurosurveillance. 2004. * DUTTA (J.K.), DUTTA (T.K.), DAS (A.K.) - Human rabies: modes of transmission. J Assoc Physicians India. 1992;40(5):322-4. * DUTTA (J.K.) Rabies transmission by oral and other non-bite routes. J Indian Med Assoc.1998;96(12):359.* MANSFIELD (K.L.), MCELHINNEY (L.M.), HUBSCHLE (O.), METTLER (F.), SABETA (C.), NEL (L.), FOOKS (A.R.) - A molecular epidemiological study of rabies epizootics in kudu (Tragelaphus strepsiceros) in Namibia. Biomedical Central Veterinary Research. 2006:2;2.* JOHNSON (N.), PHILLPOTTS (R.), FOOKS (A.R.) - Airborne transmission of Lyssaviruses. J Med Microbiol. 2006:55(6);785790.* FEKADU (M.), SHADDOCK (J.H.), BAER (G.M.) - Excretion of rabies virus in the saliva of dogs. J. Infect. Dis. 1982:145:715-719.* RUPPRECHT (C.E.), CHILDS (J.E.) - Feline rabies. Feline Pract. 1996:24(5);15-19.* TEPSUMETHANON (V.), LUMLERTDACHA (B.), MITMOONPITAK (C.), SITPRIJA (V.), MESLIN (F.X), WILDE (H.) - Survival of naturally infected rabid dogs and cats. Clin Infect Dis. 2004:39(2);27880.* WILLOUGHBY (R.E.) JR, TIEVES (K.S.), HOFFMAN (G.M.), et al. Survival after treatment of rabies with induction of coma. N Engl J Med. 2005:352;2508–2514. * WARRELL (M.J.) - Emerging aspects of rabies infection: with a special emphasis on children. J Clin Microbiol. 2008:21(3);2517.* 230 Diagnostic tools for the detection of rabies virus - L. M. McElhinney, A. R. Fooks, A.D. Radford [42] CLIQUET (F.), MCELHINNEY (L.M.), SERVAT (A.), BOUCHER (J.M.), LOWINGS (J.P.), GODDARD (T.), MANSFIELD (K.L.), FOOKS (A.R.) - Development of a qualitative indirect ELISA for the measurement of rabies virus-specific antibodies from vaccinated dogs and cats. J Virol Methods. 2004 117, 1-8. * [43] SERVAT (A.), WASNIEWSKI (M.), CLIQUET (F.) - Tools for rabies serology to monitor the effectiveness of rabies vaccination in domestic and wild carnivores (Review). Dev Biol. 2006;125:914.* [44] SERVAT (A.), FEYSSAGUET (M.), MORIZE (J.L.), SCHEREFFER (J.L), BOUE (F.), CLIQUET (F.) - A quantitative indirect ELISA to monitor the effectiveness of rabies vaccination in domestic and wild carnivores. J Immunol Methods. 2007:318(1-2);1-10.* [45] FEYSSAGUET (M.), DACHEUX (L.), AUDRY (L.), COMPOINT (A.), MORIZE (J.L.), BLANCHARD (I.), BOURHY (H.) - Multicenter comparative study of a new ELISA, PLATELIATM RABIES II, for the detection and titration of anti-rabies glycoprotein antibodies and comparison with the rapid fluorescent focus inhibition test (RFFIT) on human samples from vaccinated and non-vaccinated people. Vaccine. 2007. 25(12):2244-51.* [46] WRIGHT (E.), TEMPERTON (N.J.), MARSTON (D.A.), MCELHINNEY (L.M.), FOOKS (A.R.), WEISS (R.A.) - Investigating antibody neutralization of lyssaviruses using lentiviral pseudotypes: a crossspecies comparison. J Gen Virol. 2008:Sep;89(Pt 9):2204-13. [47] RUDD (R.J.), SMITH (J.S.), YAGER (P.A.), ORCIARI (L.A.), TRIMARCHI (C.V.) - A need for standardized rabies-virus diagnostic procedures: Effect of cover-glass mountant on the reliability of antigen detection by the fluorescent antibody test. Virus Res. 2005:111;83–88. * [35] HUGHES (G.J.), SMITH (J.S.), HANLON (C.A.), RUPPRECHT (C.E.) - Evaluation of a TaqMan PCR assay to detect rabies virus RNA: influence of sequence variation and application to quantification of viral loads. J Clin Microbiol. 2004:42;299-306. * [36] WACHARAPLUESADEE (S.), SUTIPANYA (J.), DAMRONGWATANAPOKIN (S.), PHUMESIN (P.), CHAMNANPOOD (P.), LEOWIJUK (C.), HEMACHUDHA (T.) - Development of a TaqMan real-time RT-PCR assay for the detection of rabies virus. J Virol Methods. 2008:151(2):317-20.* [37] WAKELEY (P.R), JOHNSON (N.), MCELHINNEY (L.M.), MARSTON (D.), SAWYER (J.) AND FOOKS (A.R.) - Development of a realtime, TaqMan reverse transcription-PCR assay for detection and differentiation of lyssavirus genotypes 1, 5 and 6. J Clin Microbiol. 2005:43;2786-2792.* [38] FOOKS (A.R.), MCELHINNEY (L.M.), MARSTON (D.A.), SELDEN (D.), JOLLIFFE (T.A.), WAKELEY (P.R.), JOHNSON (N.), BROOKES (S.M.) - Identification of a European Bat Lyssavirus type-2 in a Daubenton’s bat (Myotis daubentonii) found in Staines, Surrey, United Kingdom. Vet Rec. 2004:155;434-435.* [39] FOOKS (A.R.), HARKESS (G.), GODDARD (T.), MARSTON (D.A.), MCELHINNEY (L.M.), BROWN (K.), MORGAN (D.), PAUL (R.), THOMAS (P.J.), SMITH (B.) - Rabies virus in a dog imported to the UK from Sri Lanka. Vet Rec. 2008:159;598. * [40] WACHARAPLUESADEE (S.), HEMACHUDHA (T.) - Nucleic-acid sequence based amplification in the rapid diagnosis of rabies. Lancet. 2001:358;892-893.* [41] SUGIYAMA (M.), ITO (N.), MINAMOTO (N.) - Isothermal amplification of rabies virus gene. J Vet Med Sci. 2003:65;10638. * 231 ZOONOSES Canine leishmaniosis a challenging zoonosis L. Solano-Gallego(1), G. Baneth(2) SUMMARY Leishmania infantum is the causative agent of canine leishmaniosis (CaNL) and human visceral leishmaniasis in the Mediterranean basin and some other parts of the world. Dogs are the major reservoir for this infection. Surveys have revealed that dog infection rates reach 70% in some foci in the Mediterranean basin. Canine leishmaniosis is a good example of a disease in which infection does not equal clinical illness due to the high prevalence of subclinical infection. Population studies have shown that a low proportion of the canine population develops a severe disease while another fraction has persistent subclinical infection. Susceptibility to CaNL has been associated with genetic factors and mutations in several loci. The main clinical signs associated with CaNL are skin lesions, lymphadenomegaly, splenomegaly, ocular abnormalities, abnormal nails growth (onychogryphosis) and poor body condition. Additional findings include: epistaxis, renal failure, decreased appetite, polyuria and polydypsia, vomiting, melena and lameness. The diagnosis of CaNL is performed by microscopic detection of parasites, serology and PCR. Several drugs including allopurinol, meglumine antimoniate, amphotericin B, and miltefosine are used for treatment of CaNL. However, although most dogs recover following therapy, complete elimination of the parasite is usually not achieved and infected dogs may relapse following treatment. Prevention of CaNL includes the use of topical insecticides against sand flies, such as pyrethroid collars, spot-on formulations and sprays. In addition, new canine vaccines are being developed and commercially introduced or evaluated in several countries. the third most important vector-borne disease after malaria and lymphatic filariasis. Based on clinical signs in humans, the disease can be divided into cutaneous, mucocutaneous and visceral forms. An estimated 12 million human cases of leishmaniosis exist worldwide, with an estimated number of 1.5-2 million new cases occurring annually: 1-1.5 million cases of cutaneous leishmaniosis and 500.000 cases of visceral leishmaniosis [5]. Epidemiologically, two different situations occur: (i) The zoonotic form of visceral leishmaniosis found in the Mediterranean basin and South America, with the dog as the main source of L. infantum infection for the female sand fly; and (ii) the anthroponotic form found in East Africa, Bangladesh, India and Nepal where L. donovani transmission is passed from person to person through the sand fly vector [5]. Anthroponotic visceral leishmaniosis caused by L. donovani is responsible for a large part of the fatalities due to the visceral disease in people [6]. This paper was commissioned by FECAVA for publication in EJCAP. Introduction Leishmaniosis has a long history of association with mankind. The disease is known to have been present in Africa and India since at least the mid-eighteenth century [1]. In 1903, Leishman and Donovan separately described the protozoan now termed Leishmania donovani in splenic tissue from human patients in India [2, 3]. In 1908, Nicolle and Comte in Tunisia reported infection by Leishmania in dogs for the first time [4]. Leishmania infections caused by different Leishmania species are present in a variety of biogeographical regions of the Old and New Worlds. These are responsible for the wide spectrum of clinical illnesses observed in people. Human leishmaniasis is Visceral leishmaniosis caused by L. infantum in the Mediterranean basin was traditionally predominantly a disease of young children (1) Laia Solano-Gallego. Department of Pathology and Infectious Diseases, Royal Veterinary College, University of London, United Kingdom (2) Gad Baneth. School of Veterinary Medicine, Hebrew University, P.O. Box 12, Rehovot 76100, Israel. Corresponding author address: Laia Solano-Gallego. Department of Pathology and Infectious Diseases Hawkshead Lane, North Mymms, Hatfield, Herts AL9 7TA, United Kingdom, Tel. +44(0)1707666326, Fax. +44(0)1707661464, E-mail: lsolano@rvc.ac.uk 232 Canine leishmaniosis - a challenging zoonosis - L. Solano-Gallego, G. Baneth Transmission of canine leishmaniosis and the name of the causative agent of this disease reflects the predilection to infants. Malnutrition has been recognized as risk factor for infantile leishmaniosis, and may explain why this disease is more prevalent among children in poor countries as compared with affluent ones despite high prevalence rates in the dog populations [6]. With the appearance of the AIDS epidemic, adult HIV+ patients are now the predominant risk group for human visceral leishmaniosis in Southern Europe. The co-infection of HIV and leishmaniosis reported from more than 33 countries where these infections geographically overlap has been described as a “deadly gridlock” that does not respond well to therapy [7, 8]. Female sand flies from the genera Phlebtomus (Old World) or Lutzomyia (New World) are the principal agents of transmission of Leishmania in humans and dogs [27]. The activity of the adult sand flies is crepuscular and nocturnal from early spring to late autumn in the Mediterranean basin and all year round in South America [27]. Leishmania is a diphasic parasite that completes its life cycle in two hosts, a sand fly which harbors the flagellated extracellular promastigotes and a mammal where the intracellular amastigote parasite forms develop. Dogs are infected by Leishmania promastigotes deposited in the skin during the bites of infected female sand fly vectors. The promastigotes invade host cells and replicate as intracellular amastigotes. The transmission of Leishmania metacyclic promastigotes by phlebotomine sandflies is extensively reviewed elsewhere [28]. CaNL is a major zoonotic disease endemic in more than 70 countries in the world. It is present in regions of southern Europe, Africa, Asia, South and Central America [9] and has recently emerged in the USA [10, 11]. CaNL is also an important concern in non-endemic countries where imported disease constitutes a veterinary and public health problem [12]. Dogs are the main reservoir for human visceral leishmaniosis and the disease is usually fatal if not treated in people and dogs. Phlebotomine sand flies are the proven vectors of Leishmania infantum, the causative agent of CaNL in the Old World and for its New World synonym Leishmania chagasi. The majority of epidemiological studies about the prevalence and incidence of Leishmania infection in dogs have been based on serologic surveys. The seroprevalence in the Mediterranean basin ranges from 10% to 40% depending on the region [13]. Surveys employing other methods to calculate the prevalence of Leishmania infection by detecting Leishmania DNA in different tissues [14-16] or by detecting specific-Leishmania cellular immunity (17-19) have revealed even higher infection rates approaching 70% in some foci. It has been estimated based on seroprevalence studies from Italy, Spain, France and Portugal that 2.5 million dogs in these countries are infected [20] and L. infantum infection is spreading north in Europe reaching the foothills of the Alps in Northern Italy [21]. The number of infected dogs in South America is also estimated in millions with high infection rates in some areas of Brazil and Venezuela [22]. An important medical and epidemiological question is whether dogs found positive to the Leishmania parasite with different techniques such as serology and/or PCR are also infective to phlebotomine sand flies. This information would provide the best indicator of the burden of infectiousness in a dog population in an endemic area. Seropositive dogs with and without clinical signs are infectious to sand flies [29-31]. The rate of infected sand flies increased with the appearance and severity of the clinical signs [30] and with the decrease in CD4+ T cells counts [32]. No studies have been published on transmission from seronegative subclinically infected dogs. Further studies are needed to ascertain the potential of seronegative subclinically infected dogs to transmit Leishmania to vector sand flies. Other less common transmission routes have been reported in dogs. The transmission of L. infantum through blood products has been reported in dogs that received blood transfusions from infected donors in North America [33, 34]. Vertical in-utero transmission from dam to its offspring has been documented in one study [35] but disputed by other authors [36]. In addition, ticks and fleas have been proposed as alternative vectors of Leishmania transmission but evidence of such transmission is lacking [37, 38]. Direct transmission without involvement of a hematophageous vector has been suspected in some cases of infection in areas where vectors of the disease are apparently absent [11]. Public health considerations Transmission of L. infantum from dogs or wildlife animal reservoirs via sand flies is the main route for human infection. People in endemic regions share the same habitat and are frequently in close physical contact with infected dogs [23]. Several studies have investigated the association between canine and human leishmaniosis in the same region and examined to what degree infection in dogs poses a risk for human disease [23]. These studies reported that (i) increased prevalence in canine population is associated with increased incidence of human leishmaniosis [22, 24], (ii) poor socioeconomical conditions are risk factors for the association between canine and human infections [22] and (iii) dog density and infected dog ownership are risk factors for infantile human leishmaniosis [25, 26]. Therefore, effective control of canine leishmaniosis could lead to a decrease in human leishmaniosis in endemic regions. Control measures of CaNL could include effective vaccines, topical insecticides, and environmental control of sand flies. Disease versus infection by L. infantum The classical stages of an infectious disease process include the initial infection, an incubation period and a clinical disease. Numerous studies on these stages in relation to Leishmania have indicated that Leishmania infection and disease are not always considered synonymous in rodents, humans and dogs. The concept that all dogs infected with Leishmania infantum will eventually develop severe clinical leishmaniosis after a variable incubation period [39, 40] has been disproved. Specific antiLeishmania cellular immunity was demonstrated in apparently healthy dogs naturally infected with Leishmania [18, 41]. Leishmania infantum infection in dogs can display a broad 233 EJCAP - Vol. 18 - Issue 3 December 2008 spectrum of immune responses and clinical manifestations from a clinically healthy condition to a severe clinical disease. This is similar to findings in human infection where clinical disease represents one pole and subclinical infection the other pole of infection [42]. [48, 49] but decreased or absent leishmanial specific lymphocyte proliferation and delayed type hypersensitivity (DTH) reaction [41, 50, 51]. Conversely, healthy infected dogs (resistant) produce specific lymphocyte proliferation, strong DTH reaction, variable anti-parasite antibody levels [17-19, 41] and lower parasite loads when compared with sick or susceptible dogs [48, 49]. Leishmania infection in dogs may vary between a subclinical infection and a severe fatal disease [9]. In dogs, the two opposite extreme poles of this spectrum are characterized by: (i) protective immunity that is T cell mediated and (ii) disease susceptibility that is associated with the production of a marked humoral non-protective immune response and a reduced or depressed cell mediated immunity (CMI) [9, 13, 44]. For example, clinical disease can range from a mild papular dermatitis associated with specific cellular immunity and low humoral responses [43] to a severe/fatal disease characterized by renal pathology with glomerulonephritis due to immune complex deposition mainly with an extensive humoral response and high parasite loads [45-47]. Specific immune responses play a major role in susceptibility and resistance to infection. An experimental model of cutaneous leishmaniosis in mice infected with L. major has shown that a susceptible mice strain (BALB/c) which typically develops a T helper type 2 cellular response will succumb to infection with secretion of specific cytokines such as IL-4 and IL-10, and production of a significant antibody response. Another mice strain (C3H) that responds with a different set of cytokines typical of a T helper type 1 response such as IFN-γ; IL-2, is resistant to infection [52]. This general model appears to be only partially valid for CaNL and human visceral disease where studies of the immune response have shown mixed Th-1 and Th-2 types of reaction. An update on canine immune responses in L. infantum infection is reviewed elsewhere [9, 44]. Population studies in Leishmania-endemic areas have shown that a low proportion of the canine population develops a severe disease and another fraction has subclinical infection. The immune responses mounted by dogs at the time of infection and thereafter appear to be the most important factor in determining if they will develop a lasting infection and whether and when it will progress from a subclinical state into a disease condition. Animals that are predisposed and will develop severe disease are considered “susceptible”. Dogs able to control/ restrict the infection and remain constantly subclinical, have been termed “clinically resistant” [18, 19]. However, subclinical infection is not necessarily permanent and factors such as immunosuppressive conditions or concomitant disease could break the equilibrium and lead to the progression of clinical disease in dogs as observed in human patients with AIDS and Leishmania co-infection [7]. Immune-mediated mechanisms are responsible for much of the pathological findings in CaNL. Circulating immune complexes [53] and antinuclear antibodies [54, 55] have been detected in animals with CaNL. Glomerulonephritis associated with the deposition of immune complexes in the kidneys is a hallmark of CaNL [45-47]. Vasculitis induced by immune complexes which activate the complement cascade is an important pathological mechanism responsible for tissue necrosis and accountable for dermal, visceral and ocular lesions found in this disease [56-58]. Dogs with severe disease or progressing toward overt disease have high antibody levels, high parasite load in numerous tissues It is not known for certain what mechanisms in dogs are responsible for protection or susceptibility, nor the way factors such as age, gender, nutrition, host genetics, coinfections and concomitant disease, immunosuppressive conditions, cytokine environment, parasitic burden, nature of Leishmania antigens or different Leishmania strains, previous infections and way of Fig. 1 Exfoliative dermatitis, hypotrichosis and alopecia of the face and pinna in a dog with leishmaniosis. Fig. 2 Exfoliative dermatitis and alopecia on the ear tips of a dog with leishmaniosis. 234 Canine leishmaniosis - a challenging zoonosis - L. Solano-Gallego, G. Baneth transmission can affect the polarity of clinical manifestations of Leishmania infection. Age seems to be an important factor with a peak in the prevalence of disease in dogs younger than 3 years or older than 8 year [5961]. Studies have indicated that several dog breeds are apparently more susceptible to disease. These include the Boxer, Cocker Spaniel, Rottweiler and German shepherd [62, 63]. Other breeds that have evolved in endemic areas such as the Ibizian Hound rarely develop the disease and present a protective predominant cellular immunity [19]. The Slc11c1 (Solute carrier family 11 member a1) gene [64] and certain dog major histocompatibility complex (MHC) class II alleles have been found to be associated with the susceptibility to CanL [65]. Further studies on specific genes and whole genome comparisons among resistant and susceptible breeds might further elucidate the mechanisms responsible for susceptibility to CaNL. alopecia which can be generalized or localized over the face, ears and limbs (Fig. 1 and 2), (ii) ulcerative dermatitis, nodular dermatitis, (iii) mucocutaneous proliferative dermatitis and (iv) papular dermatitis [43]. The most common ocular manifestations are anterior uveitis, blepharitis (exfoliative, ulcerative, or nodular) and keratoconjunctivitis, either common or sicca [70-72]. About 25% of dogs with clinical leishmaniosis have ocular and periocular lesions including keratoconjunctivitis and uveitis [72]. Ocular consequences of systemic hypertension such as retinal detachment and/or hemorrhages, retinal arterial tortuosity and hyphema are present in the disease but not diagnosed frequently [73]. The main clinicopathological findings of canine leishmaniosis are hyperglobulinemia (polyclonal gammaglobulinemia), hypoalbuminemia, decreased albumin/globulin ratio, mild to severe proteinuria, non-regenerative anemia, renal azotemia, elevated liver enzyme activities, thrombocytopenia and thrombocytopathy [68-70, 74, 75]. Clinical findings in canine leishmaniosis Some degree of renal pathology is present in most dogs with CaNL and subsequent renal failure due to immune-complex glomerulonephritis eventually develops and is believed to be the main cause of death in dogs with CaNL [45-47]. Epistaxis, ocular abnormalities or renal failure may be the only presenting clinical findings in CaNL and this disease should be considered among the differential diagnoses for these conditions in endemic areas or in dogs that have traveled or were imported from an endemic region. Marked hyperglobulinemia with no apparent cause in dogs from endemic regions should also be investigated for CaNL. The clinical features of leishmaniosis vary widely as a consequence of the numerous pathogenic mechanisms of the disease process, the different organs affected, and because of the diversity of immune responses of individual hosts, whether humans or other mammals [66]. The main clinical findings found on physical examination in classical CaNL include skin lesions (Fig. 1 and 2), local or generalized lymphadenomegaly, loss of body weight, exercise intolerance, decreased appetite, lethargy, splenomegaly, polyuria and polydypsia, ocular lesions, epistaxis, onychogryposis, lameness, vomiting and diarrhea [67-70]. It is sometimes difficult to define if dogs have clinical disease due to CaNL because they may be infected with the parasite without clinical signs and be ill due to other causes, or because other diseases may present similar clinical signs. In the past, Skin lesions are the most frequent manifestation of CaNL in dogs brought for treatment due to the disease. Several dermatological entities have been described [39]: (i) exfoliative dermatitis with Tab. 1 Diagnostic techniques used for the diagnosis of canine leishmaniosis. Diagnostic technique 1. Serology 1. Indirect fluorescent antibody test (IFAT) 2. Enzyme linked immunosorbent assay (ELISA) 3. Direct agglutination test (DAT) 4. Antigen-specific rK39 serology 5. In-house immunochromatographic devices 6. Western blot 2. Cytology 1. Skin touch preparation 3. Histopathology 1. Formalin fixed tissues stained with hematoxylin-eosin 2. Fine needle aspirates from lymph nodes, bone marrow and spleen 2. Immunohistochemistry of tissues using anti-leishmanial antibodies 4. Detection of Leishmania DNA by PCR 1. Conventional PCR targeting the kinetoplast DNA (kDNA) or genomic ribosomal RNA genes 2. Nested PCR 3. Quantitative real-time PCR 5. Parasite culture 1. Culture of aspirates from the bone marrow, spleen or lymph node in a specialized medium for the cultivation of Leishmania 235 EJCAP - Vol. 18 - Issue 3 December 2008 Fig. 3 Touch impression from the skin of the ear in a dog with leishmaniosis. Fig. 5 Fine needle aspiration from the prescapular lymph node of a dog suspected for canine leishmaniosis. researchers have used the clinical classification of asymptomatic, oligosymptomatic and polysymptomatic dogs [76] based only on physical examination. This classification has a limited value because it does not consider clinicopathological abnormalities and disregards dogs that have widespread organ dysfunction without apparent visual manifestations. The authors consider a sick dog suffering from leishmaniosis if it presents with compatible clinical signs and/or clinicopathological abnormalities and the diagnosis is confirmed by specific tests for the infection and ultrasound can assist in raising the suspicion index for this disease. Numerous diagnostic techniques have been developed to help in the diagnosis of canine leishmaniosis (Tab. 1). It is important to understand the basis of each diagnostic test, the limitations and the appropriate clinical interpretation. Leishmania amastigotes can be demonstrated by cytology from cutaneous lesions (Fig. 3), spleen (Fig. 4), lymph nodes (Fig. 5), bone marrow [13, 78] and less commonly in other tissues [7981] or body fluids such as joint, cerebrospinal and abdominal fluids [82-86] stained with Giemsa, or modified Wright’s stain, or a quick commercial stain. Detection of amastigotes by cytology is frequently unrewarding due to a low to moderate number of detectable parasites present even in dogs with a full blown clinical disease [87, 88]. Leishmania parasites may also be viewed in histophatologic formalin-fixed, paraffinembedded biopsy sections of the skin or other infected organs. Leishmania parasites should be suspected in pyogranulomatous, granulomatous or lymphoplasmacytic inflammations in different tissues [79, 80, 89-91] or lymph node reactive hyperplasia [92, 93] on cytological or histological preparations. Definite identification of parasites within tissue macrophages may be difficult and an immunohistochemical staining method can be employed to detect or verify the presence of Leishmania in the tissue [94]. Diagnosis of canine leishmaniosis CaNL is a good example of a disease in which infection does not equal clinical illness due to the high prevalence of subclinical infection. This makes CaNL a diagnostic challenge for the veterinary practitioner, clinical pathologist and public health official in endemic countries as well as non-endemic regions where imported infection is a concern [23, 77]. Accurate diagnosis of canine leishmaniosis often requires an integrated approach (clinicopathological diagnosis and specific laboratory tests). Pertinent clinical history, a thorough physical examination and several routine diagnostic tests such as CBC, biochemical profile, urinalysis, serum electrophoresis, coagulation profile, imaging of the abdomen by radiographs Various serological methods for the detection of anti-Leishmania antibodies have been developed. These include the indirect immunofluorescence assay (IFA), enzyme-linked immunosorbent assay (ELISA), direct agglutination assays (DAT) and western blotting [95]. In general, good sensitivities and specificities are gained with these methods for the diagnosis of clinical CaNL [23]. High antibody titers are usually associated with disease and a high parasite density [49, 96] and, for this reason; they are conclusive of a diagnosis of leishmaniosis. However, the presence of lower antibody levels is not necessarily indicative of patent disease and needs to be confirmed by other diagnostic method such as PCR, cytology or histology [23, 77]. Serological cross-reactivity with different pathogens is possible with some serological tests, especially those based on whole parasite antigen. Cross reactivity has been reported with other species of Leishmania [97-99], and Trypanosoma cruzi [98]. Fig. 4 Fine needle aspiration from the spleen of a dog for the diagnosis of leishmaniosis. 236 Canine leishmaniosis - a challenging zoonosis - L. Solano-Gallego, G. Baneth First line drugs Generic name 1. Meglumine antimoniate 2. Allopurinol 3. Combination of meglumine antimoniate and allopurinol 4. Amphotericin B 5. Miltefosine 6. Combination of amphotericin B or miltefosine and allopurinol Second line drugs 1. Aminosidine 2. Metronidazole and spiramycin 3. Metronidazole and enrofloxacin 4. Marbofloxacin 5. Ketoconazole 6. Pentamidine Tab. 2 First and second line drugs for the treatment of canine leishmaniosis. evaluation for dogs [23]. Immunotherapy is also under extensive investigation in dogs with promising results [116, 117]. Detection of parasite-specific DNA in tissues by PCR allows sensitive and specific diagnosis. Several different assays with various target sequences using genomic or kinetoplast DNA (kDNA) have been developed for CaNL. PCR can be performed on DNA extracted from tissues, blood or even from histopathologic specimens. Assays based on kDNA appear to be the most sensitive for direct detection in infected tissues [23, 100]. Several tissues have been studied: lymph node [88, 101, 102], bone marrow aspirates [87, 103, 104], whole blood [101. 105, 106], buffy coat [104], paraffin-embedded skin biopsies [107], spleen [108], frozen skin and eye conjunctiva [14, 15], conjunctival swab [109, 110] and urine samples [111] with varying results. PCR on bone marrow, lymph node, or skin are most sensitive and specific for the diagnosis of canine leishmaniosis [15, 87, 101, 107, 112, 113]. PCR on whole blood and urine samples seem to be less sensitive than the tissues mentioned above [101, 111, 113]. PCR on aspirates of lymph node and bone marrow have been shown to be more sensitive than stained smears or parasite culture [87, 88, 101, 114]. Currently, three different PCR techniques are available and they are listed by the level of sensitivity from less to more sensitive: conventional PCR, nestedPCR and real-time PCR [23, 100]. Keeping and treating infected dogs presents a dilemma to owners, veterinarians and public health officials especially in areas where suitable vectors are found, because of the risk of transmission to people and pets in the community. Treated dogs often remain carriers of the disease and commonly experience clinical relapses. However, it appears that they are less infectious to sandflies [118, 119]. The use of adequate treatment is advocated for the control of canine leishmaniasis and to reduce risks of L. infantum transmission [119]. Owners must receive a thorough and realistic explanation about the disease, its zoonotic potential, the prognosis for their dog, and what should be expected from treatment. Prevention of canine leishmaniosis The use of topical insecticides against CaNL [120] in collars [121] or spot-on formulation has been shown to be effective in reducing disease transmission [122, 123]. Delthamethrinimpregnated collar significantly reduced the number of dog sand fly bites under experimental conditions [124, 125] and decreased infection transmission in field studies [121]. In a study supported by the WHO in Iran, collaring of dogs in intervention and control villages significantly reduced the seroconversion rate in dogs and in children living in the intervention villages [126]. A commercial vaccine against CaNL has recently been approved in Brazil [127, 128] and several vaccine candidates are under experimental [129] or field evaluation in Europe [130]. Therapy Anti-leishmanial treatment often achieves only clinical improvement in dogs with leishmaniosis and it is frequently not associated with the elimination of the parasite. The main drugs used against canine leishmaniosis include the pentavalent antimony meglumine antimoniate which selectively inhibits leishmanial glycolysis and fatty acid oxidation and allopurinol that acts by inhibiting protein translation through interfering with RNA synthesis (Tab. 2). The combination of antimony meglumine antimoniate and allopurinol is the most common treatment protocol used against canine leishmaniosis in Europe [115]. Amphotericin B, which acts by binding to ergosterol in the parasite’s cell membrane and altering its permeability is also used but it is highly nephrotoxic. New drugs are currently under References [1] ALLISON (M.J.) - Leishmaniasis. In: Kiple KF, editor. The Cambridge history of human disease. Cambridge: Cambridge University Press, 1993. [2] LEISHMAN (W.B.) - On the possibility of the occurrens of trypanosomiasis in India. British Medical Journal, 1903, 1:1252-4. 237 EJCAP - Vol. 18 - Issue 3 December 2008 [3] DONOVAN (C.) - On the possibility of the occurrens of trypanosomiasis in India. British Medical Journal, 1903, 2:79. [4] NICOLLE (C.), COMTE (C.) - Origine canine du Kala-azar. Bulletin Société Pathologie Exotique, 1908, 1:299-301. [5] DESJEUX (P.) - Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis, 2004, Sep;27(5):305-18. [6] ALVAR (J.), YACTAYO (S.), BERN (C.) - Leishmaniasis and poverty. Trends Parasitol, 2006 Dec;22(12):552-7. [7] ALVAR (J.), APARICIO (P.), ASEFFA (A.), DEN BOER (M.), CANAVATE (C.), DEDET (J.P.), et al. - The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol, Rev. 2008 Apr,21(2):334-59. [8] ALVAR (J.), CANAVATE (C.), GUTIERREZ-SOLAR (B.), JIMENEZ (M.), LAGUNA (F.), LOPEZ-VELEZ (R.), et al. - Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol, Rev. 1997 Apr,10(2):298-319. [9] BANETH (G.), KOUTINAS (A.F.), SOLANO-GALLEGO (L.), BOURDEAU (P.), FERRER (L.) - Canine leishmaniosis- new concepts and insights on an expanding zoonosis: part one. Trends Parasitol, 2008, 24:324-330 [10] GASKIN (A.A.), SCHANTZ (P.), JACKSON (J.), BIRKENHEUER (A.), TOMLINSON (L.), GRAMICCIA (M.), et al. - Visceral leishmaniasis in a New York foxhound kennel. J Vet Intern Med, 2002 JanFeb,16(1):34-44. [11] DUPREY (Z.H.), STEURER (F.J.), ROONEY (J.A.), KIRCHHOFF (L.V.), JACKSON (J.E.), ROWTON (E.D.), et al. - Canine visceral leishmaniasis, United States and Canada, 2000-2003. Emerg Infect Dis, 2006 Mar,12(3):440-6. [12] SHAW (S.E.), LERGA (A.I.), WILLIAMS (S.), BEUGNET (F.), BIRTLES (R.J.), DAY (M.J.), et al. - Review of exotic infectious diseases in small animals entering the United Kingdom from abroad diagnosed by PCR. Vet Rec, 2003 Feb 8,152(6):176-7. [13] ALVAR J, CANAVATE C, MOLINA R, MORENO J, NIETO J. - Canine leishmaniasis. Adv Parasitol, 2004,57:1-88. [14] BERRAHAL (F.), MARY (C.), ROZE (M.), BERENGER (A.), ESCOFFIER (K.), LAMOUROUX (D.), et al. - Canine leishmaniasis: identification of asymptomatic carriers by polymerase chain reaction and immunoblotting. Am J Trop Med Hyg. 1996 Sep,55(3):273-7. [15] SOLANO-GALLEGO (L.), MORELL (P.), ARBOIX (M.), ALBEROLA (J.), FERRER (L.) - Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. J Clin Microbiol, 2001 Feb,39(2):560-3. [16] LEONTIDES (L.S.), SARIDOMICHELAKIS (M.N.), BILLINIS (C.), KONTOS (V.), KOUTINAS (A.F.), GALATOS (A.D.), et al. - A crosssectional study of Leishmania spp. infection in clinically healthy dogs with polymerase chain reaction and serology in Greece. Vet Parasitol, 2002 Oct 16,109(1-2):19-27. [17] CARDOSO (L.), NETO (F.), SOUSA (J.C.), RODRIGUES (M.), CABRAL (M.) - Use of a leishmanin skin test in the detection of canine Leishmania-specific cellular immunity. Vet Parasitol, 1998 Nov 16,79(3):213-20. [18] CABRAL M, O’GRADY (J.E.), GOMES (S.), SOUSA (J.C.), THOMPSON (H.), ALEXANDER (J.) - The immunology of canine leishmaniosis: strong evidence for a developing disease spectrum from asymptomatic dogs. Vet Parasitol, 1998 Apr 15,76(3):17380. [19] SOLANO-GALLEGO (L.), LLULL (J.), RAMOS (G.), RIERA (C.), ARBOIX (M.), ALBEROLA (J.), et al. - The Ibizian hound presents a predominantly cellular immune response against natural Leishmania infection. Vet Parasitol, 2000 Jun 10,90(1-2):37-45. [20] MORENO (J.), ALVAR (J.) - Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol, 2002 Sep,18(9):399-405. [21] MAROLI (M.), ROSSI (L.), BALDELLI (R.), CAPELLI (G.), FERROGLIO (E.), GENCHI (C.), et al. - The northward spread of leishmaniasis in Italy: evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Trop Med Int Health. 2008 Feb,13(2):256-64. [22] WERNECK (G.L.), COSTA (C.H.), WALKER (A.M.), DAVID (J.R.), WAND (M.), MAGUIRE (J.H.) - Multilevel modelling of the incidence of visceral leishmaniasis in Teresina, Brazil. Epidemiol Infect. 2007 Feb,135(2):195-201. [23] MIRO (G.), CARDOSO (L.), PENNISI (M.G.), OLIVA (G.), BANETH (G.) - Canine Leishmaniosis: New Concepts and Insights on an Expanding Zoonosis Part two. Trends Parasitol, 2008, 24:371377 [24] MARGONARI (C.), FREITAS (C.R.), RIBEIRO (R.C.), MOURA (A.C.), TIMBO (M.), GRIPP (A.H.), et al - Epidemiology of visceral leishmaniasis through spatial analysis, in Belo Horizonte municipality, state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz, 2006 Feb,101(1):31-8. [25] GAVGANI (A.S.), MOHITE (H.), EDRISSIAN (G.H.), MOHEBALI (M.), DAVIES (C.R.) - Domestic dog ownership in Iran is a risk factor for human infection with Leishmania infantum. Am J Trop Med Hyg, 2002 Nov,67(5):511-5. [26] ACEDO SANCHEZ (C.), MARTIN SANCHEZ (J.), VELEZ BERNAL (I.D.), SANCHIS MARIN (M.C.), LOUASSINI (M.), MALDONADO (J.A.), et al - Leishmaniasis eco-epidemiology in the Alpujarra region (Granada Province, southern Spain). Int J Parasitol, 1996 Mar,26(3):303-10. [27] KILLICK-KENDRICK (R.) - The biology and control of phlebotomine sand flies. Clin Dermatol. 1999 May-Jun,17(3):279-89. [28] BATES (P.A.) - Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int J Parasitol, 2007 Aug,37(10):1097106. [29] MOLINA (R.), AMELA (C.), NIETO (J.), SAN-ANDRES (M.), GONZALEZ (F.), CASTILLO (J.A.), et al - Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans R Soc Trop Med Hyg, 1994 Jul-Aug,88(4):491-3. [30] MICHALSKY (E.M.), ROCHA (M.F.), DA ROCHA LIMA (A.C.), FRANCA-SILVA (J.C.), PIRES (M.Q.), OLIVEIRA (F.S.), et al. Infectivity of seropositive dogs, showing different clinical forms of leishmaniasis, to Lutzomyia longipalpis phlebotomine sand flies. Vet Parasitol, 2007 Jun 20,147(1-2):67-76. [31] GUARGA (J.L.), LUCIENTES (J.), PERIBANEZ (M.A.), MOLINA (R.), GRACIA (M.J.), CASTILLO (J.A.) - Experimental infection of Phlebotomus perniciosus and determination of the natural infection rates of Leishmania infantum in dogs. Acta Trop, 2000 Nov 2,77(2):203-7. [32] GUARGA (J.L.), MORENO (J.), LUCIENTES (J.), GRACIA (M.J.), PERIBANEZ (M.A.), ALVAR (J.), et al - Canine leishmaniasis transmission: higher infectivity amongst naturally infected dogs to sand flies is associated with lower proportions of T helper cells. Res Vet Sci. 2000 Dec,69(3):249-53. [33] GIGER (U.), OAKLEY (D.A.), OWENS (S.D.), SCHANTZ (P.) Leishmania donovani transmission by packed RBC transfusion to anemic dogs in the United States. Transfusion. 2002 Mar, 42(3):381-3. [34] OWENS (S.D.), OAKLEY (D.A.), MARRYOTT (K.), HATCHETT (W.), WALTON (R.), NOLAN (T.J.), et al - Transmission of visceral leishmaniasis through blood transfusions from infected English foxhounds to anemic dogs. J Am Vet Med Assoc, 2001 Oct 15,219(8):1076-83. [35] ROSYPAL (A.C.), TROY (G.C.), ZAJAC (A.M.), FRANK (G.), LINDSAY (D.S.) - Transplacental transmission of a North American isolate of Leishmania infantum in an experimentally infected beagle. J Parasitol, 2005 Aug,91(4):970-2. [36] ANDRADE (H.M.), DE TOLEDO VDE (P.), MARQUES (M.J.), FRANCA SILVA (J.C.), TAFURI (W.L.), MAYRINK (W.), et al - Leishmania (Leishmania) chagasi is not vertically transmitted in dogs. Vet 238 Canine leishmaniosis - a challenging zoonosis - L. Solano-Gallego, G. Baneth Parasitol, 2002 Jan 3,103(1-2):71-81. [37] COUTINHO (M.T.), LINARDI (P.M.) - Can fleas from dogs infected with canine visceral leishmaniasis transfer the infection to other mammals? Vet Parasitol, 2007 Jul 20,147(3-4):320-5. [38] COUTINHO (M.T.), BUENO (L.L.), STERZIK (A.), FUJIWARA (R.T.), BOTELHO (J.R.), DE MARIA (M.), et al - Participation of Rhipicephalus sanguineus (Acari: Ixodidae) in the epidemiology of canine visceral leishmaniasis. Vet Parasitol, 2005 Mar 10;128(12):149-55. [39] FERRER (L.), RABANAL (R.), FONDEVILA (D.), RAMOS (J.A.), DOMINGO (M.) - Skin lesions in canine leishmaniasis. J Small Anim Pract, 1988,29:381-8. [40] SLAPPENDEL (R.J.) - Canine leishmaniasis: A review based on 95 cases in the Netherlands. Vet Quarterly, 1988,10:1-16. [41] PINELLI (E.), KILLICK-KENDRICK (R.), WAGENAAR (J.), BERNADINA (W.), DEL REAL (G.), RUITENBERG (J.) - Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect Immun, 1994 Jan,62(1):22935. [42] BADARO (R.), JONES (T.C.), CARVALHO (E.M.), SAMPAIO (D.), REED (S.G.), BARRAL (A.), et al - New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis, 1986 Dec,154(6):100311. [43] ORDEIX (L.), SOLANO-GALLEGO (L.), FONDEVILA (D.), FERRER (L.), FONDATI (A.) - Papular dermatitis due to Leishmania spp. infection in dogs with parasite-specific cellular immune responses. Vet Dermatol, 2005 Jun,16(3):187-91. [44] BARBIERI (C.L.) - Immunology of canine leishmaniasis. Parasite Immunol, 2006 Jul,28(7):329-37. [45] NIETO (C.G.), NAVARRETE (I.), HABELA (M.A.), SERRANO (F.), REDONDO (E.) - Pathological changes in kidneys of dogs with natural Leishmania infection. Vet Parasitol, 1992 Dec,45(1-2):3347. [46] POLI (A.), ABRAMO (F.), MANCIANTI (F.), NIGRO (M.), PIERI (S.), BIONDA (A.) - Renal involvement in canine leishmaniasis. A lightmicroscopic, immunohistochemical and electron-microscopic study. Nephron, 1991,57(4):444-52. [47] COSTA (F.A.), GOTO (H.), SALDANHA (L.C.), SILVA (S.M.), SINHORINI (I.L.), SILVA (T.C.), et al - Histopathologic patterns of nephropathy in naturally acquired canine visceral leishmaniasis. Vet Pathol, 2003 Nov,40(6):677-84. [48] REIS (A.B.), MARTINS-FILHO (O.A.), TEIXEIRA-CARVALHO (A.), CARVALHO (M.G.), MAYRINK (W.), FRANCA-SILVA (J.C.), et al - Parasite density and impaired biochemical/hematological status are associated with severe clinical aspects of canine visceral leishmaniasis. Res Vet Sci, 2006 Aug,81(1):68-75. [49] REIS (A.B.), TEIXEIRA-CARVALHO (A.), VALE (A.M.), MARQUES (M.J.), GIUNCHETTI (R.C.), MAYRINK (W.), et al - Isotype patterns of immunoglobulins: hallmarks for clinical status and tissue parasite density in Brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Vet Immunol Immunopathol, 2006 Aug 15,112(3-4):102-16. [50] MARTINEZ-MORENO (A.), MORENO (T.), MARTINEZ-MORENO (F.J.), ACOSTA (I.), HERNANDEZ (S.) - Humoral and cell-mediated immunity in natural and experimental canine leishmaniasis. Vet Immunol Immunopathol, 1995 Oct,48(3-4):209-20. [51] RHALEM (A.), SAHIBI (H.), GUESSOUS-IDRISSI (N.), LASRI (S.), NATAMI (A.), RIYAD (M.), et al - Immune response against Leishmania antigens in dogs naturally and experimentally infected with Leishmania infantum. Vet Parasitol, 1999 Mar 1,81(3):17384. [52] SACKS (D.), NOBEN-TRAUTH (N.) - The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol, 2002 Nov,2(11):845-58. [53] MARGARITO (J.M.), LUCENA (R.), LOPEZ (R.), MOLLEDA (J.M.), MARTIN (E.), GINEL (P.J.) - Levels of IgM and IgA circulating immune complexes in dogs with leishmaniasis. Zentralbl Veterinarmed B, 1998 Jun,45(5):263-7. [54] LUCENA (R.), GINEL (P.J.) - Immunoglobulin isotype distribution of antinuclear antibodies in dogs with leishmaniasis. Res Vet Sci, 1998 Nov-Dec,65(3):205-7. [55] SMITH (B.E.), TOMPKINS (M.B.), BREITSCHWERDT (E.B.) Antinuclear antibodies can be detected in dog sera reactive to Bartonella vinsonii subsp. berkhoffii, Ehrlichia canis, or Leishmania infantum antigens. J Vet Intern Med, 2004 Jan-Feb,18(1):47-51. [56] TORRENT (E.), LEIVA (M.), SEGALES (J.), FRANCH (J.), PENA (T.), CABRERA (B.), et al - Myocarditis and generalised vasculitis associated with leishmaniosis in a dog. J Small Anim Pract, 2005 Nov,46(11):549-52. [57] PUMAROLA (M.), BREVIK (L.), BADIOLA (J.), VARGAS (A.), DOMINGO (M.), FERRER (L.) - Canine leishmaniasis associated with systemic vasculitis in two dogs. J Comp Pathol. 1991 Oct,105(3):279-86. [58] VAMVAKIDIS (C.D.), KOUTINAS (A.F.), KANAKOUDIS (G.), GEORGIADIS (G.), SARIDOMICHELAKIS (M.) - Masticatory and skeletal muscle myositis in canine leishmaniasis (Leishmania infantum). Vet Rec, 2000 Jun 10,146(24):698-703. [59] ABRANCHES (P.), SILVA-PEREIRA (M.C.), CONCEICAO-SILVA (F.M.), SANTOS-GOMES (G.M.), JANZ (J.G.) - Canine leishmaniasis: pathological and ecological factors influencing transmission of infection. J Parasitol, 1991 Aug,77(4):557-61. [60] CARDOSO (L.), RODRIGUES (M.), SANTOS (H.), SCHOONE (G.J.), CARRETA (P.), VAREJAO (E.), et al - Sero-epidemiological study of canine Leishmania spp. infection in the municipality of Alijo (Alto Douro, Portugal). Vet Parasitol, 2004 May 7,121(1-2):21-32. [61] DANTAS-TORRES (F.), DE BRITO (M.E.), BRANDAO-FILHO (S.P) Seroepidemiological survey on canine leishmaniasis among dogs from an urban area of Brazil. Vet Parasitol, 2006 Aug 31,140(12):54-60. [62] FRANCA-SILVA (J.C.), DA COSTA (R.T.), SIQUEIRA (A.M.), MACHADO-COELHO (G.L.), DA COSTA (C.A.), MAYRINK (W.), et al, - Epidemiology of canine visceral leishmaniosis in the endemic area of Montes Claros Municipality, Minas Gerais State, Brazil. Vet Parasitol, 2003 Feb 13,111(2-3):161-73. [63] SIDERIS (V.), PAPADOPOULOU (G.), DOTSIKA (E.), KARAGOUNI (E.) - Asymptomatic canine leishmaniasis in Greater Athens area, Greece. Eur J Epidemiol, 1999 Mar,15(3):271-6. [64] SANCHEZ-ROBERT (E.), ALTET (L.), UTZET-SADURNI (M.), GIGER (U.), SANCHEZ (A.), FRANCINO (O.) - Slc11a1 (formerly Nramp1) and susceptibility to canine visceral leishmaniasis. Vet Res, 2008 Feb 29,39(3):36. [65] QUINNELL (R.J.), KENNEDY (L.J.), BARNES (A.), COURTENAY (O.), DYE (C.), GARCEZ (L.M.), et al - Susceptibility to visceral leishmaniasis in the domestic dog is associated with MHC class II polymorphism. Immunogenetics, 2003 Apr,55(1):23-8. [66] SOLBACH (W.), LASKAY (T.) - The host response to Leishmania infection. Adv Immunol, 2000,74:275-317. [67] CIARAMELLA (P.), OLIVA (G.), LUNA (R.D.), GRADONI (L.), AMBROSIO (R.), CORTESE (L.), et al - A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet Rec, 1997 Nov 22,141(21):539-43. [68] SOLANO-GALLEGO (L.), RIERA (C.), ROURA (X.), INIESTA (L.), GALLEGO (M.), VALLADARES (J.E.), et al - Leishmania infantumspecific IgG, IgG1 and IgG2 antibody responses in healthy and ill dogs from endemic areas. Evolution in the course of infection and after treatment. Vet Parasitol, 2001 Apr 19,96(4):265-76. [69] KOUTINAS (A.F.), SARIDOMICHELAKIS (M.N.), MYLONAKIS (M.E.), LEONTIDES (L.), POLIZOPOULOU (Z.), BILLINIS (C.), et al. - A randomised, blinded, placebo-controlled clinical trial with allopurinol in canine leishmaniosis. Vet Parasitol, 2001 Jul 27,98(4):247-61. 239 EJCAP - Vol. 18 - Issue 3 December 2008 [70] KOUTINAS (A.F.), POLIZOPOULOU (Z.S.), SARIDOMICHELAKIS (M.N.), ARGYRIADIS (D.), FYTIANOU (A.), PLEVRAKI (K.G.) Clinical considerations on canine visceral leishmaniasis in Greece: a retrospective study of 158 cases (1989-1996). J Am Anim Hosp Assoc, 1999 Sep-Oct,35(5):376-83. [71] NARANJO (C.), FONDEVILA (D.), LEIVA (M.), ROURA (X.), PENA (T.) - Characterization of lacrimal gland lesions and possible pathogenic mechanisms of keratoconjunctivitis sicca in dogs with leishmaniosis. Vet Parasitol, 2005 Oct 10,133(1):37-47. [72] PENA (M.T.), ROURA (X.), DAVIDSON (M.G.) - Ocular and periocular manifestations of leishmaniasis in dogs: 105 cases (1993-1998). Vet Ophthalmol, 2000,3(1):35-41. [73] CORTADELLAS (O.), DEL PALACIO (M.J.), BAYON (A.), ALBERT (A.), TALAVERA (J.) - Systemic hypertension in dogs with leishmaniasis: prevalence and clinical consequences. J Vet Intern Med, 2006 JulAug,20(4):941-7. [74] TERRAZZANO (G.), CORTESE (L.), PIANTEDOSI (D.), ZAPPACOSTA (S.), DI LORIA (A.), SANTORO (D.), et al - Presence of anti-platelet IgM and IgG antibodies in dogs naturally infected by Leishmania infantum. Vet Immunol Immunopathol, 2006 Apr 15,110(34):331-7. [75] PELAGALLI (A.), CIARAMELLA (P.), LOMBARDI (P.), PERO (M.E.), CORTESE (L.), CORONA (M.), et al - Evaluation of adenosine 5’-diphosphate (ADP)- and collagen-induced platelet aggregation in canine leishmaniasis. J Comp Pathol, 2004 FebApr,130(2-3):124-9. [76] MANCIANTI (F.), GRAMICCIA (M.), GRADONI (L.), PIERI (S.) Studies on canine leishmaniasis control. 1. Evolution of infection of different clinical forms of canine leishmaniasis following antimonial treatment. Trans R Soc Trop Med Hyg, 1988,82(4):566-7. [77] BANETH (G.), AROCH (I.) - Canine leishmaniasis: a diagnostic and clinical challenge. Vet J, 2008 Jan,175(1):14-5. [78] SARIDOMICHELAKIS (M.N.), MYLONAKIS (M.E.), LEONTIDES (L.S.), KOUTINAS (A.F.), BILLINIS (C.), KONTOS (V.I.) - Evaluation of lymph node and bone marrow cytology in the diagnosis of canine leishmaniasis (Leishmania infantum) in symptomatic and asymptomatic dogs. Am J Trop Med Hyg, 2005 Jul,73(1):82-6. [79] DINIZ (S.A.), MELO (M.S.), BORGES (A.M.), BUENO (R.), REIS (B.P.), TAFURI (W.L.), et al - Genital lesions associated with visceral leishmaniasis and shedding of Leishmania sp. in the semen of naturally infected dogs. Vet Pathol, 2005 Sep,42(5):650-8. [80] GIUNCHETTI (R.C.), MAYRINK (W.), CARNEIRO (C.M.), CORREAOLIVEIRA (R.), MARTINS-FILHO (O.A.), MARQUES (M.J.), et al - Histopathological and immunohistochemical investigations of the hepatic compartment associated with parasitism and serum biochemical changes in canine visceral leishmaniasis. Res Vet Sci, 2008 Apr,84(2):269-77. [81] SILVA (F.L.), RODRIGUES (A.A.), REGO (I.O.), SANTOS (R.L.), OLIVEIRA (R.G.), SILVA (T.M.), et al - Genital lesions and distribution of amastigotes in bitches naturally infected with Leishmania chagasi. Vet Parasitol, 2008 Jan 25,151(1):86-90. [82] DANTAS-TORRES (F.) - Presence of Leishmania amastigotes in peritoneal fluid of a dog with leishmaniasis from Alagoas, Northeast Brazil. Rev Inst Med Trop Sao Paulo, 2006 JulAug,48(4):219-21. [83] SANTOS (M.), MARCOS (R.), ASSUNCAO (M.), MATOS (A.J.) Polyarthritis associated with visceral leishmaniasis in a juvenile dog. Vet Parasitol, 2006 Nov 5,141(3-4):340-4. [84] DUBEY (J.P.), ROSYPAL (A.C.), PIERCE (V.), SCHEINBERG (S.N.), LINDSAY (D.S.) - Placentitis associated with leishmaniasis in a dog. J Am Vet Med Assoc, 2005 Oct 15,227(8):1266-9, 50. [85] AGUT (A.), CORZO (N.), MURCIANO (J.), LAREDO (F.G.), SOLER (M) - Clinical and radiographic study of bone and joint lesions in 26 dogs with leishmaniasis. Vet Rec, 2003 Nov 22,153(21):64852. [86] VINUELAS (J.), GARCIA-ALONSO (M.), FERRANDO (L.), NAVARRETE (I.), MOLANO (I.), MIRON (C.), et al - Meningeal leishmaniosis induced by Leishmania infantum in naturally infected dogs. Vet Parasitol, 2001 Oct 31,101(1):23-7. [87] ROURA (X.), SANCHEZ (A.), FERRER (L.) - Diagnosis of canine leishmaniasis by a polymerase chain reaction technique. Vet Rec. 1999, Mar 6,144(10):262-4. [88] MOREIRA (M.A.), LUVIZOTTO (M.C.), GARCIA (J.F.), CORBETT (C.E.), LAURENTI (M.D.) - Comparison of parasitological, immunological and molecular methods for the diagnosis of leishmaniasis in dogs with different clinical signs. Vet Parasitol, 2007 Apr 30,145(3-4):245-52. [89] PENA (M.T.), NARANJO (C.), KLAUSS (G.), FONDEVILA (D.), LEIVA (M.), ROURA (X.), et al - Histopathological features of ocular leishmaniosis in the dog. J Comp Pathol, 2008 Jan,138(1):32-9. [90] DOS-SANTOS (W.L.), DAVID (J.), BADARO (R.), DE-FREITAS (L.A.) - Association between skin parasitism and a granulomatous inflammatory pattern in canine visceral leishmaniosis. Parasitol Res, 2004 Jan,92(2):89-94. [91] SOLANO-GALLEGO (L.), FERNANDEZ-BELLON (H.), MORELL (P.), FONDEVILA (D.), ALBEROLA (J.), RAMIS (A.), et al - Histological and immunohistochemical study of clinically normal skin of Leishmania infantum-infected dogs. J Comp Pathol, 2004 Jan,130(1):7-12. [92] GIUNCHETTI (R.C.), MARTINS-FILHO (O.A.), CARNEIRO (C.M.), MAYRINK (W.), MARQUES (M.J.), TAFURI (W.L.), et al. Histopathology, parasite density and cell phenotypes of the popliteal lymph node in canine visceral leishmaniasis. Vet Immunol Immunopathol. 2008 Jan 15,121(1-2):23-33. [93] MYLONAKIS (M.E.), PAPAIOANNOU (N.), SARIDOMICHELAKIS (M.N.), KOUTINAS (A.F.), BILLINIS (C.), KONTOS (V.I.) - Cytologic patterns of lymphadenopathy in dogs infected with Leishmania infantum. Vet Clin Pathol, 2005 Sep,34(3):243-7. [94] FERRER (L.), RABANAL (R.M.), DOMINGO (M.), RAMOS (J.A.), FONDEVILA (D.) - Identification of Leishmania donovani amastigotes in canine tissues by immunoperoxidase staining. Res Vet Sci, 1988 Mar,44(2):194-6. [95] GOMES (Y.M), PAIVA CAVALCANTI (M.), LIRA (R.A.), ABATH (F.G.), ALVES (L.C.) - Diagnosis of canine visceral leishmaniasis: biotechnological advances. Vet J, 2008 Jan,175(1):45-52. [96] DOS-SANTOS (W.L.), JESUS (E.E.), PARANHOS-SILVA (M.), PEREIRA (A.M.), SANTOS (J.C.), BALEEIRO (C.O.), et al - Associations among immunological, parasitological and clinical parameters in canine visceral leishmaniasis: Emaciation, spleen parasitism, specific antibodies and leishmanin skin test reaction. Vet Immunol Immunopathol, 2008 Feb 16. 2008 Jun 15;123(3-4):251-9 [97] PORROZZI (R.), SANTOS DA COSTA (M.V.), TEVA (A.), FALQUETO (A.), FERREIRA (A.L.), DOS SANTOS (C.D.), et al - Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic Leishmania infantum visceral infections in dogs. Clin Vaccine Immunol, 2007 May,14(5):544-8. [98] BARBOSA-DE-DEUS (R.), DOS MARES-GUIA (M.L.), NUNES (A.Z.), COSTA (K.M.), JUNQUEIRA (R.G.), MAYRINK (W.), et al. - Leishmania major-like antigen for specific and sensitive serodiagnosis of human and canine visceral leishmaniasis. Clin Diagn Lab Immunol. 2002 Nov,9(6):1361-6. [99] FERREIRA EDE (C.), DE LANA (M.), CARNEIRO (M.), REIS (A.B.), PAES (D.V.), DA SILVA (E.S.), et al - Comparison of serological assays for the diagnosis of canine visceral leishmaniasis in animals presenting different clinical manifestations. Vet Parasitol, 2007 May 31,146(3-4):235-41. [100] GOMES (Y.M.), PAIVA CAVALCANTI (M.), LIRA (R.A.), ABATH (F.G.), ALVES (L.C.) - Diagnosis of canine visceral leishmaniasis: Biotechnological advances. Vet J, 2006 Dec 4. [101] REALE (S.), MAXIA (L.), VITALE (F.), GLORIOSO (N.S.), CARACAPPA (S.), VESCO (G.) - Detection of Leishmania infantum in dogs by 240 Canine leishmaniosis - a challenging zoonosis - L. Solano-Gallego, G. Baneth PCR with lymph node aspirates and blood. J Clin Microbiol, 1999 Sep,37(9):2931-5. [102] MANNA (L.), VITALE (F.), REALE (S.), CARACAPPA (S.), PAVONE (L.M.), MORTE (R.D.), et al. Comparison of different tissue sampling for PCR-based diagnosis and follow-up of canine visceral leishmaniosis. Vet Parasitol. 2004 Nov 10;125(3-4):251-62. [103] ASHFORD (D.A.), BOZZA (M.), FREIRE (M.), MIRANDA (J.C.), SHERLOCK (I.), EULALIO (C.), et al - Comparison of the polymerase chain reaction and serology for the detection of canine visceral leishmaniasis. Am J Trop Med Hyg, 1995 Sep,53(3):251-5. [104] FISA (R.), RIERA (C.), GALLEGO (M.), MANUBENS (J.), PORTUS (M.) - Nested PCR for diagnosis of canine leishmaniosis in peripheral blood, lymph node and bone marrow aspirates. Vet Parasitol, 2001 Aug 1,99(2):105-11. [105] LACHAUD (L.), MARCHERGUI-HAMMAMI (S.), CHABBERT (E.), DEREURE (J.), DEDET (J.P.), BASTIEN (P.) - Comparison of six PCR methods using peripheral blood for detection of canine visceral leishmaniasis. J Clin Microbiol, 2002 Jan,40(1):210-5. [106] NASEREDDIN (A.), EREQAT (S.), AZMI (K.), BANETH (G.), JAFFE (C.L.), ABDEEN (Z.) - Serological survey with PCR validation for canine visceral leishmaniasis in northern Palestine. J Parasitol, 2006 Feb,92(1):178-83. [107] ROURA (X.), FONDEVILA (D.), SANCHEZ (A.), FERRER (L.) Detection of Leishmania infection in paraffin-embedded skin biopsies of dogs using polymerase chain reaction. J Vet Diagn Invest, 1999 Jul,11(4):385-7. [108] STRAUSS-AYALI (D.), BANETH (G.), JAFFE (C.L.) - Splenic immune responses during canine visceral leishmaniasis. Vet Res, 2007 JulAug,38(4):547-64. [109] STRAUSS-AYALI (D.), JAFFE (C.L.), BURSHTAIN (O.), GONEN (L.), BANETH (G.) - Polymerase chain reaction using noninvasively obtained samples, for the detection of Leishmania infantum DNA in dogs. J Infect Dis, 2004 May 1,189(9):1729-33. [110] FERREIRA SDE (A.), ITUASSU (L.T.), MELO (M.N.), ANDRADE (A.S.) - Evaluation of the conjunctival swab for canine visceral leishmaniasis diagnosis by PCR-hybridization in Minas Gerais State, Brazil. Vet Parasitol, 2008 Apr 15,152(3-4):257-63. [111] SOLANO-GALLEGO (L.), RODRIGUEZ-CORTES (A.), TROTTA (M.), ZAMPIERON (C.), RAZIA (L.), FURLANELLO (T.), et al - Detection of Leishmania infantum DNA by fret-based real-time PCR in urine from dogs with natural clinical leishmaniosis. Vet Parasitol, 2007 Jul 20,147(3-4):315-9. [112] MAIA (C.), RAMADA (J.), CRISTOVAO (J.M.), GONCALVES (L.), CAMPINO (L.) - Diagnosis of canine leishmaniasis: Conventional and molecular techniques using different tissues. Vet J, 2007 Oct 11. [113] MATHIS (A.), DEPLAZES (P.) - PCR and in vitro cultivation for detection of Leishmania spp. in diagnostic samples from humans and dogs. J Clin Microbiol, 1995 May,33(5):1145-9. [114] ZERPA (O.), ULRICH (M.), NEGRON (E.), RODRIGUEZ (N.), CENTENO (M.), RODRIGUEZ (V.), et al - Canine visceral leishmaniasis on Margarita Island (Nueva Esparta, Venezuela). Trans R Soc Trop Med Hyg, 2000 Sep-Oct,94(5):484-7. [115] NOLI (C.), AUXILIA (S.T.) - Treatment of canine Old World visceral leishmaniasis: a systematic review. Vet Dermatol, 2005 Aug,16(4):213-32. [116] MIRET (J.), NASCIMENTO (E.), SAMPAIO (W.), FRANCA (J.C.), FUJIWARA (R.T.), VALE (A.), et al - Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime) and the recombinant Leish110f + MPL-SE vaccine to treat canine visceral leishmaniasis. Vaccine, 2008 Mar 17,26(12):1585-94. [117] BORJA-CABRERA (G.P.), CRUZ MENDES (A.), PARAGUAI DE SOUZA (E.), HASHIMOTO OKADA (L.Y.), DE ATFA, KAWASAKI (J.K.), et al - Effective immunotherapy against canine visceral leishmaniasis with the FML-vaccine. Vaccine, 2004 Jun 2,22(1718):2234-43. [118] RIBEIRO (R.R.), MOURA (E.P.), PIMENTEL (V.M.), SAMPAIO (W.M.), SILVA (S.M.), SCHETTINI (D.A.), et al - Reduced tissue parasitic load and infectivity to sand flies in dogs naturally infected by Leishmania (Leishmania) chagasi following treatment with a liposome formulation of meglumine antimoniate. Antimicrob Agents Chemother. 2008 May 5. [119] GRADONI (L.), MAROLI (M.), GRAMICCIA (M.), MANCIANTI (F.) - Leishmania infantum infection rates in Phlebotomus perniciosus fed on naturally infected dogs under antimonial treatment. Med Vet Entomol, 1987 Oct,1(4):339-42. [120] ALEXANDER (B.), MAROLI (M.) - Control of phlebotomine sandflies. Med Vet Entomol, 2003 Mar,17(1):1-18. [121] MAROLI (M.), MIZZON (V.), SIRAGUSA (C.), D’OORAZI (A.), GRADONI (L.) - Evidence for an impact on the incidence of canine leishmaniasis by the mass use of deltamethrin-impregnated dog collars in southern Italy. Med Vet Entomol, 2001 Dec,15(4):35863. [122] OTRANTO (D.), PARADIES (P.), LIA (R.P.), LATROFA (M.S.), TESTINI (G.), CANTACESSI (C.), et al - Efficacy of a combination of 10% imidacloprid/50% permethrin for the prevention of leishmaniasis in kennelled dogs in an endemic area. Vet Parasitol, 2007 Mar 31,144(3-4):270-8. [123] MIRO (G.), GALVEZ (R.), MATEO (M.), MONTOYA (A.), DESCALZO (M.A.), MOLINA (R.) - Evaluation of the efficacy of a topically administered combination of imidacloprid and permethrin against Phlebotomus perniciosus in dog. Vet Parasitol, 2007 Feb 28,143(34):375-9. [124] KILLICK-KENDRICK (R.), KILLICK-KENDRICK (M.), FOCHEUX (C.), DEREURE (J.), PUECH (M.P.), CADIERGUES (M.C.) - Protection of dogs from bites of phlebotomine sandflies by deltamethrin collars for control of canine leishmaniasis. Med Vet Entomol, 1997 Apr,11(2):105-11. [125] HALBIG (P.), HODJATI (M.H.), MAZLOUMI-GAVGANI (A.S.), MOHITE (H.), DAVIES (C.R.) - Further evidence that deltamethrinimpregnated collars protect domestic dogs from sandfly bites. Med Vet Entomol, 2000 Jun,14(2):223-6. [126] GAVGANI (A.S.), HODJATI (M.H.), MOHITE (H.), DAVIES (C.R.) - Effect of insecticide-impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children: a matchedcluster randomised trial. Lancet, 2002 Aug 3,360(9330):374-9. [127] BORJA-CABRERA (G.P.), CORREIA PONTES (N.N.), DA SILVA (V.O.), PARAGUAI DE SOUZA (E.), SANTOS (W.R.), GOMES (E.M.), et al - Long lasting protection against canine kala-azar using the FML-QuilA saponin vaccine in an endemic area of Brazil (Sao Goncalo do Amarante, RN). Vaccine, 2002 Sep 10,20(2728):3277-84. [128] DANTAS-TORRES (F.) - Leishmune vaccine: the newest tool for prevention and control of canine visceral leishmaniosis and its potential as a transmission-blocking vaccine. Vet Parasitol, 2006 Oct 10,141(1-2):1-8. [129] RAMOS (I.), ALONSO (A.), MARCEN (J.M.), PERIS (A.), CASTILLO (J.A.), COLMENARES (M.), et al - Heterologous prime-boost vaccination with a non-replicative vaccinia recombinant vector expressing LACK confers protection against canine visceral leishmaniasis with a predominant Th1-specific immune response. Vaccine, 2008 Jan 17,26(3):333-44. [130] LEMESRE (J.L.), HOLZMULLER (P.), GONCALVES (R.B.), BOURDOISEAU (G.), HUGNET (C.), CAVALEYRA (M.), et al Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: double-blind randomised efficacy field trial. Vaccine, 2007 May 22,25(21):4223-34. 241 ZOONOSES Toxoplasmosis F. van Knapen,(1) P.A.M. Overgaauw(2) SUMMARY This paper is a short introduction to the impact of Toxoplasma gondii infection in humans. Major routes of transmission occur through tissue cysts infected raw meat and furthermore oocysts shed by (merely) young cats which contaminate litterboxes, gardens, sandboxes and playgrounds. Clinical symptoms in man are rare, however long-lasting lymphadenitis and general malaise may happen. Special attention is given to congenital toxoplasmosis in which the disease burden may be dramatic. The role of dogs and cats in human toxoplasmosis is discussed. Our housecat (Felix domestica) plays an important role. In the jejunum (epithelium) Toxoplasma behaves like a real coccidium: schizogeny and gametogeny are followed by excretion of oocysts. The oocysts are shedded with the faeces into the environment, where they have to sporulate to become infectious (Fig. 3). Sporulation takes at least 2 to 3 days. Sporulated oocysts are very resistant to environmental conditions and may remain infectious for two years in for example garden soil [5]. This paper was commissioned by FECAVA for publication in EJCAP. Introduction Toxoplasma gondii is an obligate intracellular protozoan parasite and part of the Sporozoa family. There are three known stages of this parasite with different appearances. Epidemiology During an active Toxoplasma infection the free parasites (tachyzoites) replicate by division in the host cell until this cell is lysed (Fig. 1). Extracellular parasites released by the host cell lysis actively invade new host cells and cause severe tissue damage. There is no preference for cell types or organ systems. The tachyzoites are approximately 10 microns long and 2 to 3 microns wide and have a characteristic moon shape. The consumption of, tissue cysts infected, undercooked meat [2], especially from herbivores, is the most common route of infection in man and carnivorous animals. Herbivores infect themselves with oocysts from the environment. Significant variation in frequency of toxoplasmosis infected animals has been reported from different studies and countries. Especially farm animals grazing outdoors may become infected, while production animals housed indoors during their (short) life are usually not infected with Toxoplasma. Herbivores and vegetarians can also get infected by the intake of sporulated oocysts shedded by cats (via soil, hands, vegetables etc.). A second stage is characterized by tissue- and organ cysts of 10-100 microns in diameter; the cysts have a clear wall in which hundreds of slim parasites without significant metabolism are piled up (so called rest phase or latent toxoplasmosis; (Fig. 2). These cysts remain present for many years and even stay infectious for a long period after the host has died (for example in meat). Cats only shed oocysts once during their lifetime, and only during a limited period of time (2-3 weeks) when they have lost their passive immunity and have started to hunt or to eat raw meat (ingestion of tissue cysts). Most of the spontaneous excretors found in surveys are younger than 6 months of age. A third stage of T. gondii is only found in cat species (Felidae) and more specifically in the genera Felix and Lynx. (1) Diplomate of the European College of Veterinary Public Health, Certified Parasitologist, Institute for Risk Assessment Sciences (IRAS), Division Veterinary Public Health, Veterinary Faculty, Utrecht University, Yalelaan 2, NL - 3584 CM Utrecht. E-mail: f.vanknapen@uu.nl (2) Certified Veterinary Microbiologist, Certified Parasitologist, RnA Assays, Yalelaan 2, NL - 3584 CM, Utrecht. E-mail: P.overgaauw@rnassays.com * Presented by NACAM (The Netherlands) 242 Toxoplasmosis - F. van Knapen, P.A.M. Overgaauw Fig. 1 Toxoplasma tachyzoites one hour and 24 hours after infecting a macrophage cell line. (for example in the brain) no inflammatory response is visible (Fig. 2). During the excretion period they build up enough immunity to resist a new infection. There are some exceptions to this, i.e. primary infection at a very young age, long-term treatment with corticosteroids, secondary infection with Cystoisospora, etc. In these cases even mature cats may start shedding oocysts again [4, 6]. Infection of a non-immunized cat with oocysts results in a significant antibody response, although no oocysts will be shedded [10]. In the Netherlands 60% of all one-year old cats have passed this period, thus they do not longer play an important role in the epidemiology of toxoplasmosis (Van Knapen, 1974 and 1993, unpublished data). Not in all countries seroconversion is seen in the first year of the cat’s life, as this is also depending on outdoor access, contact with other cats and the source of feed they obtained [9, 3]. This results in a persistent contamination of the environment where (young) cats deposit their faeces. In man it is estimated that 30-60% of all adults have been infected with Toxoplasma. Depending on the genetic type, the amount and virulence of the ingested parasites, clinical symptoms can be expected within 2 to 3 weeks after infection. The enteroepithelial phase of T. gondii is not usually associated with enteric disturbance [13]. The intermediate tissue cyst stage, however, may produce clinical signs in the cat as well as a variety of other animals, including the dog. The clinical signs are in that case non-specific and reflect damage to the organ systems infected, including the central nervous system, musculature, liver, lungs and the eye. Since Toxoplasma-parasites show no cell type preference, the clinical signs are variable. An adult person has enough immunity to resist an infection and far most infections proceed without any clinical symptoms. Occasionally parasitaemia is seen when chronic (latent) infections are reactivated. Special attention should be paid when a latent infection flares up again due to immunosuppression. This can be the result of another (viral) infection, or from a long-term treatment with for example corticosteroids or cytostatics. When clinical symptoms occur after an acute, acquired toxoplasmosis infection, most evident signs are lymphadenitis, fever and malaise. In rare cases a severe hepatitis, splenitis, pneumonia, polymyositis or even meningo-encephalitis can occur. Fig. 2 Toxoplasma tissue cyst containing numerous bradyzoites in brain material. Pathogenesis, pathology and clinical features in man During an acute invasion of Toxoplasma-parasites (proliferative phase, tachyzoites), there is mild to major tissue damage (necrosis). Histology shows inflammatory infiltrates consisting of round cells with free parasites and cyst formation on the borders. Probably due to a combination of cellular and humoral immune responses, the parasites are forced into a resting stage (tissue cysts). This latent toxoplasmosis is characterized by more or less round cysts with a firm wall, around which 243 EJCAP - Vol. 18 - Issue 3 December 2008 The role of dogs and cats in human toxoplasmosis It is obvious that the major source of human Toxoplasma infection is the consumption of raw or improperly cooked meat [2, 15]. However, oocysts shed by cats may be a source of soil transmitted disease in man (children) like this is the source for herbivorous livestock and rodents to become infected. Indeed Taylor et al. [14] indicated a parallel in seroprevalence studies in Ireland of specific Toxocara and Toxoplasma antibodies in children between 4-18 years of age, suggesting that contaminated soil may have been a common source. Sporulated oocysts certainly contaminate the direct environment of man (garden, sandpits) although very little research is done in this field. Having a cat has never been associated with a higher risk of getting a Toxoplasma infection. On the other hand, however, having a dog does in some studies correlate with an increased risk of having antibodies to Toxoplasma [7]. Dogs definitely do not play a biological role in the transmission of Toxoplasma. Soil transmitted infections, like Toxocara have recently been demonstrated in the fur of dogs, probably because of dogs behaviour on playing grounds [11, 12]. Fig. 3 Oocyst of Toxoplasma in faeces. A special form of toxoplasmosis is congenital toxoplasmosis. If a woman receives her first exposure to Toxoplasma while pregnant, the uterus and the unborn foetus(es) can become infected. In early pregnancy this can lead to severe malformation of the foetus leading to abortion or to malformations that are not compatible with life shortly after birth. A majority of congenital infections however go undiagnosed for years after birth, before clinical symptoms related to congenital toxoplasmosis are found (mental retardation, ocular defects) [8]. If there is indication of soil transmitted infections through dogs’ fur, it cannot be excluded that the same is true for toxoplasmosis. Therefore hygiene precautions (e.g. hand washing) should be taken after contact with dogs as a preventive measure for toxoplasmosis. Prevention Diagnosis in man Toxoplasmosis in man can be prevented by not eating raw or undercooked meat (do not even taste it!). Especially pregnant women should be careful because of the possibility of transplacental transmission. Furthermore, contact with sporulated oocysts should be avoided. This can be done by cleaning the cat litter box daily (within that time no sporulation has taken place), but far more important is it to pay extra attention to personal hygiene when gardening (do not eat or smoke while gardening, wear gloves) and do not eat unwashed, raw vegetables. Wash hands after contact with dogs. Despite the high incidence of infections, clinical toxoplasmosis is rare. Because the clinical signs of toxoplasmosis resemble other infectious diseases, laboratory tests are necessary for a diagnosis. Detection of specific antibodies alone is not enough, because many individuals (people and animals) already have an antibody titer. A new infection can be distinguished by the detection of increasing amounts of antibodies (seroconversion) of the different isotypes (IgG, IgM, IgA) or by circulating antigens. The detection of free parasites (tachyzoites) in combination with clinical symptoms can confirm an infection (for example in biopsy or abortion material). The detection of tissue cysts (just like antibodies alone) does not confirm an active infection. PCR-tests are available for examination of the foetus, the placenta or amniotic fluid. Toxoplasmosis in cats can be prevented by feeding them solely pre-fabricated food or cooked meat and by preventing cats from hunting and eating prey animals. Small rodents and birds form an important source of infection in cats, just like oocysts infected soil (licked from their fur). Diagnosis in animals Since clinical symptoms are mostly absent, the only way to demonstrate toxoplasmosis in animals is by serological testing. Seropositivity is an indication for past experience and adult animals of all kinds show moderate to high seroprevalences. In cats the excretion of oocysts can be detected by examination of the faeces. Oocysts are only shed during a short period of time, so this examination is not useful when looking for the source of clinical toxoplasmosis in a family. There are no disinfectants effective against Toxoplasma oocysts. Boiling water or steam kill them immediately. 244 Toxoplasmosis - F. van Knapen, P.A.M. Overgaauw References [9]. LOPES (A.P.), CARDOSO (L.), RODRIGUES (M.) - Serological survey of Toxoplasma gondii infection in domestic cats from northeastern Portugal. Vet Parasitol, 2008, Aug 17, 155(3-4): 184-9. [10] OVERDULVE (J.P.) - The discovery of sexuality and studies on the life cycle, particularly the sexual stages in cats of the sposozoan parasite Isospora (Toxoplasma) gondii. Thesis, University of Utrecht, 1978. [11] OVERGAAUW (P.A.M.), VAN ZUTPHEN (L), HOEK (D), YAYA (F.O.), ROELFSEMA (J), PINELLI (E.), VAN KNAPEN (F), KORTBEEK (LM) Zoonotic parasites in faecal samples and fur from dogs and cats in The Netherlands. 2008, submitted. [12] RODDIE (G.), STAFFORD (P.), HOLLAND (C.), WOLFE (A.) Contamination of dog hair with eggs of Toxocara canis. Vet Parasitol, 2007, 152: 85-93. [13] SAEIJ (J.), BOYLE (J.), BOOTHROYD (J.) - Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol, 2005, 21(10): 476-81. [14] TAYLOR (M.R.), LENNON (B), HOLLAND (C.V.), CAFFERKEY (M.) - Community study of toxoplasma antibodies in urban and rural schoolchildren aged 4 to 18 years. Arch Dis Child, 1997, 77: 406410. [15] TENTER (A.M), HECKEROTH (A.R), WEISS (L.M.) - Toxoplasma gondii: from animals to Humans. Int J Parasitol, 2000, 30 (12-13): 1217-1258. [1] BUXTON (D.) - Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Vet Res, 1998, 29 (3-4): 289-310. [2] COOK (A.J.C), GILBERT (R.E.), BUFFOLANO (W.), ZUFFEREY (J.), PETERSEN (E.), JENUM (P.A.), FOULON (W.), SEMPRINI (A.E), DUNN (D.T.) - Sources of toxoplasma infection in pregnant women: European multicentre casecontrol study. Br Med J, 2000, 321: 142147. [3] CRAEYE, (S.) DE, FRANCART (A.), CHABAUTY (J.), Vriendt, (V.) DE, Steven, GUCHT (S.) VAN, LEROUX (I.), JONGERT (E.) - Prevalence of Toxoplasma gondii infection in Belgian house cats. Vet Parasitol, 2008, 157: 128–132. [4] DUBEY (J.P.) - Reshedding of Toxoplasma oocysts by chronically infected cats. Nature, 1976, 262: 213-214. [5] DUBEY (J.P.), BEATTIE (C.P.) - Toxoplasmosis of animals and man. CRC press, Boca raton, 1988. [6] DUBEY (J.P.) - Duration of immunity to shedding of Toxoplasma gondii oocysts by cats. J Parasitol, 1995, 81: 410-415. [7] FISHER (S.), REID (R.R.) - Antibodies to Toxoplasma gondii and contact with animals in the home. The Med. J. of Australia 1973, 1: 1275–1277. [8] GILBERT (R.E.), STANFORD (M.R.) - Is ocular toxoplasmosis caused by prenatal or postnatal infection? Br J Ophthalmol, 2000, 84: 224-226. 245 ZOONOSES Toxoplasmosis – an update G. Miró,(1) A. Montoya,(2) M. Fisher,(3) I. Fuentes(4) SUMMARY Toxoplasma gondii is an obligate intracellular protozoan parasite, with the potential to infect human beings and a broad spectrum of vertebrate hosts. Toxoplasmosis is an endemic disease with a worldwide distribution. Cats and wild felidae play a crucial role in the epidemiology of this disease, as they are the only species that shed the environmentally resistant oocysts in their faeces. Sporulated oocysts from infected cats and bradyzoites from tissue cysts are infectious to all warm-blooded animals including human beings. Dogs and cats may act as intermediate hosts and could also suffer clinical toxoplasmosis of variable severity. The majority of cases in immunocompetent people are asymptomatic or produce only mild symptoms, while in congenitally infected children and immunocompromised people, infection causes high rates of morbidity and mortality. Due to the potential public health risk of toxoplasmosis, veterinarians need to be able to identify, control infected and treat sick cats and recommend measures to prevent T. gondii infection in cats as well as in human beings. first feline toxoplasmosis case was not reported until 1942, by Olafson and Monlux. After a brief illness characterized by anorexia, fever and a cough, the cat died. At necropsy, tumorlike enlargements of the mesenteric lymph nodes, ulceration of the intestinal mucosa, and multiple nodules in the lungs were observed. T. gondii was identified in the lymph nodes and lungs [4]. This paper was commissioned by FECAVA for publication in EJCAP. Introduction Toxoplasmosis is an important parasitic disease caused by the protozoan Toxoplasma gondii. The parasite was first discovered by Nicolle and Manceaux in 1908, in the gundi (Ctenodactylus gundi), a North African rodent. The name Toxoplasma gondii (“toxon”= arc; “plasma”= form in Greek) is derived from its crescent shape and from the animal from which was first isolated. The complete life cycle of T. gondii was not fully described until 1970 [1,2]. Life cycle of Toxoplasma gondii The complex life cycle of Toxoplasma gondii involves two phases. The sexual phase takes part in the Felidae family, and the asexual phase in any warm-blooded animal, i.e. mammals (including humans) and birds. There are three infective stages: – Sporulated oocysts from infected cats. The oocysts are excreted unsporulated and uninfective when passed in feces. Sporulation occurs in the environment after 1-5 days (dependent on temperature and moisture; e.g. 1 day at 2425ºC, 5 days at 15ºC and 21 days at 11ºC). The first record of canine toxoplasmosis was made by Mello (1910), who described an acute toxoplasmosis in a dog in Turin, Italy. The dog presented with fever, anorexia, anemia, dyspnoea and haemorrhagic diarrhoea. T. gondii was identified in sanguineous exudates and in nodules in the lungs [3]. The 1) Guadalupe Miró. Animal Health Department, Faculty of Veterinary Medicine / Departamento de Sanidad Animal, Facultad de Veterinaria, Universidad Complutense de Madrid, Avda Puerta Hierro s/n, 28040-Madrid (Spain). Tel.: 34-91-3943711. Fax: 34-91-3943908. E-mail: gmiro@vet.ucm.es 2) A Montoya. Animal Health Department, Faculty of Veterinary Medicine, Universidad Complutense de Madrid, 28040 Madrid, Spain. 3) M. Fisher. Shernacre Cottage, Lower Howsell Road, Malvern, Worcestershire WR14 1UX, UK. 4) I. Fuentes. Department of Parasitology, National Center of Microbiology, ISCIII, 28224 Majadahonda, Madrid, Spain. 246 Toxoplasmosis – an update - G. Miró, A. Montoya, M. Fisher, I. Fuentes Fig. 1 Life cycle of T. gondii (Montoya, 2007 [51]). time to sporulation depending on environmental temperature and humidity. Sporulated oocysts are more resistant to the environmental and chemical conditions than are unsporulated oocysts; their thermic tolerance range being – 4 to 55ºC. In liquid medium they can survive for several years [6] (Fig. 1). – Bradyzoites (“brady”= slow in Greek) from tissue cysts. – Tachyzoites (“tachos”= speed in Greek), the rapidly dividing forms that multiplies in any cell of the intermediate host and non-intestinal epithelial cells of the definitive hosts. The asexual phase or extra-intestinal cycle occurs in any warmblooded animal after infection by any infectious stage. After ingestion, sporozoites are free in the intestinal lumen and divide by an asexual process (endodyogeny). Tachyzoites multiply rapidly, invading different cells during active infection. Once host immunity develops the process slows down and during chronic infection and cysts containing bradyzoites are formed [7]. The sexual phase or entero-epithelial cycle occurs in the cat’s intestine after ingestion of any infective form. Zoites penetrate the epithelial cells of the small intestine and initiate a sequence of numerous generations (termed Types A to E) [5], whereafter a variable number of schizogonies, microgamonts and macrogamonts are formed. The microgamonts fertilize the macrogamonts and oocysts are formed. Unsporulated oocysts are shed with the feces into the environment, with The prepatent period is around 18 days in cats fed infectious oocysts as compared with 3 to 10 days in cats that are fed tissues cysts [8]. Fig. 2 Epidemiological cycle of T. gondii (Courtesy Dr. M. Mateo). Epidemiology Cats and wild felidae play a crucial role in the epidemiology of T. gondii, because they are the only hosts that can shed oocysts in the environment (Fig. 2). All nonfeline hosts are intermediate hosts that harbor tissue cysts. It is generally assumed that cats probably play a major role in transmitting the parasite through faecal contamination of soil, food or water. In studies carried out so far, there is no toxoplasmosis in areas where there are no cats [9] . However, Prestud et al. (2007), reported other possible sources of infection due to T. gondii in the Arctic [10]. Cats and dogs can be infected by T. gondii by different routes: – Ingestion of water, food, vegetables contaminated with sporulated oocysts from cats faeces. – Ingestion of infected tissues with tissue cysts containing 247 EJCAP - Vol. 18 - Issue 3 December 2008 Country Nº cats Seropre- Test valence (%) Reference Pathogenesis In the majority of acute infections acquired after ingestion of tissue cysts or sporulated oocysts, the main infection route is the intestine. Tachyzoites multiply actively, and are disseminating through the lymphatic system to the different organs where they produce cell necrosis caused by the intracellular growth of the parasites. Heavily infected animals may die during this phase. During this time parasites can be excreted through different biological fluids (faeces, urine, etc.), but these tachyzoites are very labile and are easily destroyed. For that reason transmission to other hosts is very unlikely, even when the animals are very close together. This fact has been demonstrated under experimental conditions (e.g. sick mice living in the same cage). Spain Madrid 101 54,5 IFAT Aparicio et al., 1972 [19] Madrid 69 63,4 IFAT Alonso et al., 1997 [20] Madrid 279 30,8 IFAT Miró et al., 2004 [17] Barcelona 220 45 MAT Gauss et al., 2003 [21] La Rioja 306 33,7 IFAT Miró et al., 2004 [17] Germany 267 59,9 DT Knaus & Fehler, 1989 [22] Belgium 346 70,2 MAT Dorny et al., 2002 [23] Poland 105 52,5 LAT Czech republic 2.560 61,3 IFAT Smielewska & Pacon, 2002 [24] Svobodová et al., 1998 [25] Sweden 244 ELISA Uggla et al., 1990 [26] 42 The sub-acute phase of the disease is characterized by the appearance of IgA antibodies specific for T. gondii enteroepithelial stages, which eliminate tachyzoite replication in the intestinal phase but not those localized in the nervous system. DT: Sabin Feldman Dye Test; IFAT: Indirect Fluorescent Antibody Test; ELISA: Enzyme-linked Immunosorbent Assay; MAT: Modified Agglutination Test; LAT: Latex Agglutination Test. The chronic form starts with tachyzoites beginning to disappear from visceral tissues and is characterized by persistence of bradyzoites within cysts. These may be long-lasting; up to 10 months in the dog and 3 years in rats and pigeons, or maybe for life. This phase is associated with a systemic immune response, which inhibits tachyzoites proliferation in blood and tissues (liver, spleen, lungs, etc.) [27]. Tab. 1 Seroprevalence to T. gondii in cats from Europe. bradyzoites from mammals and birds. This is the most frequent form of infection. – Ingestion of infectious oocysts directly from faeces: dogs ingesting cat litter could serve as mechanical vectors for transmission to humans as they shed ingested sporulated oocysts in their stool [11]. – Congential infection: parasitaemia during pregnancy can cause transfer and spread of tachyzoites to the fetus [12, 13]. Other minor important routes of transmission include tachyzoites shed in milk and ingestion through sucking [14], and blood transfusion. Clinical signs T. gondii is the most important coccidian of the cat as it is the cause of one of the most significant zoonoses. However, clinical signs related to infection are normally not severe in the feline host, although cats may have diarrhoea alternating with normal faeces. Oocysts excretion from an infected cat occurs 3-10 days post infection, and is maintained for a period up to 3 weeks. Cats can also be asymptomatic during this phase [28]. The reason why infected dogs or cats develop clinical disease is unknown but may depend on factors such as: age, sex, strain of T. gondii infection rate, infection acquisition (post-natally acquired infection is less severe than pre-natally acquired infection), stress, concomitant illness (retrovirus, FIP, mycoplasmosis, distemper, leishmaniasis, ehrlichiosis, etc) [29-33] or immunosuppression (glucocorticoid or cyclosporine therapy) [34-36]. There are numerous studies on the seroprevalence of T. gondii in felines reported from many countries in Europe, however these studies are difficult to compare due to the differences in the diagnostic techniques used, in the size and origin of the samples, and time of the study conducted (Tab. 1). In all of these reports the frequency of finding oocysts in faeces is low, however, probably because oocysts are shed during a short period of time [15]. Cat In general, the cats that have a major influence in the epidemiology of the disease are naive and farm cats. This fact is explained by the preying habits of this group and their diet that includes wild birds, rodents and Toxoplasma-infected placentas and stillborn fetuses [15, 16]. The higher seroprevalence reported in adult cats indicates that the risk of exposure to T. gondii increases with age. Sex is not considered to be a determining factor of infection [17]. Nonetheless, some authors suggest a higher prevalence in males associated with their territorial habits, as they have a wider area of operation than females [18]. When infected cats act as intermediate hosts they may suffer a more or less severe clinical picture (clinical toxoplasmosis) such as: fever (40 to 41ºC), lethargy, dyspnoea, lymphadenomegaly, vomiting, diarrhoea, icterus, respiratory tract disease, neurologic signs (stupor, ataxia, seizures, circling, partial or total blindness, etc.) (Fig. 3), ocular signs (anterior uveitis, retinochoroiditis etc.) (Fig. 4). Cats with ocular lesions have a higher seroprevalence than cats with normal eyes [37]. These animals with toxoplasmosis have circulating immunocomplexes that may play an important role in development of ocular lesions, and ocular signs may occur without polysystemic clinical signs of disease [38]. 248 Toxoplasmosis – an update - G. Miró, A. Montoya, M. Fisher, I. Fuentes Fig. 4 Uveitis due to toxoplasmosis in a cat (Courtesy Dr. A. Rodríguez). Dog Mild infections are almost always asymptomatic, but in severe cases clinical signs in dogs include: respiratory disorders (in 50% of the cases), digestive disorders (in 25%) and neurological disorders (in 25%). This is common in young dogs with generalized toxoplasmosis and on some occasions several clinical signs may occur concurrently (unpublished observation). Because of this, it is important to consider other diseases, including distemper and neosporosis as differential diagnosis. Fig. 3 Adult cat with clinical toxoplasmosis. All these clinical signs may persist for several days up to several months. Theoretically any organ may be infected, thus clinical signs are very variable; even though generalized infections are quite rare in cats. Central nervous system toxoplasmosis is not common in cats, with only 7% of histology confirmed cases of toxoplasmosis showing neurological signs [39]. The onset of clinical toxoplasmosis may be insidious, with clinical signs such as fever, anorexia, dyspnoea, vomiting, diarrhoea, seizures and ataxia. In contrast to the frequency of ocular toxoplasmosis that occurs in cats, there are few cases described in dogs, but occasional cases appear, with clinical signs being similar to what is seen in the cat. The disease is more severe in cats co-infected with retroviruses (mainly FIV) [29, 30] or feline infectious peritonitis (FIP) [33]. Diagnosis Epidemiological studies have shown that clinical toxoplasmosis is more prevalent in stray adult cats or owner cats than have access to prey. This higher prevalence is correlated with a higher risk of being infected, but not with age or sex. Lappin (1990) suggested three criteria for diagnosing clinical toxoplasmosis: (i) clinical signs of toxoplasmosis, (ii) serological evidence of recent or active infection, and (iii) response to antiToxoplasma drugs [41]. Likewise, clinical toxoplasmosis is much more severe in kittens that have acquired the infection by the congenital route where the disease can be severe and fatal. Clinical illness varies with the stage of gestation at the time of infection, and some newborn kittens may even shed oocysts [12]. In these cases, animals may be stillborn or die within a few hours of birth [40]. The most typical clinical signs are anorexia, lethargy and sudden death. At necropsy the liver is the most frequently affected organ with diffuse hepatitis (more than 75% of the liver is destroyed due to T. gondii infection) followed by gross lesions in the lungs (diffuse edema and congestion). Clinical signs, clinico-pathological findings and complementary examinations Encephalitis in newborn kittens has been described, animals may sleep continuously or present with signs of hyperaesthesia with atypical crying and incoordination. These clinical signs prevent feeding and affected kittens can die within few days. Cases of infertility in queens have also been described. The main haematological and biochemical abnormalities that have been reported are non regenerative anaemia, neutrophilic leucocytosis, lymphocytosis and eosinophilia. Severe leucopenia could be present in animals during the acute phase of the disease [42]. Leucocytosis is mainly seen in the recovery phase. Toxoplasmosis should be suspected in dogs and cats with anorexia, fever, dyspnoea, abdominal discomfort, hepatitis with icterus, pancreatitis, anterior uveitis, retinochoroiditis and central nervous system (CNS) disease manifestations. Thoracic radiographs and abdominal ultrasonography may aid in the diagnosis of toxoplasmosis in suspected cases. 249 EJCAP - Vol. 18 - Issue 3 December 2008 are excreted by cats during a short period of time (1-2 weeks) after first infection and because the oocyst size (10x12µm) makes microscopic analysis difficult (Fig. 5). Moreover, oocysts shedding is not associated with clinical signs such as diarrohea [15, 44]. T. gondii oocysts are morphometrically indistinguishable from oocysts of Hammondia hammondi and Besnoitia sp. Oocysts of these coccidians can be differentiated by in vitro sporulation and subsequent mouse inoculation for xenodiagnosis [45]. Serological tests There are many tests for diagnosing toxoplasmosis: the Sabin Feldman Dye Test, the indirect fluorescent antibody (IFAT), enzyme-linked immunosorbent assay (ELISA), agglutination procedures and Western Blot immunoassay being the most commonly used tests (Fig. 6). Fig. 5 Unsporulated oocyst of T. gondii (x 40). IgM antibodies are detected within 1-2 weeks post-infection and titers remain high for 12-16 weeks. An IgM titer > 1:64 with negative IgG indicates active infection. When IgM antibodies remain persistently it could be associated with reactivation of a chronic infection after repeated exposure to T. gondii, glucocorticoid therapy or concomitant infectious diseases (FIV). Since the IgM titer may remain increased for long periods, the interpretation of elevated IgM can be difficult [46]. A biochaemical profile characterized by hypoproteinaemia and hypoalbuminaemia is typical of the acute phase, while hypergammaglobulinemia has been reported in cats with chronic toxoplasmosis. Dogs with hepatic necrosis present with increased serum levels of alanine aminotransferase (ALT) and alkaline phosphate (ALP), while cats that develop colangiohepatitis or hepatic lipidosis show high serum bilirubin levels. IgG antibodies do not develop until about 2 weeks postinfection and remain at high levels for several years, even for life. Diagnosis of active toxoplasmosis using IgG test requires a fourfold or greater increase in IgG antibody titer during 2-3 weeks. Animals with acute pancreatitis due to T. gondii infection may show increased serum amylase and lipase activities. Faecal examination in cats Detection of T. gondii antibodies in aqueous humor and cerebrospinal fluid have been used as an aid in the diagnosis of dogs and cats with toxoplasmic encephalitis or uveitis. Whilst some serological tests have been developed for the detection of ocular and CNS toxoplasmosis, they are not conclusive, nonetheless they maybe helpful in some cases [47, 48]. Most cats with clinical toxoplasmosis will not be excreting oocysts at the time of presentation [43]. In cats, Toxoplasma oocysts (10 x 12 μm) can be identified in feces using any of the standard fecal flotation techniques. However, T. gondii oocysts in feces have been detected in less than 1% of the cats that have been examined in numerous studies, because oocysts usually Fig. 6 Indirect Fluorescent Antibody Test (IFAT) to detect Toxoplasma infection. 250 Toxoplasmosis – an update - G. Miró, A. Montoya, M. Fisher, I. Fuentes T. gondii may also be diagnosed by cultivation techniques such as isolation in mice or cell cultures, although these methods are only available in specialized laboratories. Molecular methods of diagnosis such as polymerase chain reaction (PCR) from samples including blood, aspirates, placentas and amniotic fluid are being used in human medicine to diagnose toxoplasmosis. The PCR can detect 1-10 tachyzoites in CSF and aqueous humor and as little as 5 tachyzoites in blood samples [49-51]. Results of PCR testing in veterinary medicine indicate that it can be an aid in diagnosis, as suggested by the most recent studies done by Montoya 2006 [52] who identified T. gondii DNA by nested-PCR and real time PCR from feline brain and blood samples (Fig. 7). Treatment Treatment is recommended in cats and dogs with clinical toxoplasmosis and works against replication of T. gondii. However, the available drugs are not completely effective in killing the parasite (Tab. 2). Clindamycin is the first line drug for treatment of disseminated toxoplasmosis in both species. Animals can also be treated with a combination of sulfonamides and pyrimethamine, although they are contraindicated in pregnant queens and bitches. Spiramycin can be used in pregnant females [43]. Fig. 7 Sensitivity determination of B1 direct PCR (A) and nestedPCR (B). Clinical signs of systemic illness usually begin to resolve within 24-48 hours after institution of therapy. Bone marrow suppression can occur with the use of pyrimethamine or trimethoprim sulfonamide combinations and can be prevented or corrected with the addition of folic acid (5 mg per day) or yeast (100 mg/kg) to the cat’s diet [43]. Lastly, the detection of antibodies to diagnose neonatal toxoplasmosis is controversial. Dubey et al., 1995, showed that transplacental transfer of T. gondii does occur in cats but the frequency is not well known [12], so if the queen is seronegative and lives indoors the kittens will not have transplacental transmission. If the queen acquires the infection during pregnancy, both kittens and queen will present with high IgG titers. Treatment of suspected ocular toxoplasmosis includes controlling anterior uveitis if present with topical anti-inflammatory agents (prednisolone acetate or dexamethasone) and the administration of systemic antitoxoplasmic drug [52]. Direct detection of T. gondii T. gondii oocysts shedding by cats can be suppressed by coccidiostatics such as toltrazuril, clazuril and sulfonamides [54]. Cytologic examination of bronchoalveolar washes, aqueous humour, cerebrospinal fluid (CSF), lymph node, peritoneal or thoracic fluid aspirates can be useful to detect tachyzoites of T. gondii. A diagnosis may be established by microscopic examination of impression smears stained with Giemsa. Lastly, seropositive cats must be monitored through a routine serologic test, at least once per year, in order to detect eventual seroconversion caused by reactivation from a chronic phase, but specific treatment is not needed in asymptomatic cats. Tab. 2. Treatment of toxoplasmosis in dogs and cats. Drug Route Dose-rate Duration Clindamycin hydrochloride Per os 10-20 mg/kg BID 4 weeks Clindamycin phosphate IM, IV 12.5-25 mg/kg BID 4 weeks Pyrimethamine plus trimethoprin-sulfonamide Per os 0.25-0.5 mg/kg BID (pyrimethamine) 4 weeks 30 mg/kg BID (TMP-sulfonamides) Spiramycin Per os 8-10 mg /kg BID 10 days Toltrazuril/Clazuril Per os 5-10 mg/kg SID 5-10 days 251 EJCAP - Vol. 18 - Issue 3 December 2008 Prevention It is very important to know the T. gondii life cycle and the epidemiology of the disease to define the control measures in order to reduce feline infection and environmental contamination with oocysts. The following measures are recommended: – Do not feed cats with raw or rare meat. If raw meat is used, it can be frozen or gamma-ray irradiated to kill tissue cysts – Change litter boxes daily to avoid oocysts sporulation – Do not allow cats to bring their prey into the house – Perform annual fecal and serologic examination of cats at risk – Cats should be prevented from entering buildings where food producing animals are housed or feed is stored – Animal blood donors should be screened before transfusion – Do not allow dogs to consume cat feces – For killing parasites, use chemical disinfection with ammonia 10% for 10 minutes, for the litter trays and all surfaces, or immerse in boiling soapy water or use steam cleaning – Control different invertebrates which may act as mechanical vectors such as: bed bugs, earthworms, cockroaches, etc. [55, 56]. Fig. 8 Distribution of human toxoplasmosis (Tenter et al., 2000 [61]). occurs in 10% to 50% of patients with AIDS who are seropositive to Toxoplasma and have a low CD4+T lymphocyte count. Since the introduction of highly active antiretroviral therapy in 1996, the incidence rates have decreased in some regions. However, toxoplasmosis is a large health problem in many countries [64]. Serological tests are useful in detecting infection, but they cannot distinguish between reactivated and latent infections and may give false negative results for immunocompromised patients. Therefore, PCR detection of active parasites needs to be performed [65]. Although commercial vaccines are still not available, a candidate T-263 (bradyzoites of a mutant strain) has been designed against Toxoplasma infection in cats. Cats developed immunity without concomitant shedding of infectious oocysts following oral administration of a vaccine containing T-263 bradyzoites [57, 58]. The vaccine can only be administered to healthy cats, and it is not recommended in pregnant females [43]. Congenital toxoplasmosis occurs if the mother is infected for the first time during gestation. The parasite reaches the foetus transplacentally and the outcome of the infection depends on the virulence of the strain, on the woman’s immune response and the period of pregnancy. Risk of congenital toxoplasmosis is lower if infection occurs during the first trimester (10%25%) than if it occurs during the second or the third trimester (60%-90%) [66]. Clinical manifestation of toxoplasmosis in foetuses and neonates vary from mild disease to severe signs (abortion, hydrocephalus, chorioretinitis, intracranial calcification, hepatosplenomegaly). Most infected neonates are asymptomatic at birth but may present much later with ocular alteration or mental and psychomotor disorders. Human toxoplasmosis and public health considerations Human toxoplasmosis has a wide geographical distribution and is estimated to affect around two billion people worldwide [27]. There is a considerable range in seroprevalence (7.5% - 95%) in different parts of the world and indeed between different population groups within the same country [59]. In Europe seroprevalence varies from less than 20% in northern Europe to more than 60% in southern Europe [60] (Fig. 8). Toxoplasma gondii is transmitted to humans via a variety of routes: ingestion of raw or undercooked contaminated meat; exposure to sporulated oocysts in cat litter or soil, from unwashed fruit, vegetables, gardening or contaminated water; or congenital transmission when a primary maternal infection is passed transplacentally to the foetus. Additionally, toxoplasmosis may be acquired by organ transplant or laboratory accident [62, 63]. The diagnosis of T. gondii infection is most commonly established by detecting IgG and IgM antibodies in the blood; however, these tests cannot estimate the time of infection precisely enough to properly manage the risk to the foetus of a maternal infection. A positive IgM test result may reflect an acute infection; however, Toxoplasma-specific IgM antibodies may be found for up to several years, and thus may lead to erroneous interpretation. Recently, it has been suggested that the combination of a sensitive test for Toxoplasma-specific IgM antibodies and measurement of the avidity of IgG antibodies for T. gondii had the highest predictive value with regard to the time of infection [60]. Diagnostics by PCR is also useful. Prevention of human toxoplasmosis mainly concerns avoiding infection. Health education may decrease the incidence during pregnancy by 60% [67]. Education programs, prenatal and The majorities of cases in immunocompetent people are asymptomatic or produce only mild symptoms, but result in life-long chronic infection with parasites inside tissue cysts. Reactivation of latent infection may therefore lead to severe and life-threatening disease in immunocompromised individuals. Toxoplasmosis is the most prevalent disorder affecting the brain in HIV-patients, causing toxoplasmic encephalitis (TE). It is the second most common AIDS-related opportunistic infection and 252 Toxoplasmosis – an update - G. Miró, A. Montoya, M. Fisher, I. Fuentes [13] DUBEY (J.P.), MATTIX (M.E.), LIPSCOMB (T.P.) - Lesions of neonatally induced toxoplasmosis in cats. Vet Pathol, 1996, 33:290-295. [14] POWELL (C.C.), BREWER (M.), LAPPIN (M.R). - Detection of Toxoplasma gondii in the milk of experimentally infected cats. Vet Parasitol, 2001, 102:29-33. [15] STEVEN (L.H.), CHENEY (J.M.), TATON-ALLEN (G.F.), REIF (J.S.), BRUNS (C.), LAPPIN (M.R.) - Prevalence of enteric zoonotic organisms in cats. J Am Vet Med Assoc, 2000, 216:687-692. [16] FRENKEL (J.K.) - Transmission of toxoplasmosis and the role of immunity in limiting transmission and illness. J Am. Vet Med Assoc, 1990, 196:233-239. [17] MIRÓ (G.), MONTOYA (A.), JIMÉNEZ (S.), FRISUELOS (C), MATEO (M.), FUENTES (I.) - Prevalence of antibodies to Toxoplasma gondii in stray, farm and household cats in Spain. Vet Parasitol, 2004, 126:249-255. [18] SMITH (K.E.), ZIMMERMAN (J.J.), PATTON (S.), BERAN (G.W.), HILL (H.T.) - The epidemiology of toxoplasmosis in Iowa swine farms with an emphasis on the roles of free-living mammals. Vet Parasitol, 1992, 42:199-211. [19] APARICIO GARRIDO (J.), COUR BOVEDA (I.), BERZOSA AGUILAR (A.M.), PAREJA MIRALLES (J.) - Estudios sobre la epidemiología de la toxoplasmosis. La infección del gato doméstico en los alrededores de Madrid. Encuesta serológica y coproparasitológica. Med Trop, 1972, 48: 24-39. [20] ALONSO (A.), QUINTANILLA-GOZALO (A.), RODRÍGUEZ (M.A.), PEREIRA-BUENO (J.), ORTEGA-MORA (L.M.), MIRÓ (G.) Seroprevalencia de la infección por Toxoplasma gondii en gatos vagabundos en el área de Madrid. Acta Parasitológica Portuguesa, 1997, 4, p. 12. [21] GAUSS (C.B.L.), ALMERÍA (S.), ORTUÑO (A.), GARCIA (F.), DUBEY (J.P.) - Seroprevalence of Toxoplasma gondii antibodies in domestic cats from Barcelona, Spain. J Parasitol, 2003, 89:10671068. [22] KNAUS (B.U.), FEHLER (K.) - Toxoplasma gondii- Infektionen und Oozystenausscheidung bei Hauskatzen-Ihre Bedeutung für die Epidemiologie und Epizootiologie der Toxoplasmose. Angew Parasitol, 1989, 30:155-60. [23] DORNY (P.), SPEYBROECK (N.), VERSTRAETE (S.), BAEKE (M.), DE BECKER (A.), BERKVENS (D.), VERCRUYSSE (J.) - Serological survey of Toxoplasma gondii, feline immunodeficiency virus and feline leukaemia virus in urban stray cats in Belgium. Vet Rec, 2002, 151: 626-629. [24] SMIELESKA-LOS (E.), PACON (J.) - Toxoplasma gondii infection of cats in epizootiological and clinical aspects. Pol J Vet Sci, 2002, 5:227-230. [25] SVOBODOVÁ (V.), KNOTEK (Z.), SVOBODA (S.) - Prevalence of IgG and IgM antibodies specific to Toxoplasma gondii in cats. Vet Parasitol, 1998, 80:173-176. [26] UGGLA (A.), MATTSON (S.), JUNTTI (N.) - Prevalence of antibodies to Toxoplasma gondii in cats, dogs and horses in Sweden. Acta Vet Scand, 1990, 31:219-222. [27] MONTOYA (J.G.), LIEDSENFELD (O.) - Toxoplasmosis. Lancet, 2004, 363:1965-1976. [28] MIRÓ (G.), CORDERO (D.E.L.) CAMPILLO (M.) - Toxoplasmosis. Neosporosis. Encefalitozoonosis. En: Parasitología Veterinaria: Parasitosis del perro y el gato. M. Cordero del Campillo y F.A. Rojo Vázquez. (eds.) Mc. Graw Hill - Interamericana. Madrid. 1999. p. 665-671. [29] HEIDEL (J.R.), DUBEY (J.P.), BLYTHE (L.L.), WALKER (L.L.), DUIMSTRA (J.R.), JORDAN (J.S.) - Myelitis in a cat infected with Toxoplasma gondii and feline immunodeficiency virus. J Am Vet Med Assoc, 1990, 196:316-318. [30] O’NEIL (S.A.), LAPPIN (M.R.), REIF (J.S.) - Clinical and epidemiological aspects of feline immunodeficiency virus and Toxoplasma gondii coinfections in cats. J Am Anim Hosp Assoc, 1991, 27:211-220. screening of neonates to identify and treat congenital infection, together with animal rearing and production methods designed to reduce T. gondii contamination of meat, are principal interventions. Toxoplasma infection in humans may be prevented by the following measures: cooking meat to a sufficient temperature to kill Toxoplasma (internal temperature higher than 67ºC; microwaving does not kill T. gondii cysts); peeling or thoroughly washing fruits and vegetables before eating; steam cleaning cooking surfaces and utensils after they have been in contact with raw meat, poultry or unwashed fruits or vegetables; pregnant women or immunocompromised individuals should avoid changing cat litter, but if done, gloves should be used and the hands should be washed thoroughly afterwards [68]. Children’s sandboxes should be covered to prevent cats from defecating in them and animal care technicians who clean cats should wear protective clothing and masks [36]. If all of these recommended measures are put in practice the risk of toxoplasmosis to human beings decreases dramatically. Finally, it must be remembered that preventing human exposure to cats is not the same as preventing exposure to Toxoplasma gondii oocysts [43]. Oocysts originating from neighbour’s cat can still be present in vegetables. References [1] FRENKEL (J.K.), DUBEY (J.P.), MILLER (N.L.) - Toxoplasma gondii in cats: fecal stages identified as coccidian oocysts. Science. 1970, 167:893-6. [2] HUTCHISON (W.M.), DUNACHIE (J.F.), SIIM (J.C.), WORK (K.) Life cycle of Toxoplasma gondii. Br Med J. 1969, 4:806. [3] PANTOJA RAMOS (A.), PÉREZ GARCÍA (L.) - Reseña histórica acerca de las investigaciones relacionadas con la toxoplasmosis. Rev Cubana Med Trp. 2001, 53 (2): 111-7. [4] DUBEY( J.P.), BEATTIE (C.P.) - Toxoplasmosis of Animals and Man. Boca Raton, Fla: C.R.C. Press; 1988. [5] DUBEY (J.P.) - Direct development of enteroepithelial stages of Toxoplasma gondii in the intestines of cats fed cysts. Am J Vet Res. 1979, 40:1634-7. [6] DUBEY (J.P.) - Toxoplasma gondii oocyst survival under defined temperatures. J Parasitol. 1998a, 84:862-5. [7] DUBEY (J.P.) - Advances in the life cycle of Toxoplasma gondii. Int J Parasitol, 1998b, 28:1019-1024. [8] DUBEY (J.P.) - Infectivity and pathogenicity of Toxoplasma gondii oocyst for cats. J Parasitol, 1996, 82:957-961. [9] LINDSAY (D.S.), DUBEY (J.P.), BUTLER (J.M.), BLAGBURN (B.L.) Mechanical transmission of Toxoplasma gondii oocysts by dogs. Vet Parasitol, 1997, 73:27-33. [10] PRESTRUD (K.W.), ÅSBAKK (K.), FUGLEI (E.), MØRK (T.), STIEN (A.), ROPSTAD (E.), TRYLAND (M.), GABRIELSEN (G.W.), LYDERSEN (C.), KOVACS (K.M.), LOONEN (M.J.J.E.), SAGERUP (K.), OKSANEN (A.) - Serosurvey for Toxoplasma gondii in arctic foxes and possible sources of infection in the high Arctic of Svalbard. Vet Parasitol, 2007, 150: 6-12. [11] DUBEY (J.P.), ROLLOR (E.A.), SMITH (K.), KWOK (O.C.H.), THULLIEZ (P.) - Low seroprevalence of Toxoplasma gondii in feral pigs from a remote island lacking cats. J Parasitol, 1997, 83:839841. [12] DUBEY (J.P.), LAPPIN (M.R.), THULLIEZ (P.) - Diagnosis of induced toxoplasmosis in neonatal cats. J Am Vet Med Assoc, 1995, 207:179-185. 253 EJCAP - Vol. 18 - Issue 3 December 2008 [31] LIN (D.S.), BOWMAN (D.D.), JACOBSON (R.H.) - Immunological changes in cats with concurrent Toxoplasma gondii and feline immunodeficiency virus infections. J Clin Microbiol, 1992, 30:1724. [32] DAVIDSON (M.G.), ROTTMAN (J.B.), ENGLISH (R.V.), LAPPIN (M.R.), TOMPKINS (M.B.) - Feline immunodeficiency virus predisposes cats to acute generalized toxoplasmosis. Am J Pathol, 1993, 143:1486-1497. [33] TOOMEY (J.M.), CARLISLE-NOWAK (M.M.), BARR (S.C.), LOPEZ (J.W.), FRENCH (T.W.), SCOTT (F.W.), et al. - Concurrent toxoplasmosis and feline infectious peritonitis in a cat. J Am Anim Hosp Assoc, 1995, 31:425-428. [34] LINDSAY (D.S.), BLAGBURN (L.B.), DUBEY (J.P.) - Feline Toxoplasmosis and the importance of the Toxoplasma gondii oocyst. Parasitology, 1997, 19:448-461. [35] BEATTY (J.), BARRS (V.) - Acute toxoplasmosis in two cats on cyclosporin therapy (letter). Aust Vet J, 2003, 81:339. [36] DUBEY (J.P.), LAPPIN (M.R.) - Toxoplasmosis and Neosporosis. In: Infectious diseases of the dog and cat. C. E. Greene (ed.) Saunders Elsevier. St. Louis, Misouri. 2006. p.754-767. [37] CHAVKIN (M.J.), LAPPIN (M.R.), POWELL (C.C.), COOPER (C.M.), MUNANA (K.R.), HOWARD (L.H.) - Toxoplasma gondii-specific antibodies in the aqueous humor of cats with toxoplasmosis. Am J Vet Res, 1994, 55:1244-1249. [38] LAPPIN (M.R.), GIGLIOTTI (A), CAYATTE (S.), GIGLIOTTI (A.), COOPER (C.), ROBERTS (S.M.) - Demonstration of Toxoplasma gondii-antigen containing immune complexes in the serum of cats. Am J Vet Res, 1993, 54:415-419. [39] DUBEY (J.P.), CARPENTER (J.L.) - Histologically confirmed clinical toxoplasmosis in cats: 100 cases (1952-1990). J Am Vet Med Assoc, 1993a, 203:1556-1566. [40] DUBEY (J.P.), CARPENTER (J.L.) - Neonatal toxoplasmosis in littermate cats. J Am Vet Med Assoc, 1993b, 203:1546-1549. [41] LAPPIN (M.R.) - Challenging cases in internal medicine: what’s your diagnosis? Vet Med, 1990, 84:448-455. [42] LAPPIN (M.R.), GEORGE (J.W.), PEDERSEN (N.C.), BARLOUGH (J.E.), MURPHY (C.J.), MORSE (L.S.) - Primary and secondary Toxoplasma gondii infection in normal and feline immunodeficiency virus-infected cats. J Parasitol, 1996, 82:733-742. [43] BOWMAN (D.D.), HENDRIX (C.M.), LINDSAY (D.S.), BARR (S.C.) (eds.) - Toxoplasma gondii (Nicolle and Manceaux, 1908). In: Feline Clinical Parasitology. Iowa State University Press, Iowa. 2002. p. 14-28. [44] DUBEY (J.P.), ZAJAC (A.), OSOFSKY (S.A.), TOBIAS (L.) - Acute primary toxoplasmic hepatitis in an adult cat shedding Toxoplasma gondii oocysts. J Am Vet Med Assoc,1990, 197:1616-1618. [45] DUBEY (J.P.), GAMBLE (H.R.), HILL (D.), SREEKUMAR (C.), ROMAND (S.), THULLIEZ (P.) - High prevalence of viable Toxoplasma gondii infection in market weight pigs from a farm in Massachusetts. J Parasitol, 2002, 88:1234-1238. [46] LAPPIN (M.R.) - Feline toxoplasmosis: interpretation of diagnostic test results. Semin Vet Med Surg (Small Anim.), 1996, 11:154160. [47] LAPPIN (M.R.), ROBERTS (S.M.), DAVIDSON (M.G.), POWELL (C.C.), REIF (J.S.) - Enzyme-linked immunosobernt assays for the detection of Toxoplasma gondii-specific antibodies and antigens in the aqueous humor of cats. J Am Vet Med Assoc, 1992, 201: 1010-1016. [48] MUÑANA (K.R.), LAPPIN (M.R.), POWELL (C.C.), et al. - Sequential measurement of Toxoplasma gondii-specific antibodies in the cerebrospinal fluid of cats with experimentaly induced toxoplasmosis. Prog Vet Neurol, 1995, 6: 27-31. [49] LAPPIN (M.R.), BURNEY (D.P.), DOW (S.W.), POTTER (T.A.) Polymerase chain reaction for the detection of Toxoplasma gondii in aqueous humor of cats. Am J Vet Res, 1996, 57:1589-1593. [50] BURNEY (D.P.), CHAVKIN (M.J.), DOW (S.W.), POTTER (T.A.), LAPPIN (M.R.) - Polymerase chain reaction for the detection of Toxoplasma gondii within aqueous humor of experimentallyinoculated cats. Vet Parasitol, 1998, 79:181-186. [51] BURNEY (D.P.), LAPPIN (M.R.), SPILKER (M.), MCREYNLOLDS (L.) - Detection of Toxoplasma gondii parasitemia in experimentally inoculated cats. J Parasitol, 1999, 85:947-951. [52] MONTOYA (A.) - La infección por Toxoplasma gondii en el gato: aspectos epidemiológicos, diagnóstico y caracterización de aislados autóctonos. Tesis doctoral. Universidad Complutense de Madrid. 2006. [53] DAVIDSON (M.G.) - Toxoplasmosis. Vet Clin North Am Small Anim Pract, 2000, 30: 1051-1062. [54] DAUGSCHIES (A.) - Prevention of excretion of Toxoplasma oocysts by medication of cats with toltrazuril. EMOP VII, (Parma, Italy) 1996. p.456. [55] SAITOH (Y.), ITAGAKI (H.) - Dung beetles, Onthophagus spp, as a potential transport hosts of feline coccidian. Nippon Juigaku Zasshi, 52 (abstract). 1990. [56] SROKA (J.), CHMIELEWSKA-BADORA (J.), DUTKIEWIEZ (J.) Ixodes ricinus as a potential vector of Toxoplasma gondii. Ann Agric Environ Med, 2003, 10:121-123. [57] FRENKEL (J.K.), PFEFFERKORN (E.K.), SMITH (D.D.), FISHBACK (J.L.) - Prospective vaccine prepared from a new mutant of Toxoplasma gondii for use in cats. Am J Vet Res, 1991, 52:759-763. [58] FREYRE (A.), CHOROMANSKI (L.), FISHBACK (J.L.), PROPIEL (I.) - Immunization of cats with tissue cysts, bradyzoites, and tachyzoites of the T-263 strain of Toxoplasma gondii. J Parasitol, 1993, 79:716-719. [59] ASTHANA (S.P.), MACPHERSON (C.N.), WEISS (S.H.), STEPHENS (R.), DENNY (T.N.), SHARMA (R.N.), et al. - Seroprevalence of Toxoplasma gondii in pregnant women and cats in Grenada, West Indies. J Parasitol, 2006, 92: 644–645. [60] PINON (J.M.), DUMON (H), CHEMLA (C.), FRANCK (J.), PETERSEN (E.), LEBECH (M.), et al. - Strategy for diagnosis of congenital toxoplasmosis: evaluation of methods comparing mothers and newborns and standard methods for postnatal detection of immunoglobulin G, M, and A antibodies. J Clin Microbiol, 2001, 39:2267-71. [61] TENTER (A.M.), HECKEROTH (A.R.), WEISS (L.M.) - Toxoplasma gondii: from animals to humans. Int J Parasitol, 2000, 30:12171258. [62] DUBEY (J.P.), JONES (J.L.) - Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol, 2008, Apr 11. [63] ROSS (D.S.), JONES (J.L.), LYNCH (M.F.) - Toxoplasmosis, Cytomegalovirus, Listeriosis, and Preconception Care. Matern Child Health J. 2006, 10 (Suppl 1): 189–193. [64] SACKTOR (N.) - The epidemiology of human immunodeficiency virus-associated neurological disease in the area of highly active antiretroviral therapy. J Neuroviral, 2002, 8: 115-121. [65] COLOMBO (F.A.), VIDAL (J.E.), PENALVA DE OLIVEIRA (A.C.), HERNANDEZ (A.V.), BONASSER-FILHO (F.), NOGUEIRA (R.S.), FOCACCIA (R.), PEREIRA-CHIOCCOLA (V.L.) - Diagnosis of Cerebral Toxoplasmosis in AIDS Patients in Brazil: Importance of Molecular and Immunological Methods Using Peripheral Blood Samples. J Clin Microbiol, 2005, 43: 5044–5047. [66] MANY (A.), KOREN (G.) - Toxoplasmosis during pregnancy. Can Fam Physician. 2006. 10; 52: 29–32. [67] FOULON (W.), NAESSENS (A.), HO-YEN (D.) - Prevention of congenital toxoplasmosis. J Perinat Med, 2000, 28:337-45. [68] LOPEZ (A.), DIETZ (V.J.), WILSON (M), NAVIN (T.R.), JONES (J.L.) - Preventing congenital toxoplasmosis. MMWR Recomm Rep, 2000, 49(RR-2): 59-68. 254 ZOONOSES Echinococcus multilocularis in veterinary practice in Europe E. R. Morgan(1) SUMMARY The tapeworm Echinococcus multilocularis is relevant to veterinary practice primarily because dogs are important definitive hosts, shedding eggs that pose a significant zoonotic threat. Dogs can also occasionally become infected as intermediate hosts and develop severe disease. The major role of the veterinary clinician is to ensure adequate deworming treatment in dogs in endemic areas, and to apply protocols aimed at reducing the risk of introducing the parasite into new areas through animal movement. In both cases, up to date knowledge of the apparently changing geographical range and epidemiology is crucial. Eggs are produced into the faeces, where they are immediately infective, and can survive for many months in the environment [1]. Normal summer and winter temperatures in Europe are not sufficient to render the eggs non-infective. After ingestion by the intermediate host, usually a rodent, the eggs hatch and migrate to the tissues. The liver is the most common site for further development, and cysts are formed, with a germinal epithelium surrounded by a carbohydrate-rich outer layer which apparently protects the parasite from the host immune response. Asexual reproduction produces more immature parasite stages, known as protoscoleces, into the fluid interior of the cyst [1]. Aggregations of cysts form, with ill-defined boundaries, and ingestion of an infected intermediate host leads to a variable number of adult parasites in the gut of the definitive host. This paper was commissioned by FECAVA for publication in EJCAP. Introduction Echinococcus multilocularis is an important zoonosis in several parts of the world. In Europe, veterinary practitioners will find themselves in one of three areas: those endemic for the parasite, those with recently recognised cases of alveolar echinococcosis (AE), and those currently free of the parasite, for which exclusion is a priority. The disease is therefore relevant for all, especially since its distribution and epidemiology appears to be undergoing a period of change. Alveolar echinococcosis (AE) Infection in the gut of the definitive host is benign, and dogs can tolerate burdens of many thousands of worms without apparent ill effect [1]. Cysts in the intermediate host, however, can be highly pathogenic. Inadvertent infection of humans leads to alveolar echinococcosis (AE). Unlike its sister species Echinococcus granulosus, cysts in humans are poorly circumscribed, and expand by local tissue infiltration and possibly distant metastasis. The liver is the most common site for the cysts, and by the time tissue destruction is sufficient to provoke clinical signs, complete surgical excision is invariably difficult or impossible [1]. Although advances in diagnosis and treatment have reduced case fatality from its previous rate of more than The parasite Canids, including dogs and foxes, are the usual definitive hosts. Adult worms are small (2-5mm in length, Fig. 1) and live in the small intestine, where they attach by embedding their anterior section in the mucosa. Following infection as the definitive host, dogs produce eggs after about a month, and continue to do so for up to 4 months [1]. There is some evidence for partial acquired immunity, but this is not sufficient to prevent future infection following re-exposure. The parasite’s biotic potential is similar in dogs and foxes, but infections in cats produce very few eggs and cats are therefore not of overall significance in maintaining parasite populations [2]. (1) Eric R. Morgan. School of Biological Sciences, University of Bristol, Woodland Road, Bristol, BS8 1UG, UK. Tel: +44 117 9287485. E-mail: eric.morgan@bristol.ac.uk 255 Echinococcus multilocularis in veterinary practice in Europe - E.R. Morgan by albendazole treatment (daily 10 mg / kg per os). Provided treatment is maintained, remission periods of up to 3.5 years have been recorded, with no instances of clinical recurrence in dogs undergoing chemotherapy after surgery [1]. Clinical cases in non-travelled dogs are an indication that the parasite is completing its life cycle in the area, and people are at risk. This should trigger surveillance and re-evaluation of local recommendations for treatment of dogs. Current distribution and epidemiology The approximate current distribution of E. multilocularis in Europe is shown in Fig. 2. There is some concern that the parasite is spreading, although sparse objective records especially in the East, improved diagnostic tests in recent years, and increased awareness leading to more diagnostic effort, make this difficult to prove. Cases of autochthonous AE and infection in foxes have been recorded in several new areas in the past few years. In some endemic areas, the parasite has colonised urban fox populations [4], placing increasing numbers of people at risk. There is evidence for real increases in parasite biomass within core endemic areas [5] as well as on the edge of the known range [6], which could be linked to increases in fox populations as a result of successful control of rabies [5]. Fig. 1 Adult Echinococcus granulosus, which is very similar in appearance to E. multilocularis. The eggs are typical of taeniids and morphologically indistinguishable from those of other species in the group. The eggs (insert) 30-40 microns in diameter, are typical. In the natural or sylvatic cycle, foxes act as the definitive host and a range of rodents as the intermediate hosts. Raccoon dogs are also competent definitive hosts and may be of epidemiological importance in northern areas [5]. The most important intermediate hosts are microtine voles rather than rats or mice, although other species including wood mice and musk rats can also be infected. The risk to humans comes from inadvertent ingestion of eggs from infected faeces, with subsequent cyst development. Contamination of fingers or food with eggs in areas frequented by foxes therefore poses a risk of infection, and people working outdoors and in contact with the soil are more likely to encounter the parasite. However, since human contact with dogs is much closer than with foxes, spillover of infection into domestic dog populations constitutes a great multiplication of risk. This would occur when dogs ingest rodents or rodent remains in an area in which foxes are present and the sylvatic cycle is operating. In this context, the increasing urbanisation of fox populations in many European cities is of justified concern [4]. Work in Switzerland suggests that the suburban fringes, where elevated fox numbers coincide with high densities of intermediate hosts and green areas frequented by people and their dogs, are the area of highest risk for transmission. Dogs can also act as a mechanical carrier of infective eggs, for example after rolling in fox faeces. 95%, the disease still carries a poor prognosis, and surgery is followed by life-long parasitostatic anthelmintic chemotherapy [3]. Although cases in humans are not common, the severity of disease and the ease with which sources of infection in companion animals can be eliminated places great responsibility on the practising veterinarian for protection of public health. In recent years a number of cases of AE have also been reported in which domestic animals act as intermediate hosts and develop cysts, usually in the liver. This has been observed in pigs, several species of primate, and dogs [1]. In at least one case a dog has acted as both definitive and intermediate host at the same time. This raises questions over whether autoinfection can occur, with rupture of cysts into the intestine or hatching of eggs before leaving the host. However, the most likely general source of infection is ingestion of eggs from the environment. AE in dogs follows a similar course to that in humans, although cysts develop more quickly [1]. Cranial abdominal mass is the main presenting sign, and may be accompanied by ascites, diarrhoea, vomiting, dyspnoea, exercise intolerance and weight loss. Clinical investigation may reveal signs of liver failure if the functional capacity of the organ is sufficiently reduced. Ultrasound examination is suggestive, with the main differential diagnosis being neoplasia, and exploratory laparotomy reveals a large polycystic mass in the cranial abdomen with liver involvement. Cytology on material collected by fine needle aspirate is unlikely to be helpful, but examination of this material by antigen-ELISA, if available, is diagnostic. Treatment options are euthanasia, or surgical resection of the mass. This is rarely completely achievable due to its locally invasive nature, and might involve removal of entire liver lobes, with inadvertent rupture of the cyst leading to major complications. Surgery is followed Diagnosis and treatment in the definitive host Eggs are easily recognised on salt flotation of faeces, but cannot be differentiated from those of other taeniid tapeworm species. Definitive diagnosis is by identification of the adult parasite using sedimentation at necropsy. In vivo, expulsion of tapeworms and enumeration of burdens, for epidemiological research purposes, can be achieved by arecoline purge. In practice, however, detection of parasite antigen by ELISA, or DNA in faeces by PCR, provide accurate diagnosis [1, 4]. 256 EJCAP - Vol. 18 - Issue 3 December 2008 parasite control through protection of infection-free status in non-endemic areas and reduction of infection pressure in the endemic zone. Praziquantel is the drug of choice for prophylactic treatment in the definitive host. However, it has a very short persistence time with a half-life of only a few hours, so repeated administration every month is needed to ensure that no egg excretion occurs. Since this provokes problems of owner compliance, a more selective approach has been suggested, focusing on dogs that have the closest contact with humans or the greatest opportunity to eat rodents [1]. Routine screening by faecal flotation would also identify infected dogs, although false positives would be caused by other taeniid infections, and false negatives by low test sensitivity. Screening also presents a zoonotic hazard to practice laboratory staff. Routine treatment of all dogs in at-risk areas is therefore the safest strategy. When recommending worming regimes to clients, veterinarians in endemic areas should be wary of combination products targeted to Toxocara canis and Dirofilaria immitis along with ectoparasites such as fleas, which do not necessarily have any effect against tapeworms. Diagnosis of patent infections in dogs should prompt the owners and their families to seek medical advice, and it is recommended that they are screened for specific antibodies [1]. The pathogenesis in humans is chronic and years can go by between infection and the appearance of clinical signs, with prognosis deteriorating as the cysts grow. Early diagnosis in people at high risk of infection is therefore beneficial. Fig. 2 Approximate geographical distribution of E. multilocularis in Europe. Dark shading indicates the core endemic area, in which infection in foxes and dogs is locally common, and light shading areas in which infection is at a lower level or autocthonous cases of AE have been recently reported. Data from multiple sources [5, 8, 11, 12 and others]. Larval stages have also been identified in humans in additional countries in the Balkans and Eastern Europe [11], but without surveys of definitive and natural intermediate hosts the endemic status of these countries cannot be confirmed. Regional distributions within countries are approximate, and records to the East of the marked range are poor. Dogs travelling from endemic to uninfected areas could carry the parasite, leading to establishment in new countries [7]. Consequently, cross-border transport of dogs in some cases carries a legal requirement for treatment. The protocol is a compromise between the gut transit time (ensuring that all eggs are voided before arrival in the destination country) and the chance of re-infection (which is in theory possible almost immediately after treatment). Currently, the favoured strategy is a single dose of praziquantel between 24 and 48 hours before entry, and there are so far no confirmed reports of introduction of E. multilocularis by dogs so treated. However, there is political pressure to weaken requirements for such treatment in the interests of European harmonisation and free movement, and given the severity of disease in humans the veterinary profession has a responsibility to ensure that changes to regulations for cross-border animal movement takes due account of the risks for disease transmission. Infection of dogs visiting an endemic area can further be reduced by avoiding opportunities for ingestion of rodents or carrion. Praziquantel is the most effective drug against adult parasites, and administration at a rate of 5.0 mg / kg body weight per os or 5.7 mg / kg body weight by intramuscular injection eliminates the entire worm burden in most cases. A second administration a day later, followed by copro-antigen or copro-PCR testing after 4-5 days, provides further security [1]. Prevention of infection in dogs The veterinary practitioner has an important role in the prevention of disease and the protection of public health. In endemic areas, this should focus on identifying dogs at risk of infection as definitive hosts and preventing egg shedding by chemoprophylactic treatment, and elimination of burdens in dogs travelling to non-endemic areas. Outside the endemic zone, veterinarians should provide appropriate advice to owners of travelling dogs concerning prevention of infection and correct application of preventative measures before returning, and should also be vigilant for cases of AE in animals that might indicate introduction of the parasite. The veterinary profession also has an important responsibility to provide public education concerning the risk of infection and the importance of responsible pet ownership, and to co-ordinate multi-national strategies for Prospects for control in endemic areas In most parts of its range, E. multilocularis is maintained in a sylvatic cycle, with foxes and voles as the major hosts, although there is evidence in Asia that dogs can take over as the main definitive host [1]. Whether or not dogs can maintain the parasite cycle in the absence of foxes, infection of dogs undoubtedly leads to increased risk of human disease. In hyper-endemic areas, the lifetime incidence of E. multilocularis infection in dogs could be as high as 80% [4]. Routine treatment of dogs with praziquantel at an appropriate dose and frequency (or, in future, 257 Echinococcus multilocularis in veterinary practice in Europe - E.R. Morgan References other proven drugs) and proper disposal of dog faeces should therefore form the cornerstone of control. [1] DEPLAZES (P.), ECKERT (J.) - Veterinary aspects of alveolar echinococcosis - a zoonosis of public health significance. Vet Parasitol, 2001, 98: 65-87. [2] KAPEL (C.M.O.), TORGERSON (P.R.), THOMPSON (R.C.A.), DEPLAZES (P.) - Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, racoon dogs and cats. Int J Parasitol, 2006, 36: 79-86. [3] CRAIG (P.) - Echinococcus multilocularis. Curr Opin Infect Dis, 2003, 16: 437-444. [4] DEPLAZES (P.), HEGGLIN (D.), GLOOR (S.), ROMIG (T.) - Wilderness in the city: the urbanisation of Echinococcus multilocularis. Trends Parasitol, 2004, 20: 77-84. [5] ROMIG (T.), DINKEL (A.), MACKENSTEDT (U.) - The present situation of echinococcosis in Europe. Parasitol Int., 2006, 55: 187-191. [6] TAKUMI (K.), DE VRIES (A.), CHU (M.L.), MULDER (J.), TEUNIS (P.), VAN DER GEESEN (J.) - Evidence for an increasing prevalence of Echinococcus multilocularis in foxes in The Netherlands. Int J Parasitol, 2008, 38: 571-578. [7] GOODFELLOW (M.), SHAW (S.), MORGAN (E.) - Imported disease of dogs and cats exotic to Ireland: Echinococcus multilocularis. Irish Vet J, 2006, 59: 14-216. [8] DEPLAZES, (P.) - Ecology and epidemiology of Echinococcus multilocularis in Europe. Parassitologia, 2006, 48: 37-39. [9] MC DONALD (R.A.), DELAHEY (R.J.), CARTER (S.P.), SMITH (G.C.), CHEESMAN (C.L.) - Perturbing implications of wildlife ecology for disease control. Trends Ecol Evol. 2008, 23: 53-56. [10] MORGAN (E.R.), MILNER-GULLAND (E.J.), TORGERSON (P.R.), MEDLEY (G.F.) - Ruminating on complexity: macroparasites of wildlife and livestock. Trends Ecol Evol, 2004, 19: 181-188. [11] KOLAROVA (L.) - Echinococcus multilocularis: new epidemiological insights in Central and Eastern Europe. Helminthologia, 1999, 36: 193-200. [12] JENKINS (D.J.), ROMIG (T.), THOMSON (R.C.A.) - Emergence / reemergence of Echinococcus spp. – a global update. Int J Parasitol, 2005, 35: 1205-1219. Control in foxes or in rodents has been considered [8]. Culling foxes is unlikely to be a realistic option, not only because of logistical and ethical problems, but also because the ensuing disruption could be counterproductive. Disturbance of stable family groups with defended territories is likely to lead to increased dispersal, especially by young foxes, which carry the highest worm burdens. This could lead both to geographical spread of infection and increased opportunity for contact with humans and domestic animals. Experiences with badger culling to control bovine tuberculosis in the UK should prompt caution when considering wildlife culling as a means of disease control, especially in territorial species [9]. In practice, a sustained decrease in fox populations might reduce the force of infection, but given the high reproductive potential of foxes is unlikely to be achievable by conventional means. A more successful strategy has employed treatment, with five- to ten-fold reductions in the prevalence of E. multilocularis in fox populations given praziquantel-laced baits over a 2 year period [4]. Treatment of urban and suburban foxes in this way could directly reduce zoonotic risk, as well as the chances of spillover infections in domestic dogs. However, such treatment should be strategic and take into account the population dynamics of foxes and the parasite, and the spatial and dynamic complexities of transmission [10]. Conclusions The severity of zoonotic disease caused by E. multilocularis compels the practising veterinary surgeon to take serious responsibility for control of infection in dogs. This can be achieved by routine anthelmintic treatment in endemic areas, and strategic treatments in dogs moving from endemic to uninfected countries. Veterinary efforts to control this parasite will benefit from awareness among practitioners of its life cycle, epidemiology and diagnosis, risk factors for human and canine infection, and the presentation and diagnosis of disease in animals acting as intermediate hosts. They should also be aware of the apparent changes in epidemiology in terms of urban fox populations and expansion of the known geographical distribution. 258 ZOONOSES Toxocarosis, an important zoonosis P.A.M. Overgaauw(1) F. van Knapen(2) SUMMARY This paper reviews the current knowledge about an important zoonotic dog and cat parasite, Toxocara. A good understanding of the epidemiology is required so that effective prevention of infection in man, dogs and cats can be possible. Education of the dog and cat owner will be significant in prevention. In this review, the epidemiology, clinical symptoms, diagnosis, prevention and control in the dog, the cat and the human will be discussed. Uniform guidelines for deworming dogs and cats will be given. Toxocara infected paratenic hosts by dogs or cats, larvae will be released and in most cases develop directly into adult worms in the intestinal tract. This may explain the higher Toxocara prevalence rate in adult cats, which catch prey animals more often than dogs. In the pregnant bitch, ‘dormant’ somatic larvae are reactivated and migrate in the bitch across the placenta to infect the fetuses. New-born puppies and kittens also acquire infection through ingestion of larvae in the milk [49, 59, 60]. This paper was commissioned by FECAVA for publication in EJCAP. Introduction Toxocarosis of dogs and cats Toxocara canis and Toxocara cati are roundworms of dogs and cats and the reported infection rates in Western Europe vary from 3.5% to 17% for T. canis in dogs and 8% to 76% for T. cati in cats [8, 15, 22, 35, 39, 41, 52]. The prevalence of patent Toxocara infections is highest in young dogs and cats and much less common in adult animals. Toxocara infection follows ingestion of embryonated Toxocara eggs or larvae in a paratenic host. Migration of larvae can lead to (overt) clinical disease (toxocarosis) in the (paratenic) host. Environment Toxocara eggs are unembryonated and not infectious when passed into the environment in the faeces of dogs and cats. Within a period of 3 weeks to several months, depending on soil type and climatic conditions such as temperature and humidity, eggs will develop to an infectious stage that can survive for at least one year under optimal circumstances. Studies from all over the world demonstrated high rates (10-30%) of soil contamination with Toxocara eggs in backyards, sandpits, parks, playgrounds, lake beaches, and other public places [37]. In a survey in the Netherlands, the presence of T. canis eggs in public parks was comparable with reports from other European cities, but most of the investigated sand-boxes were polluted with T. cati eggs [27]. Epidemiology Infection of the dog and cat Adult worms in the intestinal tract of infected dogs and cats shed large numbers of eggs into the environment (Fig. 2) via the faeces where they embryonate (Fig. 3) and maybe ingested by natural hosts as well as paratenic hosts. In the intestine the larvae hatch (Fig. 3) and migrate throughout the body via blood vessels. This is called visceral larva migrans or VLM (Fig. 4 and 5). In young animals larvae migrate from the lungs up the trachea and after swallowing, mature in the intestinal tract. In paratenic hosts and most adult dogs and cats that have some degree of acquired immunity, the larvae undergo somatic migration and remain as somatic larvae in the tissues. After predation of Infection routes Tracheal migration After young dogs ingest infective Toxocara eggs, larvae migrate through the liver, the vascular system and the lungs to the trachea. After swallowing, they complete their development in the stomach and small intestine. Eggs first appear in the faeces 4 to 5 weeks post-infection [49]. Depending on previous exposure to infection, the migratory pathway and deworming (1) Certified Veterinary Microbiologist, Certified Parasitologist, RnA Assays, Yalelaan 2, NL - 3584 CM, Utrecht. E-mail: P.overgaauw@rnassays.com (2) Diplomate of the European College of Veterinary Public Health, Certified Parasitologist, Institute for Risk Assessment Sciences (IRAS), Department of Veterinary Public Health, Veterinary Faculty, Utrecht University, Yalelaan 2, NL - 3584 CM Utrecht. E-mail: f.vanknapen@uu.nl * Presented by NACAM (The Netherlands) 259 Toxocarosis, an important zoonosis - P.A.M. Overgaauw, F. van Knapen Fig. 1 Toxocara egg 400x (Photo Felix Yaya). bitch during pregnancy. Within hours of birth, the larvae that were present in the liver of the neonate, migrate to the lungs and undergo a tracheal migration. Adult worms can be found at two weeks of age and large numbers of eggs may be passed in the faeces after a minimum period of 16 days [32]. Transmammary transmission After activation, somatic Toxocara larvae in dogs and cats will also be transmitted via the colostrum and the milk (transmammary transmission, lactogenic or milk-borne infection). Following ingestion by the offspring, the larvae undergo development without tracheal migration. Larvae are found to pass in the bitch’s milk for at least 38 days after parturition [72]. This route is less important than intra-uterine transmission in the puppy, but it is the primary mode of infection in the kitten. Kittens infected by lactogenic transmission will show faecal egg excretion after 7 weeks. [12], in pups of one to two months, the probability that newly hatched T. canis larvae will develop into adult ascarids may fall to a lower level, while the probability of somatic migration progressively increases. This is called age resistance and the mechanism operates partly within the lungs. Age resistance seems to be low in older cats. An explanation is not known [69]. Infection of the dam by the offspring Infection of the lactating bitch will occur mainly by ingestion of immature fourth-stage larvae from vomit or faeces from the puppies. Larvae develop to adults without a tracheal migration. Toxocara eggs shed in the faeces of puppies or kittens can be ingested by the mother, where they pass through the digestive tract, causing a false-positive diagnosis of Toxocara infection upon faecal examination. There is no development of intestinal infection with T. canis from somatic larvae at other times during gestation and the bitch is not at higher risk of Toxocara infection during metoestrus [44]. Although the prevalence of T. canis is highest in young dogs, a certain proportion of the adult canine population can also be infected [12, 35, 43, 53], mainly by ingestion of infective Toxocara eggs from contaminated soil. Somatic migration After ingestion of infective Toxocara eggs, larvae will migrate actively by penetration of the tissues and invasion of all parts of the body. Gradually somatic larvae accumulate in the tissues, persisting for long periods in a manner similar to that seen in paratenic hosts. Larvae of T. cati prefer to migrate to the muscles, while T. canis larvae were more commonly found in the central nervous system. Transplacental migration Nearly 100% of puppies are infected by somatic larvae in utero from day 42 of the gestation [33]. This so called transplacental migration or intra-uterine infection is the most important mode of transmission in dogs. In cats, prenatal infection via the placenta does not occur. The larvae in pregnant bitches are probably reactivated by the changing hormonal status of the Transmission through paratenic hosts Paratenesis is the mode of infection of some larval nematodes like Toxocara, ensuring its continuing survival by its distribution in prey species [21]. This route of infection exists because of the development of somatic larvae in paratenic hosts, including vertebrates such as rodents and birds or invertebrates such as earthworms and insects (e.g. flies). After ingestion of an infected paratenic host, the larvae develop directly in the intestine. Cats catch and eat more prey animals than dogs and this may be the explanation why higher infection rates are found. Fig. 2 Embryonating Toxocara egg 400x (Photo RVC). Fig. 3 Hatching Toxocara larvae 400x (Photo RVC). 260 EJCAP - Vol. 18 - Issue 3 December 2008 Fig. 4 Migrating Toxocara larvae in gut wall (Photo RVC). Fig. 5 Migrating Toxocara larvae in tissue (Photo RVC). Clinical symptoms and remain infective for years. Since no practical methods exist for reducing environmental egg burdens, prevention of initial contamination of the environment is most important. This can be achieved by taking measures such as eliminating patent infections in dogs and cats, preventing defecation by pets in public areas (Fig. 8), taking hygienic precautions and education of the public [19]. Household garden soil was found to be a potentially greater source of Toxocara infection than soil in public green areas [25]. A decrease in contamination can be achieved by methods including: restriction of uncontrolled dogs and cats, cleaning up faeces from soil and on pavements by dog owners, preventing access of dogs and cats to public places (especially children’s playgrounds) and by use of strategic anthelmintic treatment of dogs and cats with emphasis on worming puppies, kittens, nursing bitches and queens. The clinical symptoms depend on the age of the animal and on the number, location and stage of development of the worms. Toxocara infection is highest in puppies and kittens up to 6 months of age. After birth, puppies can suffer from pneumonia associated with the tracheal migration and die within 2 to 3 days. At an age of 2 to 3 weeks, puppies can show emaciation and digestive disturbances, caused by mature worms in the stomach and intestine (Fig. 6 and 7). Diarrhoea, constipation, vomiting, coughing and nasal discharge can be found at clinical examination. Distension of the abdomen (‘potbelly’) can occur, probably as result of gas formation caused by dysbacteriosis [41]. Anthelmintic treatment strategy The most serious and concentrated sources of infection are found in bitches nursing a litter and puppies aged between 3 weeks and 6 months. A major aim of long-term prophylactic treatment programmes is to suppress T. canis egg-output throughout the whole of puppyhood using a multidose schedule. Puppies should be treated with appropriate anthelmintics at the age of 2 weeks and because milk transmission occurs continuously for at least 5 weeks post partum, repeated treatments are necessary [3]. Larvae that reach the intestine need at least 2 weeks to mature and start passing eggs, therefore the treatment should be repeated every 14 days. The treatment schedule therefore requires deworming at 4, 6 and 8 weeks of age and then monthly until 6 months of age. Because prenatal infection does not occur in kittens, fortnightly treatment can begin at 3 weeks of age. Nursing bitches and queens should be treated concurrently with their offspring since they may develop patent infections along with their young. Kittens are older when worms are maturing (adult from day 28 and egg producing from day 49 after birth) and tracheal migration with related symptoms does not occur. Therefore, kittens have a better chance to grow and in the meantime develop better bodily condition before problems may be seen. For this reason clinical symptoms similar to those in puppies are usually inapparent. Diagnosis Patent Toxocara infection in dogs and cats can be tentatively diagnosed from the medical history, particularly the use or otherwise of an appropriate anthelmintic schedule, and the clinical symptoms. Confirmation of the diagnosis can be obtained by finding dark brown coloured eggs with thick pitted shells in faecal samples (Fig. 1). The direct faecal smear technique is not a sensitive test and generally should never be used for recovering eggs from faecal samples. Examination of faeces by a floatation technique is a useful method for detecting helminths [7]. Control in older dogs and cats can be achieved by periodic treatments of dogs and cats with anthelmintics, or by treatments prescribed based on the results of periodic diagnostic faecal examinations. Annual or twice annual treatments have been shown not to have a significant impact on preventing patent infection within a population, so a treatment frequency of at least 4 times Control measures There are two reasons for Toxocara control: to prevent human infection and to reduce the risk of infection to pets. Toxocara eggs are very resistant to adverse environmental conditions 261 Toxocarosis, an important zoonosis - P.A.M. Overgaauw, F. van Knapen owners should routinely collect and safely dispose of their pets’ faeces, especially from children’s play areas and finally advice for children not to play in potentially contaminated environments. Although veterinarians should be the most appropriate sources of information for their clients regarding the dangers and the control of toxocarosis, surveys have demonstrated that client education on this issue is lacking [23, 42]. per year is proposed as a general recommendation. Anthelmintics at the recommended doses are not highly effective against inhibited somatic larvae [11] and treatment of bitches before mating and two weeks before the anticipated whelping date has no useful effect on prenatal transmission [10, 14]. Therefore it is generally not advised to deworm pregnant dogs and cats. In the past, uniform guidelines for the control and treatment of parasites in pet animals were developed and published by CAPC in the US (www.capcvet.org) and recently by ESCCAP in Europe (www.esccap.org). The guidelines give overviews of worms, their significance and suggest rational control measures for the most important species in order to prevent animal and/ or human infection. Human toxocarosis Infection routes Toxocarosis is a public health problem. Man acts as an unnatural host in which Toxocara larvae will not develop but will migrate and survive for a long time. The mode of transmission to humans is by oral ingestion of infective Toxocara eggs from contaminated soil (sapro-zoonosis), from unwashed hands or consumption of raw vegetables [19]. Some infections may occur from ingestion of larvae in under-cooked organ and muscle tissue of infected paratenic hosts such as chickens, cattle and sheep [2, 38, 55, 61, 62]. Hygiene For dogs and cats, hygiene can be achieved by removing the faeces and by thorough cleaning of kennels. A 20% solution of commercial bleach can be used. This does not kill the eggs, but removes their sticky outer protein coat. These decorticated eggs are then easier to remove from inaccessible areas. Any worms vomited or passed in faeces should be destroyed and predation and scavenging on carcasses by dogs and cats should be prevented. Recent studies indicate the fur of as an important source of Toxocara eggs for infection after direct contact [1, 54, 71]. Direct contact with dogs and cats that harbour a patent Toxocara infection is usually not considered a risk, since the eggs need to mature several weeks before they are infective [41, 45, 46]. Moreover, Toxocara eggs are very sticky and therefore difficult to remove from the coat of a dog or cat and this makes ingestion of a sufficient number of eggs unlikely. A low percentage of these eggs were embryonated in the studies and even in the worst case scenario of highly contaminated fur, it is necessary to ingest several grams of heavily contaminated hair to get infected [48]. Education Dog and cat owners can help to avoid contamination of the environment with Toxocara eggs and the exposure of other persons to unnecessary risks of Toxocara infections. Proper information about this zoonosis and the social concept of responsible pet ownership is required. Pet owners should be advised about deworming schemes, effective anthelmintics and to prevent their animals from defecating on children’s playgrounds [57]. Information disseminated to pet owners should include a description of T. canis and T. cati and how they affect their hosts; clearing of prenatal and transmammary transmission; how Toxocara can be transmitted to and produce damage in humans; how prevention can most effectively be achieved; how pet The importance of Toxocara cati in human toxocarosis The role of T. cati as a zoonotic parasite is not always clearly recognised. Despite the fact that differentiation between T. canis and T. cati infections is not performed in surveys, the majority of reported human cases of toxocarosis in the past have been associated with T. canis and not with T. cati [13]. The greater Fig. 6 Patent Toxocara infection resulting in death of 6 months old Border Collie (Photo Paul Overgaauw). Fig. 7 T. canis adult worms in intestine (Photo RVC). 262 EJCAP - Vol. 18 - Issue 3 December 2008 Fig. 8 Defecating cat in sandpit (Photo Frans van Knapen). Fig. 9 Granulomatous retinal lesion in human eye due to ocular larva migrans (Photo RVC). abundance of T. canis eggs in park soil and playgrounds might be considered the reason for this, but sand-boxes and garden soil (Fig. 8) are more often polluted with T. cati eggs [27]. The large number of common antigenic fractions shared between T. canis and T. cati and the similarity in the mode of infection are indications that there is no difference in zoonotic risk. Furthermore, in Islamic countries, dogs are avoided for religious reasons, while cats are favoured pets. The seroprevalence of human toxocarosis in these countries is considerable as well [58]. The role of T. cati in human toxocarosis should therefore not be underestimated [40, 58]. serum IgE concentration, the presence of allergen-specific IgE and eosinophilia is established. The occurrence of asthma or recurrent bronchitis and hospitalization due to asthma were significantly related to seroprevalence, while eczema tended to be more frequent. It was concluded that allergic phenomena in children who are predisposed to asthma, are more frequently manifested after Toxocara infection [4]. Toxocara is not a causative agent but contributes to the development of atopic diseases and the allergic manifestation of asthma [51]. General clinical symptoms often include malaise, fever, abdominal complaints (vague upper abdominal discomfort attributed to hepatomegaly), wheezing or coughing [17]. Toxocara infection should be considered in the differential diagnosis of any child with a persistent and unexplained eosinophilia or recurrent abdominal pain. Chronic ‘idiopathic’ urticaria, chronic pruritus, and miscellaneous eczema in adults and children are found strongly associated with toxocarosis [16, 50]. Infection risk to children Children are more frequently infected than adults and VLM with more severe clinical symptoms is mainly found in children of 1 to 3 years of age. This can be explained because young children play and have closer contact with potentially contaminated soil in yards and sand-pits. In addition, children may often put their fingers into their mouth and sometimes eat dirt. Severe clinical symptoms are reported including life-threatening pneumonia after massive infection [28], eosinophilic meningoencephalitis in children [36], and thrombosis of the aorta [67]. Clinical symptoms Visceral larva migrans After ingestion of infective Toxocara eggs by a human, Toxocara larvae hatch in the stomach and migrate into the mucosa of the upper small intestine and disperse throughout the body via blood and lymphatic vessels. A more marked, inflammatory, immune response is called visceral larva migrans syndrome or VLM. This multisystem invasion can be associated with varied, non-specific clinical symptoms as a result of the host’s immune response. Ocular larva migrans Migrating Toxocara larvae can induce granulomatous retinal lesions, which are characterised by complaints of loss of visual acuity, squint and ‘seeing lights’ (Fig. 9). This is called Ocular Larva Migrans syndrome (OLM) [6, 63]. In a minority of cases, total blindness of one or both eyes can result. The mean age of patients with OLM is 8 years, but it is diagnosed in adults as well [31, 56]. Ocular larva migrans is usually caused by no more than a single larva. VLM is mainly diagnosed in children between 1 to 7 years of age (mean age 2 years) and is characterised by persistent eosinophilia, leukocytosis, an elevated GT γ level and hypergamma-globulinaemia [56]. Covert toxocarosis A third clinical syndrome, called ‘covert toxocariasis’ (CT), was found in patients with a vague complex of non-specific clinical symptoms, which do not fall within the categories of VLM or OLM. Symptoms such as hepatomegaly, cough, sleep disturbances, abdominal pain, headaches and behavioural changes have been associated with raised Toxocara antibodies Eosinophilia is seen more often in children than in adults [68] and a relationship between Toxocara seroprevalence and the incidence of chronic airway disorder (asthma), elevation of 263 Toxocarosis, an important zoonosis - P.A.M. Overgaauw, F. van Knapen [64]. The diagnosis ‘Idiopathic Abdominal Pain of Childhood’ is usually made in children. To increase the awareness of potential zoonotic hazards, particularly amongst pet owners, veterinary practitioners, general practitioners and public health agencies should provide sufficient information and advice for appropriate measures to be taken to minimize the risk of infection. Several reports, however, have indicated a significant lack of knowledge within the professions [23, 42, 47]. All authors concluded that continuing education with emphasis on the zoonotic risks is still strongly recommended. Cerebral toxocarosis There are some indications that larval involvement in the human brain may have subtle public health implications, such as changes to cognitive function in children [26]. A large study to determine OLM in 120,000 Irish schoolchildren, ranging in age from 3 to 19 years, revealed a strong association between having had a convulsion and ocular toxocarosis [20]. Recommendations include the following: be careful when in contact with young dogs and cats; wash hands before eating and after contact with animals; deworm dogs and cats regularly especially puppies and kittens; prevent children from eating earth and from playing on areas soiled with animal faeces; remove pet faeces; keep children’s nails clipped. Diagnosis Direct diagnosis of Toxocara infection is not easy because patients do not excrete parasite material such as eggs or larvae. Serodiagnostic techniques (ELISA) are the most reliable tools to detect antibodies and circulating antigens [58]. Anti-Toxocara antibodies measured by ELISA were found to persist for up to 2.8 years in infected adults and their presence alone does not distinguish between current and past infections. It should therefore be accompanied by other laboratory tests for a blood eosinophil count and total serum IgE [34]. Seroprevalence for Toxocara in some reports varied between 4.6 to 7.3% in children in the USA [24], 2.5% in Germany to 83% for children in the Caribbean [65]. In the Netherlands the prevalence was found to be 19% on average: between 4% and 15% in people younger than 30 years and 30% in adults older than 45 years [5]. Regularly re-infection of adults is probably the cause of the higher prevalence. Titres fall gradually over a period of about three years but should be considered as a balance between the fading memory of the immune system and its stimulation by continuing ingestion of viable ova or reactivation of dormant larvae. A review of cases of toxocarosis (VLM and OLM) from all over the world revealed that more than half of the patients were less than three years old, one fifth were adults and 60% were males [9]. For OLM, serum antibodies are not diagnostic but the presence of intraocular antibodies appears more promising as a diagnostic aid [63]. Treatment of patients Patients with severe Toxocara infections can be treated with systemic acting and larvicidal anthelmintics [34]. Clinicians, however, should balance the risk of therapy with the severity of the disease, because treatment can lead to severe hypersensitivity reactions caused by dying larvae. Especially in OLM cases the anthelmintic dose should be increased gradually over a period of days and accompanied by the concomitant administration of steroids [34]. References [1] AYDENIZÖZ-ÖZKAYHAN (M.), YAĞCI (B.B.), ERAT (S). The investigation of Toxocara canis eggs in coats of different dog breeds as a potential transmission route in human toxocarosis. Vet Parasitol, 2008, 152: 94-100. [2] BAIXENCH (M.T.), MAGNAVAL (J.F.), DORCHIES (PH.) Epidemiology of toxocariasis in students of the National Veterinary School in Toulouse. Rev Méd Vét, 1992, 143: 749-52. [3] BARRIGA (O.O.) - Rational control of canine toxocariasis by the veterinary practitioner, J Am Vet Med Ass, 1991, 198, 216-221. [4] BUIJS (J.), BORSBOOM (G.), RENTING (M.), HILGERSOM (W.J.H.), VAN WIERINGEN (J.C.), JANSEN (G.), NEIJENS (J.) - Relationship between allergic manifestations and Toxocara seropositivity: a cross-sectional study among elementary school children. Eur Respir J, 1997, 10, 1467-1475. [5] DE MELKER (H.E.), VAN DER PEET (T.E.), BERBERS (G.A.M.), VAN DE AKKER (R.), VAN KNAPEN (F.), SCHELLEKENS (J.F.P.), CONEYNVAN SPAENDONCK (M.A.E.) - Pilot-study for the PIENTER-Project. Report nr. 213675004, National Institute of Public Health and the Environment, Bilthoven, the Netherlands. 1995, 37-38. [6] DEUTER (C.M.E.), GARWEG (J.G.), PLEYER (U.), SCHÖNHERR (U.), THURAU (S.) – Ocular toxoplasmosis and toxocariasis in childhood. Klin Monatsbl Augenheilkd, 2007, 224: 483-487. [7] DRYDEN (M.W.), PAYNE (P.A.), RIDLEY (R.), SMITH (V.) Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther, 2005, 6: 15-28 . [8] DUBNÁ (S.), LANGROVÁ (I.), NÁPRAVNÍK (J.), JANKOVSKÁ (I.), VADLEJCH (J.), PEKÁR (S.), FECHTNER (J.) - The prevalence of intestinal parasites in dogs from Prague, rural areas, and shelters of the Czech Republic. Vet Parasitol, 2007, 145: 120-128. [9] EHRHARD (T.), KERNBAUM (S.) - Toxocara canis et toxocarose humaine, Bulletin de l’Institut Pasteur, 1979, 77: 225-287. [10] EPE (C.), SCHNIEDER (T.), STOYE (M.) - Möglichkeiten und Grenzen der chemotherapeutischen Bekämpfung vertikaler Infektionen On CT or MR imaging, hepatic lesions may be seen as multiple, ill-defined, oval lesions that measure 1.0-1.5 cm in diameter. On sonography, the lesions appear as multiple, small, oval hypoechoic lesions in the liver parenchyma [30]. Magnetic resonance imaging (MRI) can be used in patients with neurological syndromes to detect granulomas located cortically or subcortically [34]. Control measures Preventive measures A zoonotic disease like toxocarosis can be prevented for the most part. Control is important from the point of view of welfare, for the quality of human life and also for the economic costs to society [66]. Prevention of toxocarosis is possible by the institution of certain measures: appropriate health care for pets including regular anthelmintic treatments; reducing the number of uncontrolled and stray pets; preventing contamination of the environment with faeces; and promoting responsible pet ownership [40, 57, 70]. 264 EJCAP - Vol. 18 - Issue 3 December 2008 [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] Tijdschr Diergeneesk, 2004, 129: 40-44. [30] LIM (J.H.). Toxocariasis of the liver: visceral larva migrans. Abdom Imaging 2008; 33(2): 151-6. [31] Ljungström (I.)., Van Knapen (F.) - An epidemiological and serological study of Toxocara infection in Sweden, Scand J Infect Dis, 1989, 21, 87-93. [32] LLOYD (S.) - Toxocara canis: the dog. In: Toxocara and Toxocariasis, clinical, epidemiological and molecular perspectives. Lewis (J.W.), and Maizels (R.M.) British Society for Parasitology and Institute of Biology, 1993: 11-24. [33] LLOYD (S.), AMERSINGHE (P.H.), SOULSBY (E.J.L.) - Periparturient immunosuppression in the bitch and its influence on infection with Toxocara canis. J Small Anim Pract, 1983, 24, 237-247. [34] MAGNAVAL (J.F.), GLICKMAN (L.T.) – Management and treatment options for human toxocariasis. In: Toxocara, the enigmatic parasite. Holland (C.V), Smith (H.V.) Eds. CABI Publishing, 2006: 113-126. [35] MARTÍNEZ-CARRASCO (C.), BERRIATUA (E), GARIJO (M.), MARTÍNEZ (J), ALONSO (F.D.), DE YBÁÑEZ (R.R.) - Epidemiological study of non-systemic parasitism in dogs in southeast Mediterranean Spain assessed by coprological and post-mortem examination. Zoonoses Public Health, 2007, 54: 195-203 [36] MIMOSO (M.G.), PEREIRA (M.C.), ESTEVÃO (M.H.), BARROSO (A.A.), MOTA (H.C.) – Eosinophilic meningoencephalitis due to Toxocara canis, Eur J Pediatr, 1993, 152: 783-784. [37] MIZGAJSKA-WIKTOR (H.), UGA (S.) – Exposure and environmental contamination. In: Toxocara, the enigmatic parasite. Holland (C.V), Smith (H.V.) Eds. CABI Publishing, 2006: 211-227. [38] NAGAKURA (K.), TACHIBANA (H.), KANEDA (Y.), KATO (Y.) Toxocariasis possibly caused by ingesting raw chicken, J Inf Dis, 1989, 160: 735-736. [39] LE NOBEL (W.E.), ROBBEN (S.R.M.), DÖPFER (D.), HENDRIKX (W.M.L), BOERSEMA (J.H.), FRANSEN (F.), EYSKER (M.E.) Infections with endoparasites in dogs in Dutch animal shelters. Tijdschr Diergeneesk, 2004, 129: 40-4 [40] OVERGAAUW (P.A.M.) - Aspects of Toxocara epidemiology: toxocarosis in the human. Crit Rev Microbiol, 1997a, 23: 215231. [41] OVERGAAUW (P.A.M.) - Aspects of Toxocara epidemiology: toxocarosis in dogs and cats. Crit Rev Microbiol, 1997b, 23: 233252. [42] OVERGAAUW (P.A.M.) - Effect of a government educational campaign in the Netherlands on awareness of Toxocara and toxocarosis. Prev Vet Med, 1996, 28: 165-174. [43] OVERGAAUW (P.A.M.) - Prevalence of intestinal nematodes of dogs and cats in the Netherlands. Vet. Quart, 1997c, 19, 14-17. [44] OVERGAAUW (P.A.M.), OKKENS (A.C.), BEVERS (M.M.), KORTBEEK (L.M.) - Incidence of patent Toxocara canis infection in bitches during the oestrous cycle. Vet Quart, 1998, 20:104-107 [45] OVERGAAUW (P.A.M.), VAN KNAPEN (F.) - Dogs and nematode zoonoses. In: Dogs, zoonoses and public health. MacPherson CNL, Meslin FX, Wandeler AI. Eds. CABI Publishing Oxon, New York. 2000: 213-45. [46] OVERGAAUW (P.A.M.), VAN KNAPEN (F.) - Negligible risk of visceral or ocular larva migrans from petting a dog. Ned Tijdschr Geneeskd 2004, 148: 1600-1603. [47] OVERGAAUW (P.A.M.), VAN KNAPEN (F.) - No effect of educational campaign among general practitioners concerning Toxocara infections in humans. Ned Tijdschr Geneeskd, 1996, 140, 2282-2285. [48] OVERGAAUW (P.A.M.), VAN ZUTPHEN (L), HOEK (D), YAYA (F.O.), ROELFSEMA (J), PINELLI (E.), VAN KNAPEN (F), KORTBEEK (LM) - Zoonotic parasites in faecal samples and fur from dogs and cats in The Netherlands. 2008, submitted. [49] PARSONS (J.C.) - Ascarid infections of cats and dogs. Vet Clin N Am, 1987, 17, 1307-1303. mit Toxocara canis und Ancylostoma caninum beim Hund, Prakt Tierarzt, 1996: 483-490. EPE (C.) – Current and future options for the prevention and treatment of canids. In: Toxocara, the enigmatic parasite. Holland (C.V), Smith (H.V.) Eds. CABI Publishing, 2006: 239-252. FAHRION (A.S.), STAEBLER (S.), DEPLAZES (P.) – Patent Toxocara canis infections in previously exposed and in helminth-free dogs after infection with low numbers of embryonated eggs. Vet Parasitol, 2008, 152: 108-115. FISHER (M.) - Toxocara cati: an underestimated zoonotic agent. Trends Parasitol, 2003, 19: 167-170 FISHER (M.A), JACOBS (D.E.), HUTCHINSON (M.J.), DICK (I.G.C.) - Studies on the control of Toxocara canis in breeding kennels. Vet Parasitol, 1994, 55: 87-92. FOK (E.), SZATMÁRI (V.), BUSÁK (K.), ROZGONYI (F.) - Prevalence of intestinal parasites in dogs in some urban and rural areas of Hungary. Vet Quart, 2001, 23: 96-98. GAVIGNET (B.), PIARROUX (R.), AUBIN (F.), MILLON (L.), HUMBERT (P.) - Cutaneous manifestations of human toxocariasis. J Am Acad Dermatol, 2008, Sep 13. GILLESPIE (S.H.) - The clinical spectrum of human toxocariasis. In: Toxocara and Toxocariasis, clinical, epidemiological and molecular perspectives. Lewis J. W., and Maizels R. M., British Society for Parasitology and Institute of Biology. 1993, 55-61. GLICKMAN (L.T.) - The epidemiology of human toxocariasis. In: Toxocara and Toxocariasis, clinical, epidemiological and molecular perspectives. Lewis J. W., and Maizels R. M., British Society for Parasitology and Institute of Biology. 1993: 3–10. GLICKMAN (L.T.), SHOFER (F.S.) - Zoonotic visceral and ocular larva migrans. Vet Clin N Am, 1987, 17: 39-53. GOOD (B.), HOLLAND (C.V.), TAYLOR (M.R.H.), LARRAGY (J.), MORIARTY (P.), O’REGAN (M.) – Ocular toxocariasis in schoolchildren. Clin Inf Dis, 2004, 39: 173-178. GRIEVE (R.B.), STEWART (V.A.), PARSONS (J.C.) - Immunobiology of larval toxocariasis (Toxocara canis): a summary of recent research, In: Toxocara and Toxocariasis, clinical, epidemiological and molecular perspectives. Lewis (J.W.) and Maizels (R.M.) British Society for Parasitology and Institute of Biology, 1993: 117-124. HABLUETZEL (A.), TRALDI (G.), RUGGIERI (S.), ATTILI (A.R.), SCUPPA (P.), MARCHETTI (R.), MENGHINI (G.), ESPOSITO (F.) - An estimation of Toxocara canis prevalence in dogs, environmental egg contamination and risk of human infection in the Marche region of Italy. Vet Parasitol, 2003, 113: 243-252. HARVEY (J.B.), ROBERTS (J.M.), SCHANTZ (P.M.) - Survey of veterinarians recommendations for treatment and control of intestinal parasites in dogs: Public health implications. J Am Vet Med Assoc, 1991, 199: 702-707. HERMANN (N.), GLICKMAN (L.T.), SCHANTZ (P.M.) Seroprevalence of zoonotic toxocariasis in the United States: 1971-1973. Am J Epidemiol, 1985, 122: 890-896. HOLLAND (C.), O’CONNOR (P.), TAYLOR (M.R.H.), HUGHES (G.), GIRDWOOD (R.W.), SMITH (H.) - Families, parks, gardens and toxocariasis, Scand J Infect Dis, 1991, 23: 225-231. HOLLAND (C.V.), HAMILTON (C.) – The significance of cerebral toxocariasis. In: Toxocara, the enigmatic parasite. Holland (C.V), Smith (H.V.) Eds. CABI Publishing, 2006: 58-74. JANSEN (J.), VAN KNAPEN (F.) - Toxocara eggs in public parks and sand-boxes in Utrecht, Tijdschr Diergeneeskd, 1993, 118: 611614. KORTBEEK (L.M.), VELDKAMP (K.E.), BARTELINK (A.K.), MEULENBELT (J.), VAN KNAPEN (F.) – Severe pneumonia due to infection with Toxocara, Ned Tijdschr Geneeskd, 1994, 138: 2581-2584. LE NOBEL (W.E.); ROBBEN (S.R.M.), DÖPFER (D.), HENDRIKX (W.M.L.), BOERSEMA (J.H.), FRANSEN (F.), EYSKER (M.E) Infections with endoparasites in dogs in Dutch animal shelters. 265 Toxocarosis, an important zoonosis - P.A.M. Overgaauw, F. van Knapen [50] PIARROUX (R.), GAVIGNET (B.), HIERSO (S.), HUMBERT (PH.) – Toxocariasis and the skin. In: Toxocara, the enigmatic parasite. Holland (C.V), Smith (H.V.) Eds. CABI Publishing, 2006: 145-157. [51] PINELLI (E.), DORMANS (J.), VAN DIE (I.) – Toxocara and asthma. In: Toxocara, the enigmatic parasite. Holland (C.V), Smith (H.V.) Eds. CABI Publishing, 2006: 42-58. [52] ROBBEN (S.R.M.), LE NOBEL (W.E.), DÖPFER (D.), HENDRIKX (W.M.L.), BOERSEMA (J.H.), FRANSEN (F.), EYSKER (M.E.) Infections with helminths and-or protozoa in cats in animal shelters in the Netherlands. Tijdschr Diergeneesk 2004, 129: 2-6. [53] RODDIE (G.), STAFFORD (P.), HOLLAND (C.), WOLFE (A.) Contamination of dog hair with eggs of Toxocara canis. Vet Parasitol, 2007, 152: 85-93. [54] RODDIE (G.), STAFFORD (P.), HOLLAND (C.), WOLFE (A.) Contamination of dog hair with eggs of Toxocara canis. Vet Parasitol, 2007, 152: 85-93. [55] SALEM (G.), SCHANTZ (P.) - Toxocaral visceral larva migrans after ingestion of raw lamb liver. Clin Infect Dis, 1992, 15: 743-744. [56] SCHANTZ (P.M.) - Toxocara larva migrans now. Am J Trop Med Hyg, 1989, 41: 21-34. [57] SCHANTZ (P.M.) – Toxocariasis: the veterinarian’s role in prevention of zoonotic transmission. In: Toxocara, the enigmatic parasite. Holland (C.V), Smith (H.V.) Eds. CABI Publishing, 2006: 253-259. [58] SMITH (H.), NOORDIN (R.) – Diagnostic limitations and future trends in the serodiagnosis of human toxocariasis. In: Toxocara, the enigmatic parasite. Holland (C.V), Smith (H.V.) Eds. CABI Publishing, 2006: 89-112. [59] SPRENT (J.F.A.) - Observations on the development of Toxocara canis (Werner. 1782) in the dog. Parasitology, 1958, 48, 184209. [60] SPRENT (J.F.A.) - The life history and development of Toxocara cati (Schrank 1788) in the domestic cat. Parasitol, 1956, 46, 54-79. [61] STÜRCHLER (D.), WEISS (N.), GASSNER (M.) - Transmission of Toxocariasis. J Infect Dis, 1990, 162: 571. [62] TAIRA (K.), SAEED (I.), PERMIN (A.), KAPEL (C.M.O.) – Zoonotic risk of Toxocara canis infection throuigh consumption of pig or poultry viscera. Vet Parasitol, 2004, 121: 115-124. [63] TAYLOR (M.R.H.) – Ocular toxocariasis. In: Toxocara, the enigmatic parasite. Holland (C.V), Smith (H.V.) Eds. CABI Publishing, 2006: 127-144. [64] TAYLOR (M.R.H.), KEANE (C.T.), O’CONNOR (P.), GIRDWOOD (R.W.A.), SMITH (H.) - Clinical features of covert toxocariasis. Scand J Infect Dis, 1987, 19: 693-696. [65] THOMSON (D.E.), BUNDY (D.A.), COOPER (E.S.), SCHANTZ (P.M.) - Epidemiological characteristics of Toxocara canis zoonotic infection of children in a Caribbean community, Bull WHO, 1986, 64: 283-290. [66] TORGERSON (P.R.), BUDKE (C.M.) – Economic impact of Toxocara spp. In: Toxocara, the enigmatic parasite. Holland (C.V), Smith (H.V.) Eds. CABI Publishing, 2006: 281-293. [67] TRABOULSI (R.), BOUEIZ (A.), KANJ (S.S.) - Catastrophic aortic thrombosis due to Toxocara infection. Scand. J Infect Dis, 2007, 39: 283-285. [68] VAN KNAPEN (F.), BUIJS (J.) - Diagnosis of Toxocara infection. In: Toxocara and Toxocariasis, clinical, epidemiological and molecular perspectives. Lewis J. W., and Maizels R. M., British Society for Parasitology and Institute of Biology. 1993, 49-53. [69] VISCO (R.J.), CORWIN (R.M.), SELBY (L.A.) - Effect of age and sex on the prevalence of intestinal parasitism in cats. J Am Vet Med Ass, 1978, 172, 797-800. [70] WESTGARTH (C.), PINCHBECK (G.L.), BRADSHAW (J.W.S.), DAWSON (S.), GASKELL (R.M.), CHRISTLEY (R.M.) - Dog-human and dog-dog interactions of 260 dog-owning households in a community in Cheshire. Vet Rec, 2008, 162: 436-442. [71] WOLFE (A.), WRIGHT (I.P.). Human toxocarosis and direct contact with dogs. Vet Rec, 2003, 152: 419-22. [72] ZIMMERMAN (V.)., LÖWENSTEIN (M.D.), STOYE (M.) Untersuchungen über die Wanderung und Streuung der Larven von Toxocara canis WERNER 1782 (Anisakidae) im definitiven Wirt (Beagle) nach Erst- und Reinfection. Z Vet Med B, 1985, 32, 1-28. 266 ZOONOSES Update on avian chlamydiosis and its public health significance D. Vanrompay (1) SUMMARY The present review gives updated information on Chlamydophila psittaci strain classification, epidemiology, transmission, clinical disease, new diagnostic tests, public health significance, present legislation on psittacosis and recommendations for prevention and control of the disease. At present, analysis of the MOMP gene encoding the outer membrane protein A (ompA) is more often used to characterize avian Cp. psittaci strains as molecular techniques can differentiate Cp. psittaci strains more precisely and serovar-specific monoclonal antibodies are not commercially available restricting their use by veterinary clinical laboratories. Analysis of the ompA gene by sequencing, genotyping real-time PCR [10] or genotyping micro array [33] allows for the detection of genotypes A to F as well as the recently discovered genotype E/B [11]. These genotypes correspond to MOMP serovars A to F. Monoclonal antibodies cannot distinguish genotype E/B from B and E. Genotype E/B was mainly isolated from ducks. This paper was commissioned by FECAVA for publication in EJCAP. Cp. psittaci strain classification In 1999, the family Chlamydiaceae was reclassified into two genera and nine species [7]. The genus Chlamydia (C) includes C. trachomatis (human), C. suis (swine), and C. muridarum (mouse, hamster). The genus Chlamydophila (Cp) includes Cp. psittaci (avian), Cp. felis (cats), Cp. abortus (sheep, goats, cattle), Cp. caviae (guinea pigs), Cp. pneumoniae (human) and Cp. pecorum (ruminants). Epidemiology All known avian strains belong to the species Chlamydophila psittaci, which includes six avian serovars A through F, and two mammalian isolates WC and M56 isolated from epizootics in cattle and muskrats, respectively. Serotyping of avian strains is performed by a micro-immunofluorescence test using monoclonal antibodies against serovar-specific epitopes of the major outer membrane protein (MOMP) [1, 42]. The avian serovars are relatively host-specific. Serovars A and B are usually associated with psittacine birds and pigeons, respectively. Serovar C has primarily been isolated from ducks and geese, and serovar D mainly from turkeys. Serovar F was isolated from a psittacine bird and from turkeys. The host range of serovar E is the most diverse of the strains: it has been isolated from pigeons, ratites, ducks, turkeys and occasionally from humans. All serovars should be considered to be readily transmissible to humans. The psittacosis outbreaks of 1929-1930 and 1930-1938 were attributed to psittacine birds. However, in the next years it became clear that Cp. psittaci infections were not limited to psittacine birds, but could affect other bird species. In 1939, the bacterium was isolated from two South African racing pigeons and soon, additional pigeon isolates were obtained, this time in California. At that time, psittacosis in two New York citizens could be attributed to contact with infected feral pigeons. In 1942, serological evidence showed ducks and turkeys to be frequently infected and within the next 3 years, human infections due to contact with infected ducks were reported in California and New York. In the early 1950s, Cp. psittaci could be isolated from turkeys and from humans in contact with infected turkeys, during severe respiratory outbreaks in the US turkey industry. The incidence of severe Cp. psittaci epidemics in US poultry declined during the 1960s. However, avian chlamydiosis (1) Daisy Vanrompay. Ghent University, Faculty of Bioscience Engineering, Department of Molecular Biotechnology, Coupure Links 653, 9000 Ghent, Belgium. Phone: +32 09 2645972. Fax: +32 09 2646219. E-mail: Daisy.Vanrompay@ugent.ne 267 EJCAP - Vol. 18 - Issue 3 December 2008 herons, egrets, pigeons, blackbirds, grackles, house sparrows, and killdeer, all of which freely intermingle with domestic birds. Seagulls and egrets could be asymptomatic carriers of highly virulent strains of Cp. psittaci excreting a high number of bacteria. Transmission Cp. psittaci is excreted in faeces and nasal discharges. Faecal shedding occurs intermittently and can be activated by stress caused by nutritional deficiencies, prolonged transport, overcrowding, chilling, breeding, egg-laying, treatment or handling. During natural infection, bacterial excretion periods vary according to strain virulence, infection dose and host immune status. However, shedding may occur for several months. Transmission of chlamydiae is mainly by inhalation of contaminated material and sometimes by ingestion. Large numbers of Cp. psittaci can be found in respiratory tract exudate and faecal material from infected birds. In turkeys, the lateral nasal glands become infected early and remain infected for more than 60 days. Choanal/oropharyngeal swabs are more consistent for isolation of the agent than faecal swabs, especially during the early stages of infection. Turkey experimentally infected with the 84/55 genotype A strain. Note the thickened abdominal airsac totally covered with fibrin cloths (arrow). remained a continuing threat to both birds and humans. During the 1980s, chlamydiosis was reported again in US turkeys and in the 1990s also in the European turkeys [14, 30, 43]. Nowadays, Cp. psittaci is nearly endemic in the Belgian turkey industry [40, 46] and probably in other European countries as well. However, devastating, explosive outbreaks like those in ‘ancient days’ are rare and respiratory signs with no or low mortality characterize outbreaks nowadays. More recently, an increase in the number of chlamydiosis outbreaks in ducks and in people in contact with infected ducks has been reported [2, 21]. In the past, human infections associated with outbreaks in ducks had already been described [2]. Avian species, including domestic poultry sharing aquatic or moist soil habitats with wild infected aquatic birds may become infected via contaminated water. Granivorous birds like pigeons, doves, pheasants and house sparrows may become infected by dust inhalation in faecal contaminated barnyards and grain storage sites. The consumption of infected carcasses may transmit Cp. psittaci to host species that are predators or scavengers of other birds. Transmission of Cp. psittaci in the nest is possible. In many species, such as Columbiformes, cormorants, egrets, and herons, transmission from parent to young may occur through feeding, by regurgitation, while contamination of the nesting site with infective exudates or faeces may be important in other species such as snow geese, gulls and shorebirds. Cp. psittaci can be transmitted from bird to bird by blood-sucking ectoparasites The list of avian species in which Cp. psittaci infections occur has rapidly expanded. Today, Cp. psittaci has been demonstrated in about 465 bird species comprising 30 different bird orders [19]. The highest infection rates are found in psittacine birds (Psittacidae) and pigeons (Columbiformes). The seroprevalence in psittacine birds ranges between 16 and 81% and a mortality rate of 50% or even higher is not unusual [6, 28]. Psittacidae are the main chlamydial reservoirs, especially under captive conditions, and nowadays these birds are extremely popular pets [4, 44]. Hematoxylin and eosin staining of the lung of a turkey experimentally infected with the Texas Turkey genotype D strain. Note the congestion and the infiltration of lymphocytes (arrow A) and the dilated parabronchi (arrow B). Data on the seropositivity of racing pigeons range from 35.9 to 60%. Thirty-eight studies on the seroprevalence of Cp. psittaci in feral pigeons conducted from 1966 to 2005, revealed a seropositivity ranging from 12.5 to 95.6% [12, 20, 25, 27, 37]. More recent studies, conducted in feral pigeons in Italy, Bosnia and Herzegovina, and FYROM revealed a seropositivity of 48.5%, 26.5% and 19.2%, respectively [3, 17, 29]. Freeliving pigeons are distributed in urban and rural areas worldwide and are in close contact with humans in most outdoor public places. Birds mostly living on sea shores and other waters, like geese, ducks, gulls and penguins are more frequently infected than hens, pheasants and quails [19]. Common reservoirs of Cp. psittaci include wild and feral birds such as seagulls, ducks, 268 Update on avian chlamydiosis and its public health significance - D. Vanrompay For isolation the following samples can be collected: pharyngeal/ choanal slit swabs, lung tissue, thickened exudate-coated air sacs and free exudates, thickened pericardial tissues and exudates, sections of enlarged spleen and liver, intestinal mucosa at sites of hyperemia, colon contents, conjunctival and nasal discharges, whole blood and peritoneal exudates. In live birds, pharyngeal/ choanal slit swabs are preferred rather than faeces or cloacal swabs, as faecal shedding is intermittent. In dead birds, samples of the respiratory tract are most suitable. such as lice, mites and flies or, less commonly, through bites or wounds. Transmission of Cp. psittaci by arthropod vectors would be facilitated in the nest environment. Vertical transmission has been demonstrated in turkeys, chickens, ducks, parakeets, seagulls and snow geese, although the occurrence appears to be fairly low. However, it could serve as a method of introducing chlamydophila into a poultry flock. Cp. psittaci can be introduced into susceptible pet birds and poultry through the wild bird population. Contaminated feed or equipment can also be a source of infection, and feed should therefore be protected from wild birds. Careful cleaning of equipment is extremely important as Cp. psittaci can survive in faeces and bedding for up to thirty days. Cleaning and disinfection with most detergents and disinfectants will inactivate Cp. psittaci, as this Gram-negative bacterium has a high lipid content. The same tissues can be collected for molecular diagnosis. The preservation of DNA from degradation is critically important. DeGraves et al., [5] suggest all specimens for PCR analysis should be collected in a DNA stabilization reagent. Such reagents are commercially available; for instance the RNA/DNA Stabilization Reagent for Blood/Bone Marrow® from Roche Applied Science. This reagent is based on the denaturation of proteins in a concentrated solution of guanidinium isothiocyanate and another agent, for the inactivation of ribonuclease during RNA isolations. However, it also works perfectly well for DNA stabilization. Clinical disease Polymerase chain reaction Molecular methods, particularly the polymerase chain reaction (PCR), permit rapid detection and identification of Chlamydophila psittaci from clinical specimens. Two different genomic target regions are usually used for amplification, namely the ribosomal RNA gene region [7, 23, 24] and the gene encoding the MOMP antigen designated ompA [15]. Depending on the chlamydial strain and the avian host, chlamydiae cause pericarditis, air sacculitis (Fig. 1), pneumonia (Fig. 2), lateral nasal adenitis, peritonitis, hepatitis, and splenitis. Generalized infections result in fever, anorexia, lethargy, diarrhoea, and occasionally shock and death. Chlamydiosis is a very common chronic infection of psittacine birds. Infections cause conjunctivitis, enteritis, air sacculitis, pneumonitis, and hepatosplenomegaly. Droppings are often green to yellow-green. Many of the birds become chronically infected but show no clinical signs until stressed. These birds often shed chlamydiae intermittently and serve as a source of infection for humans and other birds. In some cases, concentration of target organisms by physical means increases the assays’ sensitivity, but it is important to ascertain the enrichment effect [5]. Such methods include immunomagnetic, centrifugal, or filter concentration. Several in-house-made and commercial DNA extraction methods have been described [16, 31, 47]. Alternatively, commercial DNA extraction kits can be used for the extraction of chlamydial DNA. In our hands, the QIAamp® DNA Mini Kit performed well for pharyngeal swabs while the High Pure PCR Template Preparation Kit performed well for cloacal swabs and faeces. Recent developments in diagnosis Sample collection and storage of specimens The laboratory diagnosis of avian chlamydiosis includes isolation or identification of the organism from the host preferably by recently developed molecular techniques. Because Cp. psittaci requires living cells to multiply, isolation requires inoculation of cell cultures or specific pathogen free embryonated chicken eggs in a biosafety level 3 laboratory, as the infection can be aerogenically transmitted to humans. Specimens should be collected aseptically, as contaminant bacteria may interfere with isolation. A special transport medium [35] should be used consisting of a sterile sucrose– phosphate–glutamate (SPG) buffer (sucrose, 74.6 g/liter; KH2PO 4, 0.512 g/liter; K 2HPO 4, 1.237 g/liter; and L-glutamic acid, 0.721 g/liter), supplemented with foetal calf serum (10%), vancomycin and streptomycin (100 mg/ml), and nystatin and gentamicin (50 mg/ml). The medium also serves for freezing swabs and tissues at -80°C in order to preserve chlamydial viability. The stability of chlamydiae during storage depends upon the material in which it is contained. Chlamydiae in tissue specimens or yolk-sac suspension can be preserved indefinitely by storage at -80°C [2]. Swabs should be frozen in transport medium. At 4° C, the organism can survive with gradual loss of infectivity for 30 days or longer when in heavily infected tissues or in transport medium. Recently, two highly sensitive nested PCR assays have been developed to detect Chlamydophila psittaci in avian samples [31, 41]. The method of Sachse and Hotzel [31] is based on a first amplification generating a genus-specific ompA product (576-597 bp) followed by a second amplification using one species-specific and one genus-specific primer generating a Chlamydophila psittaci-specific amplicon (389-404 bp). PCR results are visualized by agarose gel electrophoresis using ethidium bromide containing gels. The sensitivity of the nested enzyme immunoassay (PCR-EIA) was established at 1 to 0.1 infection forming unit (IFU). Van Loock et al., [41] developed a nested PCR-EIA. The fluorescein–biotin labeled PCR products were immobilized on streptavidin-coated microtitre plates and detected with anti-fluorescein peroxidase conjugate and a colorimetric substrate, although detection by use of a fluorimeter is also possible using this method. The sensitivity of the nested PCR-EIA was established at 0.1 infection-forming units (IFU). Specificity was 100%. An internal inhibition control 269 EJCAP - Vol. 18 - Issue 3 December 2008 through the use of commercially available bird seed impregnated with chlortetracycline (CTC) (0.5 mg CTC/g of seed). For large psittacine birds, feed containing 1% CTC (10 mg per g diet) is recommended. CTC medicated diets are not available in every country. A medicated mash diet for psittacine birds can be prepared by cooking 2 pounds of rice, 2 pounds of hen scratch feed and 3 pints of water for 15 minutes, adding CTC to the cooled mash. Pigeons can be fed with uncooked hen feed supplemented with CTC (5000 ppm). To reduce interference with absorption the calcium content of the diet has to be reduced to <0.7%. Doxycycline is the drug of choice for administration by mouth (25 to 50 mg/kg bw) and parenteral (intramuscular) treatment (75-100 mg/kg bw) every 5-7 days for the first 4 weeks and subsequently every 5 days for the duration of the treatment (45-day period). was included to rule out the presence of inhibitors of DNA amplification. A number of real-time PCR tests have been developed. Real-time PCR systems have the advantage that their sensitivity approaches that of the nested PCR systems, and they require no additional pipetting or handling of the PCR product following the initial set-up of the PCR mix. This reduces both the time needed for the test and the incidence of contamination. Recently a SYBR Green-based real-time PCR was developed targeting the rDNA ribosomal spacer of Chlamydophila psittaci [10]. The test could detect 10rDNA copies/µL DNA extract. Geens et al., [10] also developed a genotyping real-time PCR which detects all known ompA Chlamydophila psittaci genotypes. Micro arrays Micro arrays can be coupled with PCR where they serve as a set of parallel dot-blots to enhance product detection and identification of bacterial isolates. Sachse et al., [32] developed a DNA micro array-based detection and identification method for Chlamydia and Chlamydophila spp. The test was developed using the ArrayTube platform (CLONDIAG®chip technologies). Unique species-specific hybridization patterns were obtained for all nine species of the family Chlamydiaceae. The assay proved suitable for species identification of chlamydial cell cultures and showed a potential for direct detection of these bacteria from tissue samples. Recently, an ompA-based Cp. psittaci genotyping micro array has been developed [33]. Public health significance Cp. psittaci can infect humans and should be handled carefully under conditions of bio-containment. Humans most often become infected by inhalation when urine, respiratory secretions or dried faeces of infected birds is dispersed in the air as very fine droplets or dust particles. Other sources of exposure include mouth-to-beak contact, a bite from an infected bird or handling the plumage and tissues of infected birds. Therefore, post-mortem examinations of infected birds and handling of cultures should be done in laminar flow hoods or with proper protective equipment. Human infection can result from transient exposures. The disease is of public health significance because of the popularity of psittacine birds as pets and the increased placement of these birds in child-care facilities and in homes for the aged. Not only direct contact with birds poses risk of psittacosis but also the rural environment and activities such as gardening that may expose individuals to the infectious agent [8, 38]. Person-to-person transmission of psittacosis is possible but it is believed to be rare [18]. Serology Despite the introduction of very sensitive and specific tests like PCR, the idea of serological diagnosis still lingers in the minds of several veterinary clinicians. However, serology alone is not particularly useful for diagnosis of a patent disease in birds because of the high seroprevalence of this infection in asymptomatic birds and the long-term (up to several months) persistence of anti-chlamydial antibodies. In most bird species, there is a high background rate of antichlamydial antibodies, and until we have more information on the disease pattern in certain bird species in relation to direct identification of the bacteria and serology, we are unable to comment on the real significance of antibody titres obtained. Thus, to determine if a single bird is infected, serology should always be used in conjunction with bacterial antigen or gene detection or paired sera should be examined. The latter can not be applied for immediate clinical evaluation. Treatment with antibiotics may delay and/or diminish the antibody response. Serology is very helpful in epidemiological research. Recently, a sensitive and specific recombinant ELISA has been developed based on the detection of antibodies against the chlamydial major outer membrane protein [46]. The incubation period in humans is usually 5–14 days or longer. Human infections vary from asymptomatic to severe systemic disease with interstitial pneumonia and occasionally encephalitis. The disease is rarely fatal in properly treated patients; therefore, early diagnosis is important. Symptoms usually include headache, chills, malaise and myalgia, with or without signs of respiratory involvement. Pulmonary involvement is common. Several severe psittacosis (pneumonia) cases have recently been documented [13, 26, 36] and Cp. psittaci has been associated with ocular lymphoma [9, 48]. However, many psittacosis cases in humans are presented with either mild respiratory symptoms or as asymptomatic infections [16, 44]. The public health significance of these infections is unknown. Diagnosis is usually established through testing paired sera or only one serum sample by use of the micro-immunofluorescence (MIF) test which is more sensitive and specific than the complement fixation (CF) test. However, the MIF test presents cross-reactivity with other chlamydial species. As in birds, culture or the more sensitive molecular methods can be used to specifically detect Cp. psittaci. Tetracyclines are the drugs of choice for therapy of human psittacosis. Doxycycline or tetracycline is usually administered (not Treatment Tetracyclines are the drugs of choice and can be administered orally (medicated feed or by mouth if the bird does not accept the medicated diet) or parenterally (injections). Parakeets, budgerigars, finches, canaries and rice birds can be treated 270 Update on avian chlamydiosis and its public health significance - D. Vanrompay in pregnant women and children < 9 years), where erythromycin constitutes a second choice. The duration of treatment depends on the drug. Tetracyclines should be given for at least 14 days. In most countries, psittacosis is a notifiable disease. during quarantine it is suspected or confirmed that psittaciformes are infected with Cp. psittaci, all birds of the consignment must be treated by a method approved by the competent authority and the quarantine must be prolonged for at least two months following the last recorded case”. Thus, these requirements did not involve bird species other than poultry nor were there any rules for the management of psittacosis within the EU. Therefore, Decisions 2000/666/EC and 2005/760/EC were repealed and a new Commission Regulation, EC no 318/2007 has been in place since 1 July 2007 after being published in the Official Journal of the EU. This regulation lays down the animal health conditions for imports of certain birds from third countries and parts thereof into the EU. This Regulation also lays down the quarantine conditions. For instance: 1) approved quarantine facilities and centres; 2) direct transport of birds to quarantine stations; 3) attestation by the importers or their agents; 4) quarantine for at least 30 days; 5) examination, sampling and testing to be carried out by an official veterinarian; 6) actions in case of chlamydiosis suspicion, which are treatment of all birds and prolonged quarantine for at least two months following the date of the last recorded case. Importantly, the Regulation allows for imports of birds only from approved breeding establishments. Thus, for birds other than poultry, only birds bred in captivity with an individual identification number and accompanied by an animal health certificate are allowed for importation into the EU. Prevention and control Vaccine development Recently, attention has been turned to DNA vaccination and recombinant adenoviral vaccines as a protective means against chlamydial infections [45, 49]. DNA vaccination has been more successful at inducing protective immune responses in turkeys infected with Cp. psittaci [45], than in sheep or mouse models infected with Cp. abortus [22]. Importantly, DNA vaccination can be performed in the presence of maternal antibodies [39]. However, additional research is needed to further enhance the immunogenicity of DNA vaccines and to lower the vaccination costs. There are currently no vaccines available against avian chlamydiosis although research on MOMP-based DNA vaccination in SPF turkeys has been very promising. DNA vaccination significantly reduces clinical signs and chlamydial excretion, though it did not give no full protection. Until an effective vaccine is produced, risk reduction strategies and treatment will have to be applied. Worldwide, psittacosis prevention and control recommendations and legislation are mainly focused on the decrease of human morbidity. Recommended control measures: 1) maintenance of accurate records by the seller of all bird transactions. Records should mention when the birds are sold, the name, address and phone number of the customer, date of purchase, species and band number if applicable; 2) Birds with signs compatible with chlamydiosis should not be purchased or sold; 3) Before adding new birds to a group, the birds should be quarantined and observed for a 14-30 day period and tested or prophylactically treated; 4) accurate preventive husbandary (prevent feathers, food and other materials moving from one cage to another, daily cleaning, sufficient exhaust ventilation to prevent accumulation of aerosols); 5) special husbandary during infection (isolation, non-dusty litter, keep circulation of feathers and dust to a minimum by frequent wet-mopping with disinfectants, cleaning and disinfection); 6) disinfection (1:1000 dilution of quaternary ammonium compounds, 70% isopropyl alcohol, 1% Lysol, 1:100 dilution of household bleach or chlorophenols can be used); 7) personal protection (HEPA mask in high risk situations). References [1] [2] [3] [4] EU legislation Until recently, EU legislation for the importation of birds other than poultry from third countries was encompassed in Commission Decision 2000/666/EC (CEC, 2000) and 2005/760/EG, which primarily aim to restrict the introduction of Newcastle disease or avian influenza into the EU. According to these Decisions: 1) the bird must come from a flock in the country of origin where they have been kept for at least 21 days prior to export; 2) the birds are transported in cages or crates containing only one species of bird or one species per compartment if compartmentalised and 3) the birds are moved to designated licensed quarantine premises where they are held for 30 days and subjected to at least two veterinary inspections, usually at the beginning and before release. There was only one mention of Cp. psittaci: “if [5] [6] [7] 271 ANDERSEN (A.A.) - Serotyping of Chlamydia psittaci isolates using serovar- specific monoclonal antibodies with the microimmunofluorescence test. Journal of Clinical Microbiology, 1991, 29, 707-711. ANDERSEN (A.A.), VANROMPAY (D.) - Avian Chlamydiosis (psittacosis, ornithosis). In: Said YM (ed) Diseases of Poultry 12th Edition. Iowa State University Press, in press. CEGLIE (L.), LAFISCA (S.), GUADAGNO (C.), DALLA POZZA (G.), CAPELLO (K.), BANO (L.), VICARI (N.), DONATI (M)., MION (M.), GIURISATO ( I.), LOMBARDO ( D.), POZZATO (N.), CEVENINI (R.), NATALE (A.) - Serological surveillance in north-eastern Italy for the presence of Chlamydophila spp from birds and molecular characterization of PCR isolates within the area of Venice. In: Niemczuk K, Sachse K, Sprague LD (eds). Proceeding of the 5th Annual Workshop of Cost Action 855, Animal Chlamydioses and Zoonotic Implications, Pulawy, Poland, 2007, pp. 62-67. CHAHOTA (R.), OGAWA (H.), MITSUHASHI (Y.,) OHYA (K.), YAMAGUCHI (T.), FUKUSHI (H.) - Genetic diversity and epizootiology of Chlamydophila psittaci prevalent among the captive and feral avian species based on VD2 region of ompA gene. Microbiology and Immunology, 2006, 50, 663-678. DEGRAVES (F.J.), GAO (D.), KALTENBOECK (B.) - High-sensitivity quantitative PCR platform. Biotechniques, 2003, 34, 106-10, 1125. DOVC (A.), SLAVEC (D.), LINDTNER-KNIFIC (R.), ZORMAN-ROJS (O.), RACNIK (J.), GOLJA ( J.), VLAHOVIC (K.) - The study of a Chlamydophila psittaci outbreak in budgerigars. In: Niemczuk K, Sachse K, Sprague LD (eds) Proceedings of the 5th Annual Workshop of COST Action 855 Animal Chlamydioses and Zoonotic Implications, Pulawy, Poland, 2007, p24. EVERETT (K.D.), BUSH (R.M.), ANDERSEN (A.A.) - Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and EJCAP - Vol. 18 - Issue 3 December 2008 [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] LONGBOTTOM (D), LIVINGSTONE (M.) - Vaccination against chlamydial infections of man and animals. The Veterinary Journal, 2006, 171, 263-275. [23] MADICO (G.), QUINN (T.C.), BOMAN (J.), GAYDOS (C.A.) - Touchdown enzyme time release-PCR for detection and identification of Chlamydia trachomatis, C. pneumoniae, and C. psittaci using the 16S and 16S-23S spacer rRNA genes. Journal of Clinical Microbiology, 2000, 38, 1085-93. [24] MESSMER (T.O.), SKELTON (S.K.), MORONEY (J.F.), DAUGHARTY (H.), FIELDS (B.S.) - Application of a nested, multiplex PCR to psittacosis outbreaks. Journal of Clinical Microbiology, 1997, 35, 2043-6. [25] MITEVSKI (D.), PENDOVSKI (L.), NALETOSKI (I.), ILIESKI (V.) Survellaince for the presence of Chlamydophila psittaci in pigeons and doves from several towns in Macedonia. In: Cevenini R, Sambri V, (eds) Proceedings of the 3rd Workshop; Diagnosis and Pathogenesis of Animal Chlamydioses, Siena, Italy, 2005, pp. 141-145. [26] PANDELI (V.), ERNEST (D.) - A case of fulminant psittacosis. Critical Care and Resuscitation, 2006, 8, 40-42. [27] PRUKNER-RADOVCIC (E.), HORVATEK (D.), GROZDANIC (I.C.), MAJETIC (I.), BIDIN (Z.) - Chlamydophila psittaci in turkey in Croatia. In: Cevenini R, Sambri V, (eds) Proceedings of the 3rd Workshop; Diagnosis and Pathogenesis of Animal Chlamydioses, Siena, Italy, 2005, pp. 161-166. [28] RASO (T.F.), JUNIOR (A.B.), PINTO (A.A.) - Evidence of Chlamydophila psittaci infection in captive Amazon parrots in Brazil. Journal of Zoo and Wildlive Medicine, 2002, 33, 118-121. [29] RESIDBEGOVIC (E.), KAVAZOVIC (A)., SATROVIC (E.), ALIBEGOVICZECIC (F.), KESE (D.), DOVC (A.) - Detection of antibodies and isolation of Chlamydophila psittaci in free-living pigeons (Columba livia domestica). In: Niemczuk K, Sachse K, Sprague LD (eds) Proceedings of the 5th Annual Workshop of COST Action 855 Animal Chlamydioses and Zoonotic Implications, Pulawy, Poland, 2007, pp. 81-85. [30] RYLL (M.), HINZ (K.H.), NEUMANN (U.), BEHR (K.P.) - Pilot study of the occurrence of Chlamydia psittaci infections in commercial turkey flocks in Niedersachsen. Deutsche Tierarztliche Wochenschrift, 1994, 101, 163-5 [31] SACHSE (K.), HOTZEL (H.) - Detection and differentiation of Chlamydiae by nested PCR. Methods in Molecular Biology, 2003, 216, 123-36. [32] SACHSE (K.), HOTZEL (H.), SLICKERS (P.), ELLINGER (T.), EHRICHT (R.) - DNA microarray-based detection and identification of Chlamydia and Chlamydophila spp. Molecular and Cellular Probes, 2005, 19, 41-50. [33] SACHSE (K.), LAROUCAU (K.), HOTZEL (H.), SCHUBERT (E.), EHRICHT (R.), SLICKERS (P.) - Genotyping of Chlamydophila psittaci using a new DNA microarray assay based on sequence analysis of ompA genes. BMC Microbiology, 2008, 17, 8:63. [34] SMITH (K.A.), BRADLEY (K.K.), STOBIERSKI (M.G.), TENGELSEN (L.A.). National Association of State Public Health Veterinarians Psittacosis Compendium Committee - Compendium of measures to control Chlamydophila psittaci (formerly Chlamydia psittaci) infection among humans (psittacosis) and pet birds. Journal of the American Veterinary Medical Association, 2005, 226, 0532-9. [35] SPENCER (W.N.), JOHNSON (F.W.) - Simple transport medium for the isolation of Chlamydia psittaci from clinical material. The Veterinary Record, 1983, 113, 535-6. [36] STRAMBU (I.), CIOLOAN (G.), ANGHEL (L.), MOCANU (A.), STOICESCU (I.P.) - Bilateral lung consolidations related to accidental exposure to parrots. Pneumologia, 2006, 55, 123-127. [37] TANAKA, (C.), MIYAZAWA (T.), WATARAI (M.), ISHIGURO (N.) Bacteriological survey of feces from feral pigeons in Japan. Journal of Veterinary Medical Science, 2005, 67, 951-953. [38] TELFER (B.L.), MOBERLEY (S.A.), HORT (K.P.,) BRANLEY (J.M.), standards for the identification of organisms. International Journal of Systematic Bacteriology, 1999, 49, 415-440. FENGA (C.), CACCIOLA (A.), DI NOLA (C.), CALIMERI (S.,) LO (G.D.), PUGLIESE (M.), NIUTTA (P.P.), MARTINO (L.B.) - Serologic investigation of the prevalence of Chlamydophila psittaci in occupationally-exposed subjects in eastern Sicily. Annals of Agricultural and Environmental Medicine, 2007, 14, 93-96. FERRERI (A.J.), GUIDOBONI (M.), PONZONI (M.), DE CONCILIIS (C.), DELL’ ORO (S.), FLEISCHHAUHER (K.), CAGGIARI (L.), LETTINI (A.A.), DAL CIN (E.), IERI (R.), FRESCHI (M.), VILLA (E.), BOIOCCHI (M.), COLCETTI (R.) - Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. Journal of the National Cancer Institute, 2004, 96, 586-594. GEENS (T.), DEWITTE (A.), BOON (N.), VANROMPAY (D.) Development of a Chlamydophila psittaci species-specific and genotype-specific real-time PCR. Veterinary Research, 2005a, 36, 787-797. GEENS (T.), DESPLANQUES (A.), VAN LOOCK (M.), BÖNNER (B.M.), KALETA (E.F.), MAGNINO (S.), ANDERSEN (A.A.), EVERETT (K.D.E.), VANROMPAY (D.) - Sequencing of the Chlamydophila psittaci ompA gene reveals a new genotype, E/B, and the need for a rapid discriminatory genotyping method. Journal of Clinical Microbiology, 2005b, 43, 2456-2461. HAAG-WACKERNAGEL (D.) - Feral pigeons (Columba livia) as potential source for human ornithosis. In: Cevenini R, Sambri V, (eds) Proceedings of the 3rd Workshop; Diagnosis and Pathogenesis of Animal Chlamydioses, Siena, Italy, 2005, pp. 15-16. HAAS (L.E.), TJAN (D.H.), SCHOUTEN (M.A.), VAN ZANTEN (A.R.) - Severe pneumonia from psittacosis in a bird-keeper. Nederlands Tijschrift voor Geneeskunde, 2006, 150, 117-121. HAFEZ (H.M.), STING (R) - Über das Vorkommen von ChlamydienInfektionen beim Mastgeflügel, Tierärtzliche Umschau, 1997, 52, 281–285. HEWINSON (R.G.), GRIFFITHS (P.C.), DAWSON (M.), WOODWARD (M.J.) - PCR test for Chlamydia psittaci. The Veterinary Record, 1991, 128, 599. HARKINEZHAD (T.), VERMINNEN (K.), VAN DROOGENBROECK (C.), VANROMPAY (D.) - Chlamydophila psittaci genotype E/B transmission from African grey parrots to humans. Journal of Medical Microbiology, 2007, 56, 1097-1100. ILIESKI (V.), RISTOSKI (T.), PENDOVSKI (L.), DODOVSKI (A.), MITEVSKI (D.) - Detection of Chlamydophila psittaci in freeliving birds using ELISA and immumohistochemical methods. In: Longbottom D, Rocchi M, (eds) Proceedings of the 4th Annual Workshop of COST Action 855, Animal Chlamydioses and Zoonotic Implications, Edinburgh, Scotland, UK, 2006, pp. 8687. ITO (I.), ISHIDA (T.), MISHIMA (M.), OSAWA (M.), ARITA (M.), HASHIMOTO (T.), KISHIMOTO (T.) - Familial cases of psittacosis: possible person-to-person transmission. Internal Medicine Journal, 2002, 41, 580-583. KALETA (E.F.), TADAY (E.M.) - Avian host range of Chlamydophila spp. based on isolation, antigen detection and serology. Avian Pathology, 2003, 32, 435-461. LAROUCAU (K.), MAHE (A.M.), BOUILLIN (C.), DEVILLE, (M.), GANDOUIN (C.), TOUATI (F.), GUILLOT (J.), BOULOUIS (H.J.) - Health status of free-living pigeons in Paris. In: Cevenini R, Sambri V, (eds) Proceedings of the 3rd Workshop; Diagnosis and Pathogenesis of Animal Chlamydioses, Siena, Italy, 2005, pp. 17-18. LAROUCAU (K.), DE BARBEYRAC (B.), VORIMORI (F.), CLERC (M.), BERTIN (C.), HARKINEZHAD (T.), VERMINNEN (K.), OBINICHE (K.), CAPEK (F.), BÉBÉAR (I.), VANROMPAY (D.), GARIN-BASTUJI (B.), SACHSE (K.) - Chlamydial infections in duck plants associated with human cases of psittacosis in France. Veterinary Microbiology, 2008, in press. 272 Update on avian chlamydiosis and its public health significance - D. Vanrompay [39] [40] [41] [42] [43] [44] DWYER (D.E.), MUSCATELLO (D.J.), CORRELL (P.K.), ENGLAND (J.), MCANULTY (J.M.) - Probable psittacosis outbreak linked to wild birds. Emerging Infectious Diseases, 2005, 11, 391-397. VAN LOOCK (M.), LAMBIN (S.), VOLCKAERT (G.), GODDEERIS (B.M.), VANROMPAY (D.) - Influence of maternal antibodies on Chlamydophila psittaci-specific immune responses in turkeys elicited by naked DNA. Vaccine, 2004, 22, 1616-23. VAN LOOCK, (M.), GEENS (T.), DE SMIT (L.), NAUWYNCK (H.), VAN EMPEL (P.), NAYLOR (C.), HAFEZ (H.M.), GODDEERIS (B.M.), VANROMPAY (D.) - Key role of Chlamydophila psittaci on Belgian turkey farms in association with other respiratory pathogens. Veterinary Microbiology, 2005a, 107, 91-101. VAN LOOCK (M.), VERMINNEN (K.), MESSMER (T.O.), VOLCKAERT (G.), GODDEERIS (B.M.), VANROMPAY (D.) - Use of a nested PCR-enzyme immunoassay with an internal control to detect Chlamydophila psittaci in turkeys. BMC Infectious Diseases, 2005b, 26, 5:76. VANROMPAY ( D.) , ANDERSEN (A . A .) , DUCATELLE ( R.) , HAESEBROUCK (F.) - Serotyping of European isolates of Chlamydia psittaci from poultry and other birds. Journal of Clinical Microbiology, 1993a, 31, 134-137. VANROMPAY (D.), DUCATELLE (R.), HAESEBROUCK (F.), HENDRICKX (W.) - Primary pathogenicity of an European isolate of Chlamydia psittaci from turkey poults. Veterinary Microbiology, 1993b, 38, 103-113. VANROMPAY, (D.), HARKINEZHAD (T.), MARIJKE (V.W.), BEECKMAN (D.S.), VAN DROOGENBROECK (C.), VERMINNEN (K.), LETEN (R.), MARTEL (A.), CAUWERTS (K.) - Chlamydohpila psittaci Transmission from Pet Birds to Humans. Emerging Infectious Diseases, 2007, 13, 1108-1110. [45] VERMINNEN (K.), VAN LOOCK (M.), COX (E.), GODDEERIS (B.M.), VANROMPAY (D.) - Protection of turkeys against Chlamydophila psittaci challenge by DNA and rMOMP vaccination and evaluation of the immunomodulating effect of 1 alpha, 25-dihydroxyvitamin D(3). Vaccine, 2005, 23, 4509-16. [46] VERMINNEN (K.), VAN LOOCK (M.), HAFEZ (H.M.), DUCATELLE (R.), HAESEBROUCK (F.), VANROMPAY (D.) - Evaluation of a recombinant enzyme-linked immunosorbent assay for detecting Chlamydophila psittaci antibodies in turkey sera. Veterinary Research, 2006, 37, 623-632. [47] WILSON (P.A.), PHIPPS (J.), SAMUEL (D.), SAUNDERS (N.A.) Development of a simplified polymerase chain reaction-enzyme immunoassay for the detection of Chlamydia pneumoniae. Journal of Applied Bacteriology, 1996, 80, 431-8. [48] ZHANG (G.S.), WINTER (J.N.), VARIAKOJIS (D.), REICH (S.), LISSNER (G.S.), BRYAR (P.), REGNER (M.), MANGOLD (K.), KAUL (K.) - Lack of association between Chlamydia psittaci and ocular adnexal lymphoma. Leukemia and Lymphoma, 2007, 48, 577-83. [49] ZHOU (J.), QIU (C.), CAO (X.A.), LIN (G.) - Construction and mmunogenicity of recombinant adenovirus expressing the major outer membrane protein (MOMP) of Chlamydophila psittaci in chicks. Vaccine, 2007, 25, 6367-72. 273 ZOONOSES The role of Bartonella spp. in veterinary and human medicine with special emphasis on pathogenicity mechanisms V. A. J. Kempf(1), F. Krämer(2) SUMMARY Bartonella spp. are important pathogens in human and veterinary medicine. Currently, B. henselae is considered to be the most relevant zoonotic Bartonella species responsible for cat scratch disease (CSD), bacillary angiomatosis and peliosis hepatis. Besides B. henselae, several other species have been isolated from cats, dogs and humans suffering from a variety of clinical manifestations ranging from mild infections to severe disease. The analysis of the underlying pathogenicity mechanisms will result in better understanding of the diseases and consequent vector control might allow the prevention of such Bartonella-infections. Bartonella infections of cats, dogs and humans Infectious diseases caused by Bartonella have been described for more than 1,000 years. Historically, infections with B. bacilliformis (which is endemic in South America) are known since the dynasty of the Inca. B. quintana was even detected in 4,000-year-old human tissue [15]. However, only 18 years ago, B. henselae, the most important human pathogenic species, was discovered by David Relman who identified this pathogen in 1990 by molecular techniques [41]. This paper was commissioned by FECAVA for publication in EJCAP. Introduction Bartonellosis is considered an emerging zoonosis with increasing frequency. While cats often remain asymptomatic carriers for Bartonella spp., dogs may develop clinical signs that are similar to those observed in humans [21]. Due to their way of transmission in cats and dogs via fleas and presumably also ticks [12], bartonellosis is counted into the field of canine vector borne diseases (CVBD). Cats have been confirmed as a reservoir for B. henselae. Besides this species, cats are also the main reservoir for B. clarridgeiae and are presumably the reservoir of B. koehlerae which has been isolated from the blood of cats in the USA [16] and was also detected in cat fleas in France [46]. Mostly, cats with serological, cultural or PCR-based evidence of an exposure to a certain Bartonella spp. show no particular signs of infection. Nevertheless, B. henselae infections of cats have been associated with a variety of clinical manifestations, including, e.g., uveitis [31, 32]. The ability to cause vasculoproliferative disorders or intraerythrocytic bacteraemia is a unique feature of the genus Bartonella. The pathogens are present in a broad spectrum of mammals including cats, dogs, ruminants and rodents which might either suffer from these infections [25] or serve as asymptomatic reservoir hosts for zoonotic infections [13]. Elucidating the course of Bartonella infections in animals, arthropods and humans will help to prevent such infections in future; vector control in cats and dogs might also prevent transmission to humans as well as disease in the pets themselves. The most prevalent Bartonella species in dogs, B. vinsonii subsp. berkhoffii, was initially isolated from a dog with endocarditis and intermittent epistaxis in 1993 [5, 30]. Meanwhile, this pathogen has also been associated with cardiac arrythmias, myocarditis, granulomatous rhinitis, anterior uveitis, and choroiditis [4, 5, 36, 38, 39]. B. henselae [17, 19, 23, 29, 47], B. clarridgeiae [10, (1) Volkhard AJ Kempf. University Hospital, Institute for Medical Microbiology and Hygiene, Elfriede-Aulhorn-Str. 6, D-72076 Tuebingen, Germany E-mail: volkhard.kempf@med.uni-tuebingen.de (2) Friederike Krämer. University of Veterinary Medicine Hannover, Institute for Parasitology, Bünteweg 17, D-30559 Hannover, Germany 274 The role of Bartonella spp. in veterinary and human medicine - V. A. J. Kempf & F. Krämer 23], B. washoensis [11], B. elizabethae [3] and B. quintana [25] have also been associated with pathology and clinical signs in dogs, including endocarditis, hepatic disease and sudden death. Furthermore, B. henselae has been implicated in peliosis hepatis [29], granulomatous hepatis [23], and granulomatous sialodenitis [47]. Interestingly, B. henselae and B. quintana were also detected in the blood or lymph nodes of dogs suffering from lymphoma [19]. Today, the clinically most important humanpathogenic species are B. henselae, B. quintana and B. bacilliformis. A synopsis of Bartonella spp., their reservoir hosts, vectors and the spectrum of human diseases are given in Tab. 1. Cat scratch disease (B. henselae, B. clarridgeiae) Cat scratch disease (CSD) is caused by B. henselae (less frequently by B. clarridgeiae). The pathogens are transmitted by bites or scratches of infected cats (Fig. 1). Usually, 2–3 weeks after infection, a unilateral lymphadenitis in the lymph Fig. 1 Inoculation site (forearm) of a patient suffering from a B. henselae-caused CSD. Tab. 1 Bartonella spp.: reservoirs, vectors, human diseases (modified table, taken from ref. 13). Bartonella spp. Reservoir Vector human sandfly Human diseases Human-specific spp.: B. bacilliformis Carrion’s disease, Oroya fever, verruga peruana 46 8 B. quintana human body louse (cat flea , ticks ) trench fever, endocarditis, bacillary angiomatosis B. rochalimae unknown unknown bacteraemia, fever20 B. clarridgeiae cat cat flea cat scratch disease B. elizabethae rat unknown endocarditis, neuroretinitis B. grahamii mouse, vole unknown neuroretinitis B. henselae cat cat flea (ticks?) cat scratch disease, bacillary angiomatosis, endocarditis, neuroretinitis, bacteraemia B. koehlerae cat unknown endocarditis B. vinsonii subsp. arupensis mouse tick bacteraemia, fever, endocarditis (?) B. vinsonii subsp. berkhoffii dog tick endocarditis B. washoensis ground squirrel unknown myocarditis, endocarditis (?) B. alsatica rabbit unknown unknown B. birtlesii mouse unknown unknown B. bovis (= B. weissii) cattle, cat unknown unknown B. capreoli roe deer unknown unknown B. chomelii cattle unknown unknown B. doshiae vole unknown unknown B. peromysci deer, mouse unknown unknown B. phoceensis rat unknown unknown B. rattimassiliensis rat unknown unknown B. schoenbuchensis roe deer unknown unknown B. talpae vole unknown unknown B. taylorii mouse, vole unknown unknown B. tribocorum rat unknown unknown B. vinsonii subsp. vinsonii vole unknown unknown Zoonotic spp.: Animal-specific spp.: 275 EJCAP - Vol. 18 - Issue 3 December 2008 Fig. 2 Typical histology of CSD in a lymph node of a 52-year-old patient showing granulomatous inflammation and central necrosis. Fig. 3 Bacillary angiomatosis in a 37-year-old AIDS patient located at the chest. Newly appeared vessel formations can be noted. draining region near the site of the scratch or bite occurs (Fig. 2). In ~10 % of CSD, infected lymph nodes may form a fistula through the skin draining pus. CSD is a common cause of chronic lymph node swelling in children, and these patients might also suffer from fever, headache or splenomegaly. In rare cases, oculoglandular involvement (Parinaud’s syndrome), encephalopathy, neuroretinitis or osteomyelitis can occur. Usually, the disease is self-limiting and patients do not require antibiotic treatment. Serological testing is the best evaluated method for the laboratory diagnosis of CSD. Relapsing or systemic infections might be treated with macrolides (e.g., erythromycin). lobulated proliferations of mainly capillary-sized vessels and are understood as the result of a chronic infection with B. henselae or B. quintana. The pathogen is detectable in the vasculoproliferative lesions, and bacterial eradication by antibiotic treatment results in a complete regression of the angiomatous tumors. Bloodstream infections: Trench fever (B. quintana) B. quintana, the agent of “Trench fever”, caused large epidemics in Europe during the First and Second World Wars. Trench fever reemerged at the end of the last century with increasing frequency. B. quintana bloodstream infections were reported in homeless people or patients with chronic alcoholism [7, 52]. The disease is characterized by a sudden onset and subsequent periodical feverish relapses (“five-day fever”) accompanied by an intraerythrocytic bacteraemia. The presence of B. quintana in the bone marrow (erythroblasts) has been described [45]. Humans are the only known natural reservoir host for B. quintana; body lice transmit the pathogen from patient to patient. Interestingly, the pathogen has recently been detected in cat fleas [46] and ticks [8]. B. quintana has also been detected in two dogs suffering from endocarditis [25]. Cats have been identified as the primary reservoir for B. henselae, and the domesticated species is most often associated with the transmission of the infection to humans (mainly children) by scratches or bites [3]. Between cats, the organism is transmitted by the cat flea Ctenocephalides felis [9]. Recent studies have shown a strong correlation between the prevalence of B. henselae in cats and fleas from these cats [33]. Dogs are also suggested to represent a reservoir for B. henselae as the pathogen was found to be the causative agent in a case of human osteomyelitis following a dog scratch [28]. Furthermore, a dog owner suffering from lymphadenopathy (seroreactive for B. henselae) was reported and B. henselae DNA was amplified from gingival swaps of the dog [53]. Currently, four Bartonella species have been detected in dog saliva [18]. From these findings, a transmission of Bartonella from dogs to humans cannot be excluded and it might be assumed that vector control (flea and tick) reduces the risk of transmission to dogs (e.g., via ticks [12]) and, thus, possibly from dogs to humans. Recently, B. henselae and B. vinsonii subsp. berkhoffii were detected in the peripheral blood of immunocompetent patients suffering from chronic neurological symptoms including ataxia, seizures and tremor [6]. Fig. 4 Detection of anti-B. henselae antibodies by IFA using cell culturederived antigen. Bacteria-induced neoangiogenesis: bacillary angiomatosis, peliosis hepatis (B. henselae, B. quintana) The ability to induce tumorous vasculoproliferative disorders is a unique feature of pathogens of the genus Bartonella. In humans, mainly immunosuppressed patients (e.g. AIDS patients) suffer from the vasculoproliferative disorders bacillary angiomatosis (cutaneous manifestations) (Fig. 3) or peliosis hepatis (hepatic manifestations). Both diseases are histologically characterized as 276 The role of Bartonella spp. in veterinary and human medicine - V. A. J. Kempf & F. Krämer B. henselae infections: Diagnostic procedures Concerning the zoonotic importance of Bartonella infections in cats and dogs, as well as the general relevance of Bartonella infections in humans, obviously, B. henselae represents the most important pathogenic Bartonella species. include the Bartonella adhesin A (BadA) of B. henselae [43] and the variably expressed outer membrane proteins (Vomps) of B. quintana [54]. Intraerythrocytic bacteraemia caused by Bartonella spp. Several Bartonella species cause a long-lasting intraerythrocytic bacteraemia in their mammalian reservoir host. This obviously enables the transmission of the pathogens via blood-sucking arthropods (e.g. fleas, body lice). Furthermore, a persistent bacteraemia with B. henselae in asymptomatic cats (and possibly in dogs) represents an important factor facilitating the spread of microorganism. A variety of methods for the laboratory identification of B. henselae infections have been employed including histological examination, isolation and culture or molecular and serological approaches. Nevertheless, the detection of the causative agent of CSD by histology or PCR (e.g., via amplification of the 16S-rDNA) requires invasive procedures (biopsy, fine-needle aspiration). Unfortunately, cultivation of the slowly growing and fastidious pathogens from patient specimen is normally not successful. Therefore, the most widely used diagnostic method is the serological testing for B. henselae antibodies. Here, an indirect immunofluorescence assay using whole cell antigen from B. henselae co-cultivated with Vero cells is used (Fig. 4). Most of the knowledge of intraerythrocytic Bartonella infections was gained using a B. tribocorum rat-infection model which mimics Trench fever [48]. Here, the pathogens are rapidly cleared from the circulating blood after an intravenous infection. Five days later, the bacteria start to be periodically released from their unknown niche into the bloodstream. B. tribocorum persists several weeks in the circulating blood in an immunoprivileged intracellular niche (erythrocyte). This haematotropic infection strategy is probably a specific adaptation of the pathogen to the transmission by blood-sucking arthropod vectors and is shared by many Bartonella species. From all Bartonellae, only B. bacilliformis causes a massive haemolysis of infected erythrocytes often resulting in a fatal haemolytic anaemia [13]. Using human haematopoietic stem cells, it could be demonstrated that Bartonella spp. are able to infect and to persist in erythroid-differentiating stem cells suggesting that haematopoietic stem cells might serve as a potential primary niche in Bartonella infections [35]. The seroprevalence of anti-B. henselae antibodies in cats ranges from 0% in Norway [2] over 35% in Alabama, Maryland and Texas [33] and 71% in Spain [50], up to 93% in North Carolina [37], depending on the region, the method of examination and the type of cats examined (e.g., feral/stray or pet cats). In dogs the seroprevalence of anti-B. henselae antibodies has been reported between 2% in Brazil [14] and 3% in the UK [1] over 5% in southern Ontario and Quebec [22] and 17% in Spain [51], up to 27% in south-eastern USA [49] and 28% in Italy [40]. The seroprevalence of anti-B. henselae antibodies in humans is between 5-30% and, therefore, much higher compared with the seroprevalence of antibodies against, e.g. Borrelia burgdorferi (causing Lyme disease) and many other pathogens. 13% of cervical lymph node swellings are caused by Bartonella spp. [42]. In the United States, the incidence of Bartonella infections was 9.3 per 100,000 inhabitants in the year 1993 [24]. Vasculoproliferative diseases caused by Bartonella spp. B. henselae, B. quintana and B. bacilliformis are able to cause vasculoproliferative disorders. This feature is especially reported from humans; however, less known, also dogs can develop such vasculoproliferations (e.g. peliosis hepatis [29]). Mechanisms leading to this neoangiogenic lesions are best investigated for B. henselae. Currently, it is speculated that Bartonella species may cause vasculoproliferations by at least three different mechanisms which may act synergistically: (i) triggering the proliferation of endothelial cells directly, (ii) inhibition of endothelial cell apoptosis (via the VirB/D4 T4SS), and (iii) angiogenic reprogramming of infected host cells. This bacterially induced angiogenic gene program is regulated via the activation of “hypoxia-inducible factor (HIF)-1” (which represents the key transcription factor involved in the induction of angiogenesis) and subsequent secretion of angiogenic cytokines (e.g., vascular endothelial growth factor, VEGF), all known to be crucially involved in neoangiogenic processes. Both, HIF-1 activation and VEGF secretion are induced after infection of cultured host cells with B. henselae in vitro and where also demonstrated in patient’s bacillary angiomatosis tissue lesions in vivo. Pathogenicity of Bartonella spp. Two aspects are in the focus of Bartonella research: the ability of the bacteria to induce vessel formation (“neoangiogenesis”) and their ability to cause a long-lasting bacteraemia, both being highly interesting phenomena, especially of interest for developing new strategies of inducing therapeutic vessel growth, possibly influencing angiogenesis research. Strategies of how bacteria cause chronic intraerythrocytic infections are also fascinating as the underlying mechanisms are largely unknown. However, Bartonella research is hampered by the fact that the bacteria are slow growing and no sufficient liquid medium is available. With the recently described “BAPGM”-medium [34] or our new liquid medium [44] new promising tools for liquid cultivation of Bartonella spp. now exist. Mainly two pathogenicity factors of Bartonella spp. have been investigated in the past: (i) the so-called “VirB/D4 type-4 secretion system” (T4SS), a molecular nanomachine injecting Bartonella effector proteins (Beps) into human host cells and (ii) the so-called “trimeric autotransporter adhesins” which Based on our own previous work, we favour a “two-step”pathogenicity model suggesting that host-cell-derived vasculoproliferative factors play a crucial role in the pathogenesis 277 EJCAP - Vol. 18 - Issue 3 December 2008 of neoangiogenesis in Bartonella infections. The resulting proliferation of endothelia might be understood as bacteriumtriggered promotion of its own niche: the endothelial cell. These proliferating host cells promote persistence and growth of B. henselae by supplying the bacterium with yet unknown, possibly proteinacous compounds [27]. It is tempting to speculate that by affecting host-cell metabolism, B. henselae creates its own endothelial habitat [26]. [9] CHOMEL (B.B.), KASTEN (R.W.), FLOYD-HAWKINS (K.), CHI (B.), YAMAMOTO (K.), ROBERTS-WILSON (J.), GURFIELD (A.N.), ABBOTT (R.C.), PEDERSEN (N.C.), KOEHLER (J.E.) - Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol, 1996, 34:1952-6. [10] CHOMEL (B.B.), MAC DONALD (K.A.), KASTEN (R.W.), CHANG (C.C.), WEY (A.C.), FOLEY (J.E.), et al - Aortic valve endocarditis in a dog due to Bartonella clarridgeiae. J Clin Microbiol, 2001, 39:3548-54. [11] CHOMEL (B.B.), WEY (A.C.), KASTEN (R.W.) - Isolation of Bartonella washoensis from a dog with mitral valve endocarditis. J Clin Microbiol, 2003, 41:5327-32. [12] COTTE (V.), BONNET (S.), LE RHUN (D.), LE NAOUR (E.), CHAUVIN (A.), BOULOUIS (H.J.), et al - Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis, 2008, 14:1074-80. [13] DEHIO (C.) - Bartonella-host-cell interactions and vascular tumour formation. Nat Rev Microbiol, 2005, 3:621-31. [14] DINIZ (P.P.), MAGGI (R.G.), SCHWARTZ (D.S.), CADENAS (M.B.), BRADLEY (J.M.), HEGARTY (B.), et al - Canine bartonellosis: serological and molecular prevalence in Brazil and evidence of coinfection with Bartonella henselae and Bartonella vinsonii subsp. berkhoffii. Vet Res, 2007, 38:697-710. [15] DRANCOURT (M.), TRAN-HUNG (L.), COURTIN (J.), LUMLEY (H.), RAOULT (D.) - Bartonella quintana in a 4000-year-old human tooth. J Infect Dis, 2005, 191:607-11. [16] DROZ (S.), CHI (B.), HORN (E.), STEIGERWALT (A.G.), WHITNEY (A.M.), BRENNER (D.J.) - Bartonella koehlerae sp. nov., isolated from cats. J Clin Microbiol, 1999, 37:1117-22. [17] DUNCAN (A.W.), MAGGI (R.G.), BREITSCHWERDT (E.B.) - A combined approach for the enhanced detection and isolation of Bartonella species in dog blood samples: pre-enrichment liquid culture followed by PCR and subculture onto agar plates. J Microbiol Methods, 2007, 69:273-81. [18] DUNCAN (A.W.), MAGGI (R.G.), BREITSCHWERDT (E.B.) Bartonella DNA in dog saliva. Emerg Infect Dis, 2007, 13:194850. [19] DUNCAN (A.W.), MARR (H.S.), BIRKENHEUER (A.J.), MAGGI (R.G.), WILLIAMS (L.E.), CORREA (M.T), et al - Bartonella DNA in the blood and lymph nodes of Golden Retrievers with lymphoma and in healthy controls. J Vet Intern Med. 2008, 22:89-95. [20] EREMEEVA (M.E.), GERNS (H.L.), LYDY (S.L.), GOO (J.S.), RYAN (E.T.), MATHEW (S.S.), et al - Bacteremia, fever, and splenomegaly caused by a newly recognized Bartonella species. N Engl J Med, 2007, 356:2381-7. [21] FENOLLAR (F.), SIRE (S.), RAOULT (D.) - Bartonella vinsonii subsp. arupensis as an agent of blood culture-negative endocarditis in a human. J Clin Microbiol, 2005, 43:945-7. Erratum in: J Clin Microbiol, 2005, 43:4923. [22] GARY (A.T.), WEBB (J.A.), HEGARTY (B.C.), BREITSCHWERDT (E.B.) - The low seroprevalence of tick-transmitted agents of disease in dogs from southern Ontario and Quebec. Can Vet J, 2006, 47:1194-200. [23] GILLESPIE (T.N.), WASHABAU (R.J.), GOLDSCHMIDT (M.H.), CULLEN (J.M.), ROGALA (A.R.), BREITSCHWERDT (E.B.) Detection of Bartonella henselae and Bartonella clarridgeiae DNA in hepatic specimens from two dogs with hepatic disease. J Am Vet Med Assoc, 2003, 222:47-51, 35. [24] JACKSON (L.A.), PERKINS (B.A.), WENGER (J.D.) - Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health, 1993, 83:1707-11. [25] KELLY (P.), ROLAIN (J.M.), MAGGI (R.), SONTAKKE (S.), KEENE (B.), HUNTER (S.), et al - Bartonella quintana endocarditis in dogs. Emerg Infect Dis, 2006, 12:1869-72. [26] KEMPF (V.A.), HITZIGER (N.), RIESS (T.), AUTENRIETH (I.B.) Do plant and human pathogens have a common pathogenicity Conclusion Bartonella spp. are important pathogens in human and veterinary medicine. B. henselae is currently considered to be the most relevant zoonotic pathogenic Bartonella species responsible for CSD, bacillary angiomatosis and other diseases. Besides B. henselae, several other Bartonella species have been isolated from cats, dogs and humans with a variety of clinical manifestations, from symptomless to severe disease. The ability to cause vasculoproliferative disorders and intraerythrocytic bacteraemia are unique features of the genus Bartonella. Obviously, the VirB/D4 T4SS and BadA are key virulence factors of B. henselae responsible for host-cell infection, inhibition of endothelial cell apoptosis and induction of angiogenic gene programming. Elucidating the course of Bartonella infections in animals, arthropods and humans will help to understand and thus possibly prevent such infections in future. Taking into account the current knowledge about Bartonella infections, their zoonotic nature and the transmission risk by arthropods, it can be assumed that vector control strategies reduce the ectoparasite – host interactions thus limiting pathogen transmission. References [1] BARNES (A.), BELL (S.C.), ISHERWOOD (D.R.), BENNETT (M.), CARTER (S.D.) - Evidence of Bartonella henselae infection in cats and dogs in the United Kingdom. Vet Rec, 2000, 147:673-7. Comment in: Vet Rec, 2001, 148:219. [2] BERGH (K.), BEVANGER (L.), HANSSEN (I.), LØSETH (K.) - Low prevalence of Bartonella henselae infections in Norwegian domestic and feral cats. APMIS, 2002, 110:309-14. [3] BOULOUIS (H.J.), CHANG (C.C.), HENN (J.B.), KASTEN (R.W.), CHOMEL (B.B.) - Factors associated with the rapid emergence of zoonotic Bartonella infections. Vet Res, 2005, 36:383-410. [4] BREITSCHWERDT (E.B.), ATKINS (C.E.), BROWN (T.T.), KORDICK (D.L.), SNYDER (P.S.) - Bartonella vinsonii subsp. berkhoffii and related members of the alpha subdivision of the Proteobacteria in dogs with cardiac arrhythmias, endocarditis, or myocarditis. J Clin Microbiol, 1999, 37:3618-26. [5] BREITSCHWERDT (E.B.), KORDICK (D.L.), MALARKEY (D.E.), KEENE (B.), HADFIELD (T.L.), WILSON (K.) - Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol, 1995, 33:154-60. [6] BREITSCHWERDT (E.B.), MAGGI (R.G.), NICHOLSON (W.L.), CHERRY (N.A.), WOODS (C.W.) - Bartonella sp. bacteremia in patients with neurological and neurocognitive dysfunction. J Clin Microbiol, 2008, 46:2856-61. [7] BROUQUI (P.), LASCOLA (B.), ROUX (V.), RAOULT (D.) - Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med, 1999, 340:184-9. [8] CHANG (C.C.), CHOMEL (B.B.), KASTEN (R.W.), ROMANO (V.), TIETZE (N.) - Molecular evidence of Bartonella spp. in questing adult Ixodes pacificus ticks in California. J Clin Microbiol, 2001, 39:1221-6. 278 The role of Bartonella spp. in veterinary and human medicine - V. A. J. Kempf & F. Krämer strategy? Trends Microbiol, 2002, 10:269-75. [27] KEMPF (V.A.), SCHALLER (M.), BEHRENDT (S.), VOLKMANN (B.), AEPFELBACHER (B.), CAKMAN (I.), et al - Interaction of Bartonella henselae with endothelial cells results in rapid bacterial rRNA synthesis and replication. Cell Microbiol, 2000, 2:431-41. [28] KERET (D.), GILADI (M.), KLETTER (Y.), WIENTROUB (S.) - Catscratch disease osteomyelitis from a dog scratch. J Bone Joint Surg Br, 1998, 80:766-7. [29] KITCHELL (B.E.), FAN (T.M.), KORDICK (D.), BREITSCHWERDT (E.B.), WOLLENBERG (G.), LICHTENSTEIGER (C.A.) - Peliosis hepatis in a dog infected with Bartonella henselae. J Am Vet Med Assoc, 2000, 216:519-23, 517. [30] KORDICK (D.L.), SWAMINATHAN (B.), GREENE (C.E.), WILSON (K.H.), WHITNEY (A.M.), O’CONNOR (S.), et al - Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int J Syst Bacteriol, 1996, 46:704-9. [31] LAPPIN, (M.) - Canine and feline vector-borne diseases with special emphasis on Bartonella spp. and Haemobartonella spp. Proceedings of the 1st Canine Vector-Borne Disease (CVBD) Symposium; 2006 Apr 18-20; Billesley, Alcester, UK. pp. 14-19. [32] LAPPIN (M.R.), BLACK (J.C.) - Bartonella spp. infection as a possible cause of uveitis in a cat. J Am Vet Med Assoc, 1999, 214:1205-7. [33] LAPPIN (M.R.), GRIFFIN (B.), BRUNT (J.), RILEY (A.), BURNEY (D.), HAWLEY (J.), et al - Prevalence of Bartonella species, haemoplasma species, Ehrlichia species, Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the blood of cats and their fleas in the United States. J Feline Med Surg, 2006, 8:85-90. [34] MAGGI (R.G.), DUNCAN (A.W.), BREITSCHWERDT (E.B.) - Novel chemically modified liquid medium that will support the growth of seven Bartonella species. J Clin Microbiol, 2005, 43:2651-5. [35] MÄNDLE (T.), EINSELE (H.), SCHALLER (M.), NEUMANN (D.), VOGEL (W.), AUTENRIETH (I.B.), et al - Infection of human CD34+ progenitor cells with Bartonella henselae results in intraerythrocytic presence of B. henselae. Blood 2005, 106:1215-22. [36] MICHAU (T.M.), BREITSCHWERDT (E.B.), GILGER (B.C.), DAVIDSON (M.G.) - Bartonella vinsonii subspecies berkhoffi as a possible cause of anterior uveitis and choroiditis in a dog. Vet Ophthalmol, 2003, 6:299-304. [37] NUTTER (F.B.), DUBEY (J.P.), LEVINE (J.F.), BREITSCHWERDT (E.B.), FORD (R.B.), STOSKOPF (M.K.) - Seroprevalences of antibodies against Bartonella henselae and Toxoplasma gondii and fecal shedding of Cryptosporidium spp, Giardia spp, and Toxocara cati in feral and pet domestic cats. J Am Vet Med Assoc, 2004, 225:1394-8. [38] PAPPALARDO (B.L.), BROWN (T.), GOOKIN (J.L.), MORRILL (C.L.), BREITSCHWERDT (E.B.) - Granulomatous disease associated with Bartonella infection in 2 dogs. J Vet Intern Med, 2000, 14:37-42. [39] PAPPALARDO (B.L.), BROWN (T.T.), TOMPKINS (M.), BREITSCHWERDT (E.B.) - Immunopathology of Bartonella vinsonii (berkhoffii) in experimentally infected dogs. Vet Immunol Immunopathol, 2001, 83:125-47. [40] PINNA PARPAGLIA (M.L.), MASU (G.), MASALA (G.), PORCU (R.), ZOBBA (R.), PINTORI (G.), et al - Seroprevalence of Bartonella henselae in dogs and cats in Sassari. Vet Res Commun, 2007, 31 Suppl 1:317-20. [41] RELMAN (D.A.), LOUTIT (J.S.), SCHMIDT (T.M.), FALKOW (S.), TOMPKINS (L.S.) - The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med, 1990, 323:1573-80. [42] RIDDER (G.J.), BOEDEKER (C.C.), TECHNAU-IHLING (K.), GRUNOW (R.), SANDER (A.) - Role of cat-scratch disease in lymphadenopathy in the head and neck. Clin Infect Dis, 2002, 35:643-9. [43] RIESS (T.), ANDERSSON (S.G.), LUPAS (A.), SCHALLER (M.), SCHÄFER (A.), KYME (P.), et al - Bartonella adhesin A mediates a proangiogenic host cell response. J Exp Med, 2004, 200:126778. [44] RIESS (T.), DIETRICH (F.), SCHMIDT (K.V.), KAISER (P.O.), SCHWARZ (H.), SCHÄFER (A.), KEMPF (V.A.) - Analysis of a novel insect-cell culture medium-based growth medium for Bartonella species. Appl Environ Microbiol, 2008, 74:5224-7. [45] ROLAIN (J.M.), FOUCAULT (C.), BROUQUI (P.), RAOULT (D.) Erythroblast cells as a target for Bartonella quintana in homeless people. Ann NY Acad Sci, 2003, 990:485-7. [46] ROLAIN (J.M.), FRANC (M.), DAVOUST (B.), RAOULT (D.) Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerg Infect Dis, 2003, 9:338-42. [47] SAUNDERS (G.K.), MONROE (W.E.) - Systemic granulomatous disease and sialometaplasia in a dog with Bartonella infection. Vet Pathol, 2006, 43:391-2. [48] SCHÜLEIN (R.), SEUBERT (A.), GILLE (C.), LANZ (C.), HANSMANN (Y.), PIEMONT (Y.), et al - Invasion and persistent intracellular colonization of erythrocytes. A unique parasitic strategy of the emerging pathogen Bartonella. J Exp Med, 2001, 193:1077-86. [49] SOLANO-GALLEGO (L.), BRADLEY (J.), HEGARTY (B.), SIGMON (B.), BREITSCHWERDT (E.) - Bartonella henselae IgG antibodies are prevalent in dogs from southeastern USA. Vet Res, 2004, 35:585-95. [50] SOLANO-GALLEGO (L.), HEGARTY (B.), ESPADA (Y.), LLULL (J.), BREITSCHWERDT (E.) - Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Vet Microbiol, 2006, 118:274-7. [51] SOLANO-GALLEGO (L.), LLULL (J.), OSSO (M.), HEGARTY (B.), BREITSCHWERDT (E.) - A serological study of exposure to arthropod-borne pathogens in dogs from northeastern Spain. Vet Res, 2006, 37:231-44. [52] SPACH (D.H.), KANTER (A.S.), DOUGHERTY (M.J.), LARSON (A.M.), COYLE (M.B.), BRENNER (D.J.), et al - Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med, 1995, 332:424-8. [53] TSUKAHARA (M.), TSUNEOKA (H.), IINO (H.), OHNO (K.), MURANO (I.) - Bartonella henselae infection from a dog. Lancet, 1998, 352:1682. [54] ZHANG (P.), CHOMEL (B.B.), SCHAU (M.K.), GOO (J.S.), DROZ (S.), KELMINSON (K.L.), et al - A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc Natl Acad Sci USA, 2004, 101:13630-5. 279 ZOONOSES Meticillin-resistant staphylococci in companion animals T. Nuttall(1), N. Williams(2), R. Saunders(1), S. Dawson(2) SUMMARY Meticillin resistant (MR) Staphylococcus aureus (MRSA), S. intermedius/pseudintermedius, S. schleiferi coagulans, S. schleiferi schleiferi and other coagulase negative staphylococci have been associated with infections and colonisation in many animal species. Dogs and cats are usually colonised with the most prevalent human MRSA isolates, whereas other animal species are colonised with clone types unique to those species. MR staphylococci are resistant to β-lactams, cefalosporins, fluoroquinolones and clindamycin. They are frequently sensitive to tetracyclines, sufonamides, fucidic acid and mupirocin, but multi-drug resistant S. intermedius and S. pseudintermedius have been identified. The prevalence in healthy animals appears to be low. Risk factors for infection include repeated courses of broad spectrum antibacterials, surgery, joint infections, non-healing wounds and catheterisation. Most infected animals survive, provided that the primary condition can be managed. It is likely that there is two-way exchange of MR staphylococci between humans and animals. Animals are therefore at risk of colonisation from contact with veterinary staff and premises, and colonised animals may act as reservoirs for colonisation of in-contact humans. True zoonotic infections remain rare and are associated with defined risk factors. Antibacterial use guidelines may limit the development and spread of multi-drug resistant bacteria. Strict hygienic precautions will help prevent contamination and dissemination in veterinary clinics. MRSA in humans This paper was commissioned by FECAVA MRSA is of little concern to healthy people. Approximately 2030% of people are colonised with meticillin sensitive S. aureus (MSSA) but probably less than 1% carry MRSA. The carriage rate in healthcare workers, however, may be 5-10%. Veterinary personnel have also been shown to have higher carriage rates than the general population [1-4] . for publication in EJCAP. Introduction MRSA (meticillin resistant Staphylococcus aureus) and other meticillin resistant (MR) staphylococci are major medical and veterinary health care concerns. MRSA and other meticillin resistant staphylococcal species have been isolated from dogs, cats, rabbits, birds, horses and farm animals. Animals could be at risk of colonisation or infection in veterinary premises and/or act as reservoirs for colonisation or infection of in-contact humans. Colonisation in this context refers to the non-pathogenic temporary (also referred to as ‘contamination’) or persistent (or ‘carriage’) presence of MR staphylococci at mucosal surfaces or on the skin. The aim of this review is to give veterinary clinicians and nurses or technicians an overview of the current state of MR staphylococci in companion animals, and an update on the practical and clinical aspects of controlling these organisms. There has been a dramatic increase in MRSA infections in recent years, especially in patients who have skin or mucosal barrier defects, are immuno-compromised, undergo extensive surgery or are long term in-patients. MRSA clones can be differentiated by several techniques including pulsed field gel electrophoresis (PFGE), multi-locus sequence typing (MLST) and antibiotic resistance patterns. In the UK, the majority are hospital acquired (HA) epidemic (E) strains 15 and 16 [5]. EMRSA-15 is resistant to -lactams, fluoroquinolones and macrolides, whilst EMRSA-16 can be resistant to further antimicrobials. EMRSA-15 is also prevalent elsewhere in Europe. Based on blood culture isolates, the prevalence of MRSA infections varies greatly across Europe, 1) Department of Veterinary Clinical Science University of Liverpool Faculty of Veterinary Science, Leahurst Campus, Neston, Cheshire, UK. 2) Department of Veterinary Pathology, National Centre for Zoonosis Research, Leahurst Campus, Neston, Cheshire, UK. Corresponding author: Dr Tim Nuttall. The University of Liverpool Small Animal Teaching Hospital, Leahurst Campus, Neston, Cheshire, CH64 7TE, UK. Tel. - +44 151 795 6216. Email. timn@liv.ac.uk 280 Meticillin-resistant Staphylococci in companion animals - T. Nuttall, N. Williams, R. Saunders, S. Dawson Canada and the USA (Weese personal communication). The PVL gene has also been detected in 50% of US MRSA isolates from animals [31]. The majority of isolates from horses, rabbits and other animals in the UK, Ireland, Austria, Germany, Japan, US and Canada, in contrast, are largely restricted to these animal species and are only rarely found in the human population [14, 16, 20, 23, 32, 33]. Equine isolates are predominantly identical or closely related to Canadian MRSA-5 (USA500), which is rare in the general human population but has been isolated from in-contact humans [32, 34]. Fig. 1 70% alcohol hand rub worn on a nurse’s uniform. All our staff and students wear these for quick and easy hand disinfection between patients. Organic debris must be washed off with a detergent though. An MRSA type identified as sequence type (ST) 398 by multilocus sequence typing has come to prominence because of its association with pig farming, farmers and veterinarians in the Netherlands and Germany [21, 35, 36]. ST398 has also been isolated from horses in Germany and Canada, and neonatal humans in Scotland. from <1% in Scandinavia and the Netherlands to >40% in southern and western Europe [6]. MR S. intermedius/pseudintermedius MR has also been seen in other staphylococci more commonly associated with animals, particularly S. intermedius [37-39], although it is now thought that most, if not all, canine isolates are actually S. pseudintermedius [40-42]. In a recent Japanese study, 17/18 MR staphylococcal isolates from dogs were S. pseudintermedius [41]. In Germany, 12 identical or closely related S. intermedius strains, resistant to at least five antimicrobial classes including penicillins, cefalosporins and fluoroquinolones, were isolated from skin and ear infections in 11 dogs and one cat [43]. Community acquired (CA) clones are genetically distinct from HAMRSA clones [7]. CA-MRSA are usually isolated from individuals without healthcare contact or other risk factors. Infections are rare and sporadic but can be severe and life-threatening. Antibiotic resistance is less marked but CA-MRSA infections are often associated with Panton Valentine Leucocidin (PVL). PVL can result in extensive tissue necrosis and severe disease [8]. The designations HA and CA do not necessarily refer to the source of colonisation or infection: HA-MRSA can be acquired in the community [9] and CA-MRSA clones have been associated with hospital acquired infections [10]. Other MR staphylococci in animals Other MR staphylococci isolated from animals include the coagulase positive species (CPS) S. schleiferi subsp. coagulans, particularly in the US [37-39], and the coagulase negative staphylococci (CNS) S. schleiferi subsp. schleiferi, S. haemolyticus, S. vitulinus, S. sciuri, S. epidermidis, and S. warneri [44]. CNS area usually regarded as non-pathogenic, commensal species, as they lack many of the virulence factors associated with CPS. CNS, particularly S. schleiferi subsp. schleiferi, have nevertheless been isolated from pyoderma and wound infections in animals [38, 39, 45, 46], although it can be difficult to determine their importance in mixed infections. Commensal CNS will also be exposed to antibacterials, and could act as reservoirs of mobile genetic elements encoding for antimicrobial resistance (see below). Further research on the clinical relevance of CNS and the impact of antibacterials on commensal bacteria is therefore warranted. MR staphylococci in animals MR staphylococci have been isolated from healthy and diseased individuals in many animal species [11-21]. The epidemiology varies between staphylococcal isolates, animal species and geographic location, reflecting differences in staphylococcal species, animal-human interaction, antimicrobial use, and species susceptibility. MRSA in companion animals MRSA has been the most frequent MR staphylococcal species isolated from animals in the UK, Ireland and Canada [14-16, 19, 22, 23]. British, Irish and German small animal isolates are mostly identical or closely related to EMRSA-15 [14, 16, 23-25]. EMRSA-16 has also been isolated from dogs in the UK, but is less common [26]. In contrast, canine isolates unrelated to human clones have been occasionally identified in the UK, the Netherlands and Germany [20, 27, 28]. Origins and dissemination of MR staphylococci Several studies have associated identical or very closely related MR staphylococcal isolates with particular veterinary premises in the UK, Denmark, Austria, Germany, Ireland, Japan and Canada [22, 23, 33, 35, 41, 43, 47, 48]. The genetic diversity of MR S. pseudintermedius and intermedius isolates suggests that colonisation can be acquired either in veterinary clinics or In the US and Canada the predominant human strain, Canadian epidemic MRSA-2, (USA100) is most common in animals [15, 29, 30]. More recently PVL-positive Canadian epidemic MRSA10 (USA-300) has been isolated from small animals in both 281 EJCAP - Vol. 18 - Issue 3 December 2008 Fig. 2 Fully waterproof and washable keyboard for use in clinical areas. in the community [41, 43], but the similarity of isolates from the staff and patients in particular premises suggests dissemination in veterinary clinics occurs. A US study of 27 veterinary teaching hospitals, however, could not associate specific clones with individual hospitals and concluded that colonisation was probably community acquired [49]. will be ineffective. Third line drugs are those very important to humans and animals (e.g. fluoroquinlones, anti-Pseudomonas penicillins, ceftazidime, imipenem etc.). They should only be used where culture indicates they are necessary. In one study the adoption of antibacterial use guidelines lead to a significant fall in the use of second and third line drugs with first line drugs accounting for approximately 90% of treatment [53]. Antibacterial use and the emergence of MR staphylococci It is likely that antibacterials played a role in the emergence of MR staphylococci in animals. One study concluded that the prevalence of MR-CNS in companion animals reflected national and local patterns of antimicrobial use [47]. A significantly higher prevalence of resistance to two or more antibacterials was reported in S. intermedius isolates from dogs and domestic pigeons compared to those from wild birds [50]. Prior use of antibacterials is also a risk factor for MRSA infection in humans and horses [18, 51], and recent studies by the authors and others have shown that courses of broad-spectrum antibacterials are a risk factor for MRSA infection in dogs. The prevalence of MR staphylococci in companion animals Colonisation in healthy animals It is likely that the colonisation rate in healthy animals is low. One Slovenian study did not isolate any MR-CPS from 200 healthy dogs [46]. A UK study failed to isolate MRSA from dogs sampled in the community over a two month period [16], and only 1/255 dogs was positive for MRSA in another [54]. Rational and responsible use of antibacterials Measures to minimise the development and spread of antimicrobial resistant bacteria should be adopted [52]. It is beyond the scope of this article to discuss these in detail, but issues to address include: Colonisation and infection in sick animals The colonisation rate in non-healthy animals seems to be much higher, although not all reports distinguish colonisation and infection. One UK study found that MRSA was carried by up to 10% of dogs referred to a small animal veterinary hospital without MRSA infections [24], although these had received treatment including antibacterials prior to referral. MRSA was also isolated from approximately 3% of clinical submissions to a UK veterinary diagnostic laboratory [25]. In Ireland, MRSA was recovered from 25 animals (14 dogs, eight horses, one cat, one rabbit and a seal) and from 10 in-contact veterinary personnel [14]. Of 869 submissions from a German small animal hospital over a 20 month period, 3% (approximately 0.05% of the total caseload) were MRSA [20]. Similar proportions of samples were from dogs (3%) and cats (2.7%), with MRSA also isolated from a bird, rabbit, guinea pig, turtle and bat. In the authors’ institution MR-CPS infections comprised 0.04% of all cases and 0.5% of all bacterial infections from 2004-2007. Twelve MR S. intermedius isolates identified in one report accounted for 23% of all S. intermedius submissions from a German dermatology referral clinic during an 18 month period [43]. MR S. pseudintermedius, S. aureus and S. schleiferi coagulans were isolated from 2.1%, 0.5% and 0.5% respectively of 193 dogs referred to a Canadian Veterinary Teaching Hospital [55]. – Ensuring that the patient has a bacterial infection – Ensuring that the infection warrants systemic or topical antibacterial therapy – Selecting the most appropriate drug – Using an efficacious dose for an adequate treatment period – Maximising compliance It can be helpful to divide antibacterials into first, second and third line drugs, although this will depend on the patient, target tissue and type of bacteria. First line drugs include older antibacterials and/or narrow spectrum drugs such as simple penicillins, tetracyclines, and sulfonamides. These are no less potent than other antibacterials in the appropriate circumstances. Second line drugs include newer, broad spectrum products that are more important for humans and animals and/or more prone to resistance (e.g. broad spectrum β-lactamase resistant penicillins, cefalosporins and macrolides). These should be used where culture or good empirical evidence indicates that first line drugs 282 Meticillin-resistant Staphylococci in companion animals - T. Nuttall, N. Williams, R. Saunders, S. Dawson MR staphylococcal infections in animals There is probably little risk to healthy animals but vulnerable pets could include immunocompromised animals, long-term inpatients, and animals with open wounds, implants and major surgery. A wide variety of skin, soft-tissue and other infections have been reported [13, 24, 43]. In the authors’ clinic MRSA infections are significantly more likely to be associated with post-operative infections and non-healing wounds compared to meticillin sensitive CPS infections, whereas primary skin, ear and soft-tissue infections were less common. In US reports, MR S. intermedius was more likely to be associated with superficial and first-line infections, whereas MR S. schleiferi were more common in recurrent infections following antimicrobial therapy, and MRSA were associated with opportunistic soft-tissue infections and/or septicaemia [30, 38, 39]. One retrospective study, comparing infection with MRSA versus MSSA, showed the presence of a urinary catheter, joint infection and prior treatment with fluoroquinolones to be significant risk factors [59]. Fig. 3 Tape-strip impression smear from a staphylococcal pyoderma. Note the degenerate neutrophils with intra- and extracellular cocci. Diff-Quik stain, magnification x 400. A prevalence study at a UK referral hospital isolated MRSA from 6% of staff and 6% of dogs (2/3 had MRSA infections) but no cats [16, 56]. A single day prevalence study at another UK referral hospital found that 18% of staff, 9% of dogs, 10% of environmental samples and again no cats were colonised by MRSA [24]. In Japan, MR-CPS were isolated from 15% of staff, 46% of canine in-patients and 19% of canine out-patients [41]; 2/3 isolates from staff were MRSA, but only one of the isolates from the dogs was MRSA and the remaining 17 were MR S. pseudintermedius. There is very little data on risk factors for colonisation and infection in dogs and cats, but the following are currently considered to be at risk: – Patients from known MRSA positive households or that belong to owners with recent or frequent healthcare contact. – Patients with non-healing wounds. – Patients with non-antibiotic responsive infections where previous cytology and/or culture indicates that staphylococci are involved. – Nosocomial or secondary infections, especially in at-risk patients. In contrast, US studies have identified MR staphylococci at much higher frequency. They have been isolated from 15% of healthy cats and 38% of dogs with recurrent pyoderma; MR rates for S. aureus was 35%, 17% for S. intermedius and 40% for S. schleiferi [37]. More recently, however, the reported prevalence has been lower with MR staphylococci isolated from 15% dogs with inflammatory skin disease (MR in 17% of S. aureus, 8% of S. intermedius, 20% of S. schleiferi schleiferi and 20% of S. schleiferi coagulans) and 5% of healthy dogs (MR in 0% of S. aureus, 3% of S. intermedius, 50% of S. schleiferi coagulans) [57]. In another report, 20% of 1,772 staphylococci were MR. These comprised 15.6% of the S. intermedius, 46.6% of the S. schleiferi and 23.5% of the S. aureus isolates [58]. A six month surveillance study of 27 veterinary teaching hospitals identified 65 S. aureus isolates, of which 13% were MRSA [49]. Detection of MR staphylococcal infections Any Staphylococcus with unusual (e.g. clavulanate potentiated amoxicillin, cefalosporin or fluoroquinolone) antibacterial resistance is suspicious, and resistance to oxacillin and identification of the mecA gene is definitive. It is important to use a reputable laboratory as some techniques overestimate resistance and not using enrichment may miss 8-13% of MR isolates (M. Rich, personal communication). Further genetic typing to identify the strain is important in epidemiology. Swabs should be taken from clinical lesions and implants as appropriate in each case. Swabs should be taken from both the nose and perineum, as single swabs miss 29-56% of dogs colonised with CPS (J. Fazakerley, accepted for publication). Nosocomial and community acquired colonisation Very few studies have differentiated nosocomial colonisation from animals colonised at admission. One study found that the rate of community-associated colonisation in horses (27 per 1,000 admissions) was comparable to that of nosocomial colonisation (23 per 1,000 admissions) [18], although horses colonized at admission were significantly more likely to develop clinical MRSA infections. A survey of three large UK equine veterinary hospitals, in contrast, could not detect any MRSA on admission or discharge [56]. Treatment MR staphylococci are usually resistant to all penicillins, cefalosporins and fluoroquinolones [60]. Most MRSA isolated from animals also appear to have inducible clindamycin resistance [25]. Tetracyclines, trimethoprim-sulfonamides, fucidic acid and mupirocin are often effective. Recently, however, S. pseudintermedius and S.intermedius isolates resistant to five or more classes of antibiotics including -lactams, cefalosporins, fluoroquinlones, macrolides, trimethoprim-sulfonamides, fucidic acid and mupirocin have been reported [41, 43]. 283 EJCAP - Vol. 18 - Issue 3 December 2008 Antibiotics should be chosen following bacterial culture and sensitivity tests. These are, however, less reliable in predicting the response to topical antibiotics as direct application can achieve local concentrations in excess of the minimum inhibitory concentration break points established for systemic therapy. For example, of 11 multi-drug resistant S. intermedius in one study, seven responded to a combination of systemic and topical antibiotics, and four to topical therapy only [43]. – Before surgery, it may be possible to decontaminate the patient (see above). – Covering wounds with impermeable dressings and preventing licking (using collars etc.) may help reduce wound infection. Routine measures to prevent the spread of MRSA (and other infectious diseases) 1. Hand washing and disinfection of surfaces and equipment between patients. Alcohol gel pouches on uniforms and kennels are a visual cue and can be quickly used immediately after handling an animal. Remember that alcohol will not be effective in the presence of organic matter - this must be removed by hand washing with a detergent. 2. Avoid materials at hand touch sites that cannot be cleaned use washable keyboards or keyboard covers etc. Alcohol wipes are effective and easy to use on equipment and keyboards, but soiled surfaces must be cleaned with a detergent. 3. Cover wounds or skin lesions with waterproof dressings. 4. High standards of aseptic technique: minimise theatre staff; sterile gowns, gloves, hats and masks; sterilisation of equipment; and single patient use. 5. High standards of cleaning: clean cages and bedding at least once daily, and thoroughly clean and disinfect between patients. Antimicrobial impregnated materials have not been shown to prevent colonisation in clinical settings. The authors recently found that no difference in bacterial contamination of standard and tolnaftate/triclosan impregnated veterinary bedding material. 6. Segregation of all waste and contaminated material. 7. Ensure that all staff understand and adhere to infection control. It is most important to identify the underlying cause. Further treatment depends on the nature of the primary problem (e.g. removing implants and or the use of gentamicin impregnated beads, collagen sponges, activated silver dressings etc.) [13]. The majority of animals with MR staphylococcal infections survive, and in about half of the fatal cases, death is attributable to the underlying disease. In the authors’ clinic the mortality rate with MR staphylococcal infections is comparable to that in meticillin sensitive CPS infections. Discharged patients and decolonisation Patients should be discharged as soon as they are clinically fit. If the animal remains colonised potential risks and precautions must be discussed with the owner. Decolonisation of humans often involves repeated cycles of intranasal fucidic acid or mupirocin and chlorhexidine washes. Treatment clears 91-99% of patients but re-colonisation rates are up to 26%. Experience suggests that dogs require longer courses of treatment of 2-3 weeks. At present, non-antibiotic decolonisation methods are preferred. Hygiene, cleanliness and removal from the source are most important and effective. Preventing contamination with MR staphylococci in veterinary clinics Monitoring and surveillance This is a very controversial and no figures for acceptable microbiological levels in medical or veterinary premises have been established. If surveillance is used, it is essential that it is conducted following advice, is done with a reputable laboratory and that the policy has clear aims. Admission Admit known or suspected cases directly into a consultation room to avoid contamination and contagion in the waiting room. To minimise contact and contamination procedures should be scheduled for the end of the day, discharging wounds covered with an impermeable dressing and trolleys used to move patients. Surfaces and equipment should be cleaned and disinfected before they are used again. Fig. 4 MRSA wound infection. Hospitalisation – Isolate MRSA patients as far as possible from other patients. – Use disposable gloves, gowns, hats and mask. Long hair should be tied back and sleeves rolled up to the elbow. Eye protection should be worn if there is a risk of splashing or aerosols. Overshoes, however, may actually increase contamination (presumably from having to balance and handle shoes). – Strict washing of the hands and forearms after handling the patient. – Equipment should be used with the affected patient only and then disposed of or disinfected. – Bathing every 2-3 days with an antibacterial wash may reduce mucosal and cutaneous carriage, but may be not be possible with all individuals or species, increases staff or owner contact and could increase environmental aerosols. 284 Meticillin-resistant Staphylococci in companion animals - T. Nuttall, N. Williams, R. Saunders, S. Dawson Routine environmental screening could be used to monitor cleanliness – of 82-91% hospital surfaces judged to visually clean only 30-45% were microbiologically clean. MRSA contamination rates have declined where cleaners have been trained to think about microbiological cleanliness. One Canadian study isolated MRSA from 10% of samples (including tables, floors, telephones, keyboards, taps, kennels, stethoscopes and otoscopes) [61]. skin infection in three and nasal colonisation in 10 veterinary staff treating a foal with Canadian epidemic MRSA-5 [70]. Transmission occurred despite barrier nursing precautions and resulted in infection in healthy individuals without risk factors for opportunistic infection. References Surveillance of staff is highly controversial and involves problems with consent, confidentiality, stigmatisation and further action. Screening can also miss transiently contaminated staff, which may still be a source of infection if they fail to observe hygienic precautions. Screening should therefore be done to identify problems in infectious disease control, and not to apportion blame or as a substitute for control measures [62]. [1] [2] [3] Transmission of MRSA between animals and humans Recent studies suggest that up to 45% of owners of dogs with pyoderma can be colonised by S. intermedius [63] and that concurrent human-animal colonisation occurs in 20% of S. aureus and 67% of S. intermedius positive households [64]. MRSA has also been isolated from up to 17% of veterinary staff and 11% of owners in contact with infected animals, compared to 5% and 0% in contact with MSSA infected animals (Annette Loeffler, personal communication). In another recent case, identical PVL-positive MRSA strains were isolated from three humans and one dog in a household [65]. MRSA colonisation has also been found in 1/1 cats and 16/88 humans in contact with eight dogs and three cats with MRSA infections [29]. [4] MRSA colonization may be an occupational risk for veterinary staff. MRSA has been isolated from the nares of 7% of vets and 12% of nurses sampled at a North American veterinary congress [1] and 10% of vets and nurses sampled at a British congress [2]. At the American meeting, working in large animal practice was significantly associated with colonisation. The pattern of colonisation reflected the strains most commonly seen in animals; Canadian epidemic MRSA-2 was isolated from 11 small animal but only two large animal personnel, whereas Canadian epidemic CMRSA-5 was only isolated from large animal clinicians [1]. In the Netherlands, 4.6% of veterinary staff working with pigs were found to be colonised with porcine-associated MRSA strains that are otherwise rare in the human population [3]. Another study at an equine meeting isolated MRSA from 10% of the participants [4]. Contact with an MRSA patient increased the risk of MRSA colonisation, whereas hand washing between patients and farms was protective. Work conducted by the authors found a prevalence of 8% in veterinary personnel attending a British equine congress. Approximately half the strains were typical of those seen common in small companion animals, with the other halve typical of equine associated strains, which are less common in the human population. [7] [5] [6] [8] [9] [10] [11] [12] [13] MRSA colonised animals can be reservoirs for re-colonisation of in-contact humans [65-69]. True zoonotic infections, however, are rare and sporadic, and associated with accepted risk factors for opportunistic infection. There is a report, however, describing [14] 285 HANSELMAN (B.), ROUSSEAU (J.), KRUTH (S.), WEESE (J.S.) Methicillin-resistant Staphylococcus aureus (MRSA) colonization in veterinary professionals. J Vet Int Med. 2006, 20: 761. WILLIAMS (N. J.), DEACON V.), PINCHBECK, G.), DAWSON (S). - A pilot survey of the prevalence of methicllin resistant Staphylococcus aureus (MRSA) and other methicillin resistant staphylococci (MRS) nasal carriage in small animal veterinary personnel. British Small Animal Veterinary Association; 2007, Birmingham. 501. WULF (M.), VAN NES (A.), EIKELENBOOM-BOSKAMP (A.), DE VRIES (J.), MELCHERS, (W.), KLAASSEN, (C.) et al. Methicillinresistant Staphylococcus aureus in veterinary doctors and students, the Netherlands. Emerg Infect Dis. 2006, 12: 1939-41. ANDERSON (M.E.C.), LEFEBVRE (S.L.), WEESE (J.S.) - Evaluation of prevalence and risk factors for methicillin-resistant Staphylococcus aureus colonization in veterinary personnel attending an international equine veterinary conference. Vet Microbiol. 2008, 129: JOHNSON (A.P.), AUCKEN (H.M.), CAVENDISH (S.), GANNER (M.), WALE (M.C.J.), WARNER (M.) et al. - Dominance of EMRSA-15 and-16 among MRSA causing nosocomial bacteraemia in the UK: analysis of isolates from the European Antimicrobial Resistance Surveillance System (EARSS). J Antimicrobial Chemo. 2001, 48: 143-4. TIEMERSMA (E.W.), BRONZWAER (S.L.A.M.), LYYTIKAINEN (O.), DEGENER (J.E.), SCHRIJNEMAKERS (P.), BRUINSMA (N.) et al. Methicillin-resistant Staphylococcus aureus in Europe, 1999-2002. Emerg Infect Dis. 2004, 10: 1627-34. HEROLD (B.C.), IMMERGLUCK (L.C.), MARANAN (M.C.), LAUDERDALE (D.S.), GASKIN (R.E.), BOYLE-VAVRA, (S.) et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. J Amer Med Assoc. 1998, 279: 593-8. HOLMES (A.), GANNER (M.), MCGUANE (S.), PITT (T.L.), COOKSON (B.D.), KEARNS (A.M.) - Staphylococcus aureus isolates carrying panton-valentine leucocidin genes in England and Wales: Frequency, characterization, and association with clinical disease. J Clin Microbiol. 2005, 43: 2384-90. COOKSON (B.D.) - Methicillin-resistant Staphylococcus aureus in the community: New battlefronts, or are the battles lost? Infection Control Hosp Epidemiol. 2000, 21: 398-403. PATEL (M.), KUMAR (R.A.), STAMM (A.M.), HOESLEY (C.J.), MOSER (S.A.), WAITES (K.B.) - USA300 genotype communityassociated methicillin-resistant Staphylococcus aureus as a cause of surgical site infections. J Clin Microbiol. 2007, 45: 3431-3. PAK (S.I.), HAN (H.R.), SHIMIZU (A.) - Characterization of methicillin-resistant Staphylococcus aureus isolated from dogs in Korea. J Vet Med Sci. 1999, 61: 1013-8. SEGUIN (J.C.), WALKER (R.D.), CARON (J.P.), KLOOS (W.E.), GEORGE (C.G.), HOLLIS (R.J.) et al. - Methicillin-resistant Staphylococcus aureus outbreak in a veterinary teaching hospital: potential human-to-animal transmission. J Clin Microbiol. 1999, 37: 1459-63. TOMLIN (J.), PEAD (M.J.), LLOYD (D.H.), HOWELL (S.), HARTMANN (F.), JACKSON (H.A.) et al. - Methicillin-resistant Staphylococcus aureus infections in 11 dogs. Vet Rec. 1999, 144: 60-4. O’MAHONY (R.), ABBOTT (Y.), LEONARD (F.C.), MARKEY (B.K.), QUINN (P.J.), POLLOCK (P.J.) et al. - Methicillin-resistant EJCAP - Vol. 18 - Issue 3 December 2008 [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] 26. [27] [28] [29] [30] Staphylococcus aureus (MRSA) isolated from animals and veterinary personnel in Ireland. Vet Microbiol. 2005, 109: 28596. WEESE (J.S.) - Methicillin resistant Staphylococcus aureus: an emerging pathogen in small animals. J Amer An Hosp Assoc. 2005, 41: 150-7. BAPTISTE (K.E.), WILLIAMS (K.), WILLAMS (N.J.), WATTRET (A.), CLEGG (P.D.), DAWSON (S.) et al. - Methicillin-resistant staphylococci in companion animals. Emerg Infect Dis. 2005, 11: 1942-4. STROMMENGER (B.), KEHRENBERG (C.), KETTLITZ (C.), CUNY (C.), VERSPOHL (J.), WITTE (W.) et al. - Molecular characterization of methicillin-resistant Staphylococcus aureus strains from pet animals and their relationship to human isolates. J Antimicrobial Chemo. 2006, 57: 461-5. WEESE (J.S.), ROUSSEAU (J.), WILLEY (B.M.), ARCHAMBAULT (M.), MCGEER (A.), LOW (D.E.) - Methicillin-resistant Staphylococcus aureus in horses at a veterinary teaching hospital: Frequency, characterization, and association with clinical disease. J Vet Int Med. 2006, 20: 182-6. LEONARD (F.C.), MARKEY (B.K.) - Meticillin-resistant Staphylococcus aureus in animals: A review. Vet J. 2008, 175: 2736. WALTHER (B.), WIELER (L.H.), FRIEDRICH (A.W.), HANSSEN (A.M.), KOHN (B.), BRURMBERG (L.) et al. - Methicillin-resistant Staphylococcus aureus (MRSA) isolated from small and exotic animals at a university hospital during routine microbiological examinations. VetMicrobiol. 2008, 127: 171-8. VAN DUIJKEREN (E.), HOUWERS (D.J.), SCHOORMANS (A.), BROEKHUIZEN-STINS (M.J.), IKAWATY (R.), FLUIT (A.C.) et al. Transmission of methicillin-resistant Staphylococcus intermedius between humans and animals. Vet Microbiol. 2008, 128: 213-5. LEONARD (F.C.), ABBOTT (Y.), ROSSNEY (A.), QUINN (P.I.), O’MAHONY (R.), MARKEY (B.K.) - Methicillin-resistant Staphylococcus aureus isolated from a veterinary surgeon and five dogs in one practice. Vet Rec. 2006, 158: 155-9. MOODLEY (A.), STEGGER (M.), BAGCIGIL (A.F.), BAPTISTE (K.E.), LOEFFLER (A.), LLOYD (D.H.) et al. - Spa typing of methicillinresistant Staphylococcus aureus isolated from domestic animals and veterinary staff in the UK and Ireland. J Antimicrobial Chemo. 2006, 58: 1118-23. LOEFFLER (A.), BOAG (A.K.), SUNG (J.), LINDSAY (J.A.), GUARDABASSI (L.), DALSGAARD (A.) et al. - Prevalence of methicillin-resistant Staphylococcus aureus among staff and pets in a small animal referral hospital in the UK. J Antimicrobial Chemo. 2005, 56: 692-7. RICH (M.) - Staphylococci in animals: Prevalence, identification and antimicrobial susceptibility, with an emphasis on methicillinresistant Staphylococcus aureus. Brit J Biomed Sci. 2005, 62: 98105. WALLER (A.) - The creation of a new monster: MRSA and MRSI - Important emerging veterinary and zoonotic diseases. Vet J. 2005, 169: 315-6. ENOCH (D.A.), KARAS (J.A.), STATER (J.D.), EMERY (M.M.), KEARNS (A.M.), FARRINGTON (M.) - MRSA carriage in a pet therapy dog. J Hosp Infect. 2005, 60: 186-8. VAN DUIJKEREN (E.), BOX (A.T.A.), HECK (M.E.O.C.), WANNET (W.J.B.), FLUIT (A.C.) - Methicillin-resistant staphylococci isolated from animals. Vet Microbiol. 2004, 103: 91-7. WEESE (J.S.), DICK (H.), WILLEY (B.M,. MCGEER (A.), KREISWIRTH (B.N.), INNIS (B.) et al. - Suspected transmission of methicillinresistant Staphylococcus aureus between domestic pets and humans in veterinary clinics and in the household. Vet Microbiol. 2006, 115: 148-55. MORRIS (D.O.), MAULDIN (E.A.), O’SHEA (K.), SHOFER (F.S.), RANKIN (S.C.) - Clinical, microbiological, and molecular [31] [32] [33] [34] [35] [36] [37] [38] [39] [40] [41] [42] [43] [44] [45] [46] 286 characterization of methicillin-resistant Staphylococcus aureus infections of cats. Amer J Vet Res. 2006, 67: 1421-5. RANKIN (S.), ROBERTS (S.), O’SHEA (K.), MALONEY (D.), LORENZO (M.), BENSON (C.E.) - Panton valentine leukocidin (PVL) toxin positive MRSA strains isolated from companion animals. Vet Microbiol. 2005, 108: 145-8. WEESE (J.S.), ROUSSEAU (J.), TRAUB-DARGATZ (J.L.), WILLEY (B.M.), MCGEER (A.J.), LOW (D.E.) - Community-associated methicillin-resistant Staphylococcus aureus in horses and humans who work with horses. J Amer Vet Med Assoc. 2005, 226: 580-3. CUNY (C.), KUEMMERLE (J.), STANEK (C.), WILLEY (B.), STROMMENGER (B.), WITTE (W.) - Emergence of MRSA infections in horses in a veterinary hospital: strain characterisation and comparison with MRSA from humans. Euro Surveill. 2006, 11: 44-7. WEESE (J.S.), ARCHAMBAULT (M.), WILLEY (B.M.), DICK (H.), HEARN (R.), KREISWIRTH (B.N.) et al. - Methicillin-resistant Staphylococcus aureus in horses and horse personnel, 20002002. Emerg Infect Dis. 2005, 11: 430-5. KHANNA (T.), FRIENDSHIP (R.), DEWEY (C.), WEESE (J.S.) Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet Microbiol. 2008, 128: 298-303. MEENIKEN (D.), CUNY (C.), WITTE (W.), EICHLER (U.), STAUDT (R.), BLAHA (T.) - Occurrence of MRSA in pigs and in humans involved in pig production - Preliminary results of a study in the Northwest of Germany. Deut Tierarzt Woch. 2008, 115: 132-9. MORRIS (D.O.), ROOK (K.A.), SHOFER (F.S.), RANKIN (S.C.) Screening of Staphylococcus aureus, Staphylococcus intermedius, and Staphylococcus schleiferi isolates obtained from small companion animals for antimicrobial resistance: a retrospective review of 749 isolates (2003-04). Vet Dermatol. 2006, 17: 332-7. FRANK (L.A.), KANIA (S.A.), HNILICA (K.A.), WILKES (R.P.), BEMIS (D.A.) - Isolation of Staphylococcus schleiferi from dogs with pyoderma. J Amer Vet Med Assoc. 2003, 222: 451-4. KANIA (S.A.), WILLIAMSON (N.L.), FRANK (L.A.), WILKES (R.P.), JONES (R.D.), BEMIS (D.A.) - Methicillin resistance of staphylococci isolated from the skin of dogs with pyoderma. Amer J Vet Res. 2004, 65: 1265-8. BANNOEHR (J.), BEN ZAKOUR (N.L.), WALLER (A.S.), GUARDABASSI (L.), VAN DEN BROEK (A.H.M.), THODAY (K.L.) et al. - Population genetic structure of the Staphylococcus intermedius group: insights into agr diversification and the emergence of methicillin-resistant strains. J Bacteriol. 2007, 8685-92. SASAKI (T.), KIKUCHI (K.), TANAKA (Y.), TAKAHASHI (N.), KAMATA, (S.), HIRAMATSU (K.) - Methicillin-resistant Staphylococcus pseudintermedius in a veterinary teaching hospital. J Clin Microbiol. 2007, 45: 1118-25. DEVRIESE (L.A.), VANCANNEYT (M.), BAELE (M.), VANEECHOUTTE (M.), DE GRAEF (E.), SNAUWAERT (C.) et al. - Staphylococcus pseudintermedius sp nov.), a coagulase-positive species from animals. Int J System Evo Microbiol. 2005, 55: 1569-73. LOEFFLER (A.), LINEK (M.), MOODLEY (A.), GUARDABASSI (L.), SUNG (J.M.L.), WINKLER (M.) et al. - First report of multiresistant, mecA-positive Staphylococcus intermedius in Europe: 12 cases from a veterinary dermatology referral clinic in Germany. Vet Dermatol. 2007, 18: 412-21. BUSSCHER (J.F.), VAN DUIJKEREN (E.), OLDRUITENBORGHOOSTERBAAN (M.M.S.) - The prevalence of methicillin-resistant staphylococci in healthy horses in the Netherlands. Vet Microbiol. 2006, 113: 131-6. KLOOS (W.E.), BANNERMAN (T.L.) - Update on clinical significance of coagulase-negative staphylococci. Clin Microbiol Rev. 1994, 7: 117-40. VENGUST (M.), ANDERSON (M.E.C.), ROUSSEAU (J.), WEESE (J.S.) - Methicillin-resistant staphylococcal colonization in clinically normal dogs and horses in the community. Lett App Microbiol. 2006, 43: 602-6. Meticillin-resistant Staphylococci in companion animals - T. Nuttall, N. Williams, R. Saunders, S. Dawson [47] BAGCIGIL (F.A.), MOODLEY (A.), BAPTISTE (K.E.), JENSEN (V.F.), GUARDABASSI (L.) - Occurrence, species distribution, antimicrobial resistance and clonality of methicillin- and erythromycin-resistant staphylococci in the nasal cavity of domestic animals. Vet Microbiol. 2007, 121: 307-15. [48] WEESE (J.S.), FAIRES (M.), ROUSSEAU (J.), BERSENAS (A.M.E.), MATHEWS (K.A.) - Cluster of methicillin-resistant Staphylococcus aureus colonization in a small animal intensive care unit. J Amer Vet Med Assoc. 2007, 231: 1361-4. [49] MIDDLETON (J.R.), FALES (W.H.), LUBY (C.D.), OAKS (J.L.), SANCHEZ (S.), MNYON (J.M.) et al. - Surveillance of Staphylococcus aureus in veterinary teaching hospitals. J Clin Microbiol. 2005, 43: 2916-9. [50] FUTAGAWA-SAITO (K.), BA-THEIN (W.), FUKUYASU (T.) - High occurrence of multi-antimicrobial resistance in Staphylococcus intermedius isolates from healthy and diseased dogs and domesticated pigeons. Res Vet Sci. 2007, 83: 336-9. [51] COIA (J.E.), DUCKWORTH (G.J.), EDWARDS (D.I.), FARRINGTON (M.), FRY (C, HUMPHREYS (H.) et al. - Guidelines for the control and prevention of meticillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. J Hosp Infect. 2006, 63: S1-S44. [52] MORLEY (P.S.), APLEY (M.D.), BESSER (T.E.), BURNEY (D.P.), FEDORKA-CRAY (P.J.), PAPICH (M.G.) et al. - Antimicrobial drug use in veterinary medicine. J Vet Int Med. 2005, 19: 617-29. [53] WEESE (J.S.) - Investigation of antimicrobial use and the impact of antimicrobial use guidelines in a small animal veterinary teaching hospital: 1995-2004. J Amer Vet Med Assoc. 2006, 228: 553-8. [54] RICH (M.), ROBERTS (L.) - MRSA in companion animals. Vet Rec. 2006, 159: 535-6. [55] HANSELMAN (B.A.), KRUTH (S.), WEESE (J.S.) - Methicillinresistant staphylococcal colonization in dogs entering a veterinary teaching hospital. Vet Microbiol. 2008, 126: 277-81. [56] MEEHAN (L.) - Survey of methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative staphylococci in UK equine hospitals [MSc Thesis]. The University of Liverpool. 2005. [57] GRIFFETH (G.C.), MORRIS (D.O.), ABRAHAM (J.L.), SHOFER (F.S.), RANKIN (S.C.) - Screening for skin carriage of methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Vet Dermatol. 2008, 19: 142-9. [58] JONES (R.D.), KANIA (S.A.), ROHRBACH (B.W.), FRANK (L.A.), BEMIS (D.A.) - Prevalence of oxacillin- and multidrug-resistant staphylococci in clinical samples from dogs: 1,772 samples (20012005). J Amer Vet Med Assoc. 2007, 230: 221-7. 59. FAIRES (M.), WEESE (J.S.) - Risk factors associated with MRSA infection in dogs. ASM Conference on antimicrobial resistance in zoonotic bacteria and foodborne pathogens; 2008, Copenhagen, Denmark. [60] RICH (M.), DEIGHTON (L.), ROBERTS (L.) - Clindamycin-resistance in methicillin-resistant Staphylococcus aureus isolated from animals. Vet Microbiol. 2005, 111: 237-40. [61] WEESE (J.S.), DACOSTA (T.), BUTTON (L.), GOTH (K.), ETHIER (M.), BOEHNKE (K.) - Isolation of methicillin-resistant Staphylococcus aureus from the environment in a veterinary teaching hospital. J Vet Int Med. 2004, 18: 468-70. [62] MCLEAN (C.L.), NESS (M.G.) - Meticillin-resistant Staphylococcus aureus in a veterinary orthopaedic referral hospital: staff nasal colonisation and incidence of clinical cases. J Small An Prac. 2008, 49: 170-7. [63] GUARDABASSI (L.), LOEBER (M.E.), JACOBSON (A.) - Transmission of multiple antimicrobial-resistant Staphylococcus intermedius between dogs affected by deep pyoderma and their owners. Vet Microbiol. 2004, 98: 23-7. [64] HANSELMAN (B.), KRUTH (S.A.), WEESE (J.S.) - Evauation of coagulase-positive staphylococcal carriage in people and their household pets. Proceedings of the First International Conference on MRSA in Animals; 2006, June 18-19; Liverpool, UK. Liverpool: The University of Liverpool; 2006. p. 32-3. [65] VAN DUIJKEREN (E.), WOLFHAGEN (M.J.H.M.), HECK (M.E.O.C.), WANNET (W.J.B.) - Transmission of a Panton-Valentine leucocidinpositive, methicillin-resistant Staphylococcus aureus strain between humans and a dog. J Clin Microbiol. 2005, 43: 620911. [66] SCOTT (G.M.), THOMSON (R.), MALONELEE (J.), RIDGWAY (G.L.) - Cross-infection between animals and man: possible feline transmission of Staphylococcus aureus infections in humans? J Hosp Infect. 1988, 12: 29-34. [67] CEFAI (C.), ASHURST (S.), OWENS (C.) - Human carriage of methicillin-resistant Staphylococcus aureus linked with pet dog. Lancet. 1994, 344: 539-40. [68] MANIAN (F.A.) - Asymptomatic nasal carriage of mupirocinresistant, methicillin-resistant Staphylococcus aureus (MRSA) in a pet dog associated with MRSA infection in household contacts. Clin Infect Dis. 2003, 36: E26-E28. [69] VITALE (C.B.), GROSS (T.L.), WEESE (J.S.) - Methicillin-resistant Staphylococcus aureus in cat and owner. Emerg Infect Dis. 2006, 12: 1998-2000. [70] WEESE (J.S.), CALDWELL (F.), WILLEY (B.M.), KREISWIRTH (B.N.), MCGEER (A.), ROUSSEAU (J.) et al. - An outbreak of methicillinresistant Staphylococcus aureus skin infections resulting from horse to human transmission in a veterinary hospital. Vet Microbiol. 2006, 114: 160-4. 287