Biodegradability PAO
advertisement

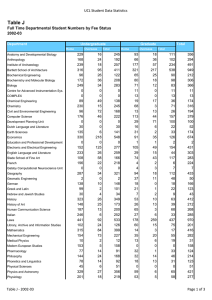
Stevens Ecology Project Report PREPARED FOR: PROJECT NUMBER: Biodegradability Testing of Ten Oils by PREPARED BY: 09 September, 2013 Biosynthetic Technologies 4521 OFFICE 1710 State Road Mosier, OR 97040 USA VOICE (541) 478-0594 (866) 942-7601 OECD 301B / ASTM D5864 INTERNET www.stevensecology.com EMAIL info@stevensecology.com Todd O. Stevens, Ph.D. Table of Contents List of Figures ........................................................................................................................................3 List of Tables ..........................................................................................................................................4 Project Summary and Certification .................................................................................................5 Project Description .............................................................................................................................6 Task 1. Aerobic Biodegradation by ASTM D 5864 / OECD 301B ...........................................8 Experimental Protocol ........................................................................................................................8 Results ..................................................................................................................................................12 Discussion of Results ........................................................................................................................15 Conclusions ........................................................................................................................................17 References Cited ...............................................................................................................................18 Appendix: Data Tables .....................................................................................................................19 Stevens Ecology Project Report 4521 rev.1 Page 2 List of Figures Figure 1. Appearance of Samples as Received Figure 2. Schematic Drawing of Experimental Apparatus Figure 3. Method 301B Microcosms in the Incubator Figure 3. Inorganic Carbon Production Figure 4. Biodegradation in Project Microcosms Stevens Ecology Project Report 4521 rev.1 Page 3 List of Tables Table 1. Sample Properties Table 2. Experimental Design Table 3. Biodegradation Summary Stevens Ecology Project Report 4521 rev.1 Page 4 Stevens Ecology Biogeochemistry Research and Analysis Project Summary and Certification Ten samples of oil, submitted by Biosynthetic Technologies, were tested for aerobic biodegradability by OECD 301B (ASTM method D 5864-05). Sample 4521.9 (Diester Hatcol 2901) was mineralized by more than 60% during a 10-day window and can be considered “Readily Biodegradable.” Sample 4521.7 (POE Hatcol 2926) and the reference material were mineralized to inorganic carbon by 60% or more during a 28-day incubation period under stringent standardized conditions and can be considered “Ultimately Biodegradable.” Samples 4521.2 (POE Hatcol 5150), 4521.4 (Group II 220R), 4521.5 (Group III Yubase 6), 4521.6 (PAO Synfluid 7), 4521.8 (PAG OSP-32), and 4521.10 (Bright Stock) were mineralized by 20% or more and can be considered “Inherently Biodegradable.” Samples 4521.1 (PAO SpectraSyn 40) and 4521.3 (PAG OSP - 150) were not mineralized or mineralized less than 20%. However, this assay was more stringent than “Ultimate” biodegradability assays, and it is possible that the last two groups might perform better in a OECD 302-series test or equivalent. Particularly since some samples required long lag periods before mineralization began. OFFICE 1710 State Road Mosier, OR 97040 USA VOICE (541) 478-0594 (866) 942-7601 These conclusions are based on the following report of research that was conducted under my supervision. INTERNET www.stevensecology.com EMAIL info@stevensecology.com Sample Reference 4521.1 4521.2 4521.3 4521.4 4521.5 4521.6 4521.7 4521.8 4521.9 4521.10 Label Canola Oil PAO (SpectraSyn 40) POE (Hatcol 5150) PAG (OSP-150) Group II (220R) Group III (Yubase 6) PAO (Synfluid 7) POE (Hatcol 2926) PAG (OSP-32) Diester (Hatcol 2901) Bright Stock Percent Degraded 70.1 4.3 25.3 0.0 33.6 37.8 29.1 61.6 26.6 76.2 37.0 Biodegradati Estimated Halfon Rate Life 0.0250 0.0026 0.0090 0.0000 0.0120 0.0135 0.0104 0.0219 0.0095 0.0272 0.0135 16 days 442 days 67 days 47 days 41 days 56 days 20 days 63 days 14 days 42 days Estimated Persistence 49 -106 days 1 - 8 years 111 - 445 days indefinite 71 - 312 days 68 - 272 days 96 - 372 days 45 - 132 days 105 - 419 days 37 - 93 days 74 - 279 days Todd O. Stevens, Ph.D. Stevens Ecology Project Report 4521 rev.1 Page 5 Designation Ultimately Biodegradable not biodegradable Inherently Biodegradable not biodegradable Inherently Biodegradable Inherently Biodegradable Inherently Biodegradable Ultimately Biodegradable Inherently Biodegradable Readily Biodegradable Inherently Biodegradable Project Description Ten samples of non-aqueous liquid materials, submitted by Biosynthetic Technologies were subjected to aerobic biodegradability testing by the OECD 301B open-bottle aerobic biodegradation assay. This protocol simulates an aerobic aquatic environment and is considered indicative of biodegradability in all aerobic environments. Biodegradation is measured in relationship to a reference material known to be biodegradable. The corresponding OECD guidelines provide several benchmark “pass” levels. Substances that pass the “readily biodegradable” criteria are considered likely to be completely mineralized during standard waste-water treatment. Ready biodegradability tests may also yield sufficient data for the “inherently biodegradable” and “ultimately biodegradable” designations, but are not designed specifically for that purpose. Sample Description Nine samples of clear oils were received in the laboratory on 10 June, 2013 and one on 5 August, 2013. The appearance of the samples as received is shown in Figure 1. The samples were colorless oils of varying viscosity, except that samples 4521.2 and 4521.7 had a slight yellow color, while sample 4521.10 was amber-colored. Sample descriptions are shown in Table 1. Figure 1. Samples As Received A standard reference material, known to be ultimately biodegradable, was used as a positive control. For this assay, the reference was Low-Erucic Acid Rapeseed Oil, or Canola Oil (Aldrich W530228). Stevens Ecology Project Report 4521 rev.1 Page 6 Sample Preparation Aliquots of all samples were submitted to Midwest Microlabs, LLC (Indianapolis, IN) for elemental analysis. C, H, and N were determined by combustion at 990°C with an elemental analyzer. Oxygen was determined by Pyrolysis via the Unterzaucher method. Results are shown in Table 1, and were used to calculate biodegradation from inorganic carbon production. Density was determined gravimetrically. sample Label Referen Canola Oil ce 4521.1 PAO (SpectraSyn 40) 4521.2 POE (Hatcol 5150) 4521.3 PAG (OSP-150) 4521.4 Group II (220R) 4521.5 Group III (Yubase 6) 4521.6 PAO (Synfluid 7) 4521.7 POE (Hatcol 2926) 4521.8 PAG (OSP-32) 4521.9 Diester (Hatcol 2901) 4521.10 Bright Stock water Elemental Composition, Percent Density C H O N S P Cl Na 0.87 78.07 11.68 10.22 0.20 - Ash total 0 101.04 0.86 85.20 12.98 - 0.00 - - - - 0 98.18 0.97 78.62 12.43 - 0.00 - - - - 0 91.05 0.96 68.22 0.86 85.20 0.85 85.50 10.67 13.60 13.50 - 0.00 0.00 0.00 - - - - 0 0 0 78.89 98.80 99.00 0.88 85.60 1.05 77.20 13.56 11.99 - 0.00 0.00 - - - - 0 0 99.16 89.19 0.94 66.24 0.91 74.90 9.98 11.61 - 0.00 0.00 - - - - 0 0 76.22 86.51 0.80 85.92 1.00 0.00 14.04 0.00 11.11 88.89 0.00 0.00 0.00 0.00 0.00 0.00 99.96 0 100.00 Table 1. Properties of Project Samples Since the substrates could not be diluted in water to make stock solutions, stock solutions were prepared by dilution in hexane. Aliquots were then added to microcosms and the solvent allowed to evaporate. Solvent alone was added to control microcosms in a similar way. Seed material, the source of microorganisms for this assay, was collected from the municipal sewage treatment plant in The Dalles, Oregon. Aerobic inoculum was “mixed liquor” aerated effluent. Inoculum was transported to the laboratory, diluted to achieve 3 g l-1 suspended solids, and aerated for five days at 25°C, to reduce background metabolizable carbon. Stevens Ecology Project Report 4521 rev.1 Page 7 Task 1. Aerobic Biodegradation by ASTM D 5864 / OECD 301B This experiment measured the mineralization of the test sample to CO2 in “open” aerated microcosms that simulated an aerobic aquatic environment, with microorganisms seeded from a waste-water treatment plant. This is considered representative of most aerobic environments that are likely to receive waste materials. Samples were incubated at 25°C in a mineral salts medium containing mature activated sewage solids in an apparatus that provided continuous aeration, agitation, and trapping of emitted CO2 (Figure 2). Treatments included media with test material, media with reference material, or media alone. Compressed air, free of CO2, was continuously sparged through each test vessel, then bubbled through a chain of three CO2 traps, containing .05M NaOH. As microorganisms from the inoculum gradually degraded the test substrates, the resulting CO2 was swept into the trap bottles and converted to carbonates. The carbonate accumulated in the traps was measured periodically to determine Biodegradation. Experimental Protocol An inoculum of activated “mixed liquor” sewage effluent was obtained from a municipal sewage treatment plant in The Dalles, Oregon. This material was conditioned in the laboratory as described above. The inoculum was diluted 1:100 in a mineral salts solution containing, per liter: KH2PO4 K2HPO4 . Na2HPO4 2H2O NH4Cl . CaCl2 7H2O MgSO4.7H2O FeCl3.6H2O 0.00850g 0.02175g 0.03340g 0.0050g 0.0364g 0.0225g 0.0025g Substrates were added to microcosms in amounts that yielded approximately 25 mg of carbon per liter, as shown in Table 2. Slight variations are due to corrections for density of the samples. Stevens Ecology Project Report 4521 rev.1 Page 8 House Air input Air purification section Bubbler flasks containing 1N NaOH Distribution Manifold & Flow meters Incubator section vent Test Vessel Trap Bottles containing 1N NaOH vent Trap Bottles containing 1N NaOH Test Vessel vent Trap Bottles containing 1N NaOH Test Vessel Figure 2. Schematic drawing of experimental apparatus. Stevens Ecology Project Report 4521 rev.1 Page 9 TREATMENT AMENDMENT AMOUNT NUMBER CHAMBER 4521C none 23.1 mg 3 1 4521R 25 mg Canola oil 22.9 mg 3 1 4521.1T PAO (SpectraSyn 40) 22.8 mg 3 1 4521.2T POE (Hatcol 5150) 25.8 mg 3 1 4521.3T PAG (OSP-150) 25.5 mg 3 1 4521.10T Bright Stock 21.3 mg 3 1 4521.4T Group II (220R) 22.9 mg 3 2 4521.5T Group III (Yubase 6) 22.6 mg 3 2 4521.6T PAO (Synfluid 7) 23.4 mg 3 2 4521.7T POE (Hatcol 2926) 27.9 mg 3 2 4521.8T PAG (OSP-32) 25.0 mg 3 2 4521.9T Diester (Hatcol 2901) 24.2 mg 3 2 Table 2. Design of Aerobic Biodegradation Experiment Three flasks were prepared for each treatment, each containing 1 liter of inoculated medium and a test substrate as shown in Table 2. To each flask, a train of three CO2-trap bottle was connected. Because this experiment required more apparatus than would fit into a single environmental chamber, they were divided into two groups, as shown in the table. At each time point, the lead trap bottle was removed from the train, and the remaining two flasks moved up one position. A fresh trap bottle was added to the end of the chain. The sacrificed bottle was sealed with a butyl rubber stopper and and acidified to pH <2 by the injection of 0.5 ml 10% H2SO4 with a hypodermic needle and syringe. After agitation and equilibration for one hour, headspace samples were removed with a gas-tight syringe and hypodermic needle, and analyzed by gas chromatography. The instrument used was a Hewlett-Packard 5880A equipped with dual packed columns (carboseive II, Supelco, Inc.) and a two-channel thermal conductivity detector, or a Carle GC 8700 equipped with a packed column (carboseive II, Supelco, Inc.) and a single thermal conductivity detector. Carrier and reference gases were helium. Instruments were calibrated using mixed-gas standards. The amount of CO2 produced in each microcosm was used to calculate the percentage of the test substrate that was mineralized by microorganisms. %D = (TICt – TICb) X 100 TOC Where TICt = mg inorganic carbon in test bottle at time t TICb = mg inorganic carbon in blank bottles at time t TOC = mg organic carbon added initially to the test vessel Stevens Ecology Project Report 4521 rev.1 Page 10 At the end of the incubation, the test vessels were acidified with 2 ml of acid solution, to volatilize any carbonates sequestered in solution. After four hours, the carbonate present in all remaining trap bottles was determined as described above, and the results added together for the final time point. Figure 3. Method 301B Microcosms in the Incubator Stevens Ecology Project Report 4521 rev.1 Page 11 Results Mineralization of reference substances began immediately after the start of incubation, as determined by accumulation of inorganic carbon in the trap bottles (Figure 4). After approximately 10 days, the emissions from the test samples diverged into three groups. Samples 1, 3, 6, and 8 produced approximately the same amount of inorganic carbon (IC) as the negative control flasks, until the final time point, when samples 6 and 8 began producing IC. Samples 7 and 9 produced IC at about the same rate as the canola oil reference material. Samples 2, 4, 5, and 10 produced IC at intermediate rates. The amount of CO2 produced by controls was subtracted from that in test and reference microcosms and compared with the theoretical CO2 yield to calculate percent biodegradation as shown in FIgure 5. The high-rate samples corresponded to the “ultimately biodegradable” designation. The intermediate rate samples corresponded to the “inherently biodegradable” designation. The two lowest-rate samples did not appear to be significantly biodegradable under the conditions of this assay, however between the last two time points, samples 6 and 8 began to mineralize at a great enough rate that they achieved the “inherently biodegradable” bench mark. See discussion of lag periods below. Although the samples were divided into two incubation chambers, this does not appear to have affected which rate group that a sample fell into. That is, both incubators contained samples in all three groups and the rates of the samples within each of these groups were very similar. Stevens Ecology Project Report 4521 rev.1 Page 12 Group 1 CO2 3000 2250 1500 750 0 0 7.5 4521C 4521.10 4521R 15.0 4521.1 22.5 30.0 4521.2 4521.3 Group 2 CO2 Inorganic Carbon µmol 3000 2250 1500 750 0 0 7.5 15.0 22.5 30.0 Days Incubation 4521.4 4521.9 4521.5 4521.6 4521.7 4521.8 Figure 4. Inorganic carbon production. Stevens Ecology Project Report 4521 rev.1 Page 13 Group 1 Biodegradation 100.00 75.00 50.00 25.00 0 0 7.5 4521R 4521.1 15.0 4521.2 22.5 30.0 4521.3 4521.10 Group 2 Biodegradation Percent Mineralization 100.00 75.00 50.00 25.00 0 0 7.5 15.0 22.5 30.0 Days Incubation 4521.4 4521.9 4521.5 4521.6 4521.7 4521.8 Figure 5. Calculated Biodegradation Stevens Ecology Project Report 4521 rev.1 Page 14 Discussion of Results The reference material was mineralized normally, and the inorganic carbon accumulation in control microcosms was less than 20% of that in reference microcosms. The elemental analysis did not account for all of the mass of samples 4521.3 and 4521.8. There may be one or more elements present that were not analyzed. However, since little or no inorganic carbon production was observed from 4521.3, the discrepancy does not affect the calculated results. If the carbon analysis is in error, the value for the final time point of 4521.8 could be over-estimated. The OECD designation of “ready biodegradability” is the most stringent defined by OECD protocols. It requires at least 60% mineralization of a substance within a 10-day window during a 28-day incubation. This is depicted by the box superimposed on Figure 5. To meet the “ready biodegradability” criteria, the degradation curve must enter the box through the lower left corner and exit through the top of the box. This criteria was satisfied for only one of the samples, 2521.9 which could be considered “readily biodegradable.” The OECD guidelines also provide less stringent designations. “Inherently Biodegradable” materials are those for which unequivocal evidence for mineralization is available. Typically, 20% mineralization, with no time limit is required. “Ultimately Biodegradable” materials are those for which there is a reasonable expectation that complete mineralization will eventually be achieved under optimized conditions. Typically 60% mineralization, with no time limit, and with pre-adapted cultures is required. Although this stringent experiment was not designed to test “inherent” and “ultimate” biodegradability, it can be seen that some of the materials passed these criteria. A wide range of biodegradation rates was obtained from these samples. The reference material, and Sample 4521.7 passed the “Ultimately Biodegradable” criteria. The observed rates were typical for vegetable oils. Samples 4521.2, 4521.4 and 4521.5, 4521.6, 4521.8, and 4521.10 passed the “Inherently Biodegradable” criteria. This is the typical range for mineral oils. It is possible that with pre-acclimated cultures and extended incubation periods, as allowed for OECD 302 series tests, these three samples would meet the criteria for “ultimately biodegradable,” but that cannot be seen from this present experiment. The remaining samples, 4521.1 and 4521.3, did not produce significantly more inorganic carbon than the negative controls, and do not appear to be biodegradable under the conditions of the ready biodegradability assay. Several of the sample curves in this experiment exhibited a “lag period,” or an initial period of low or no mineralization, before biodegradation began. This also occurred in a prior run of the experiment, except that the lag periods were approximately one week longer. Lag periods can be attributed to several possible causes. In some cases, time is required for cells to biochemically acclimate to the substrate - genes must be turned on and proteins synthesized. Sometimes only a very few cells capable of degrading the substrate are initially present, and degradation rates are low until those cells proliferate. These sorts of lag Stevens Ecology Project Report 4521 rev.1 Page 15 periods are characteristic of the inoculum. Lag periods might also be due to catabolite repression. In this case, the enzymes necessary for substrate utilization are suppressed until some other more energetically-favorable substrate is consumed. Catabolite repression would seem to be an unlikely cause of lag in this experiment, since no other carbon substrate was present. However it is at least possible that the substrate itself might repress some step in mineralization until some initial transformation step (e.g. removal of a sidechain or functional group) has been completed. The exact cause of the lag period is outside the scope of the current experiment. Note that in dedicated “inherent biodegradability” tests (OECD 302 series) lag periods are presumed to be reduced or eliminated by preincubating the substrate with aliquots of the inoculum, however that is not allowed for OECD 301-series assays. A summary of the results and estimated biodegradation rates are shown in Table 3. Estimated persistence was calculated for a “best case” scenario as a straight-line extrapolation and for a worst-case scenario with a half-life kinetic model. The actual rates in the environment would depend on the starting concentration, potential toxic effects at higher concentrations, and the environmental conditions. However, the data suggest that in this concentration range, samples 4521.7, and 4521.9 could require 1 to 4 months for complete biodegradation, while samples 4521.4, 4521.5, and 4521.10 could require 2 to 14 months, samples 4521.2, 4521.6, and 4521.8 could require 3 to 15 months, and samples 4521.1, and 4521.3 might persist for many years. Note that these assays assume that the material being tested is a pure substance. The assay may not provide information about minor components present at a few percent or less. The OECD guidelines permit the treatment of mixtures of closely related substances - such as oils - to be considered as pure substances. Stevens Ecology Project Report 4521 rev.1 Page 16 Sample Label Reference Canola Oil 4521.1 PAO (SpectraSyn 40) 4521.2 POE (Hatcol 5150) 4521.3 PAG (OSP-150) 4521.4 Group II (220R) 4521.5 Group III (Yubase 6) 4521.6 PAO (Synfluid 7) 4521.7 POE (Hatcol 2926) 4521.8 PAG (OSP-32) 4521.9 Diester (Hatcol 2901) 4521.10 Bright Stock Percent Biodegr Estimated Degraded adation Half-Life Rate 70.1 0.0250 16 days Estimated Persistence Designation 49 -106 days Ultimately Biodegradable 4.3 0.0026 442 days 1 - 8 years not biodegradable 25.3 0.0090 67 days 111 - 445 days Inherently Biodegradable 0.0000 - indefinite not biodegradable 33.6 0.0120 47 days 71 - 312 days Inherently Biodegradable 37.8 0.0135 41 days 68 - 272 days Inherently Biodegradable 29.1 0.0104 56 days 96 - 372 days Inherently Biodegradable 61.6 0.0219 20 days 45 - 132 days Ultimately Biodegradable 26.6 0.0095 63 days 105 - 419 days Inherently Biodegradable 76.2 0.0272 14 days 37 - 93 days Readily Biodegradable 37.0 0.0135 42 days 74 - 279 days Inherently Biodegradable 0.0 Table 3. Biodegradation Summary Conclusions Sample 4521.9 (Diester Hatcol 2901) was mineralized by more than 60% during a 10-day window and can be considered “Readily Biodegradable.” Sample 4521.7 (POE Hatcol 2926) and the reference material were mineralized to inorganic carbon by 60% or more during a 28-day incubation period under stringent standardized conditions and can be considered “Ultimately Biodegradable.” Samples 4521.2 (POE Hatcol 5150), 4521.4 (Group II 220R), 4521.5 (Group III Yubase 6), 4521.6 (PAO Synfluid 7), 4521.8 (PAG OSP-32), and 4521.10 (Bright Stock) were mineralized by 20% or more and can be considered “Inherently Biodegradable.” Samples 4521.1 (PAO SpectraSyn 40) and 4521.3 (PAG OSP - 150) were not mineralized or mineralized less than 20%. However, this assay was more stringent than “Ultimate” biodegradability assays, and it is possible that the last two groups might perform better in a OECD 302-series test or equivalent. Particularly since some samples required long lag periods before mineralization began. Stevens Ecology Project Report 4521 rev.1 Page 17 References Cited ASTM. 2005. Standard test method for determining aerobic aquatic biodegradation of lubricants or their components. ASTM International. West Conshohocken, PA. Bitton, G. 1999. Wastewater Microbiology. 2nd ed. Wiley-Liss. New York. Clesceri, L.S., Greenberg, A.E., and Trussell, R.R. 1989. Standard Methods for the Examination of Water and Wastewater. 17th ed. American Public Health Association, Washington, D.C. OECD. 2003. OECD guidelines for the testing of chemicals: Principles and strategies related to the testing of degradation of organic chemicals. Organization for Economic Cooperation and Development, Paris. OECD. 1992. OECD guidelines for the testing of chemicals: Biodegradability in Seawater. Organization for Economic Cooperation and Development, Paris. Wackett, L.P. and Hershberger, C.D. 2001. Biocatalysis and biodegradation. Microbial transformation of organic compounds. American Society for Microbiology, Washington, D.C. Stevens Ecology Project Report 4521 rev.1 Page 18 Appendix: Data Tables Date Days Incubation C C C R R R Aug 7, 2013 0 139.23 142.8 135.66 149.94 146.37 Aug 12, 2013 5 114.24 107.1 117.81 467.67 756.84 628.32 Aug 16, 2013 9 199.92 178.5 260.61 1074.57 1278.06 1088.85 Aug 21, 2013 14 364.14 285.6 310.59 1602.93 1710.03 1406.58 Aug 28, 2013 21 253.47 253.47 410.55 1888.53 1895.67 1995.63 Sep 4, 2013 28 403.41 374.85 392.7 2074.17 2188.41 2227.68 T1 T1 T1 T2 T2 135.66 T2 132.09 146.37 142.8 153.51 135.66 142.8 339.15 207.06 271.32 385.56 303.45 424.83 253.47 267.75 314.16 610.47 517.65 560.49 310.59 349.86 381.99 896.07 842.52 724.71 360.57 439.11 442.68 1024.59 971.04 985.32 514.08 478.38 521.22 1095.99 1088.85 1071 T3 T3 T3 T4 T4 T4 149.94 142.8 146.37 142.8 139.23 135.66 232.05 164.22 149.94 381.99 260.61 349.86 342.72 157.08 157.08 456.96 528.36 406.98 435.54 282.03 249.9 667.59 717.57 689.01 446.25 267.75 392.7 892.5 963.9 1003.17 514.08 310.59 274.89 1253.07 1370.88 1313.76 T5 T5 T5 T6 T6 T6 146.37 149.94 139.23 135.66 142.8 146.37 581.91 449.82 424.83 264.18 242.76 196.35 881.79 639.03 603.33 324.87 317.73 317.73 1342.32 860.37 399.84 442.68 403.41 674.73 1595.79 967.47 656.88 467.67 474.81 803.25 1806.42 1145.97 1278.06 1024.59 1228.08 1367.31 T7 T7 T7 T8 T8 T8 146.37 149.94 142.8 139.23 142.8 146.37 867.51 428.4 778.26 253.47 146.37 149.94 1663.62 885.36 1345.89 346.29 324.87 324.87 2363.34 1888.53 1920.66 399.84 381.99 399.84 2199.12 2145.57 2045.61 674.73 364.14 499.8 2288.37 2166.99 2113.44 960.33 910.35 949.62 T9 T9 T9 T10 T10 T10 210.63 135.66 146.37 135.66 142.8 146.37 274.89 499.8 456.96 321.3 285.6 257.04 1278.06 1295.91 978.18 546.21 474.81 414.12 2102.73 2356.2 1974.21 949.62 738.99 446.25 2034.9 2320.5 2345.49 1281.63 996.03 813.96 2156.28 2259.81 2238.39 1592.22 1338.75 1113.84 Daily CO2 Evolved Stevens Ecology Project Report 4521 rev.1 Page 19 Days Incubation 4521C 4521R 4521.1 4521.2 4521.3 4521.4 4521.5 4521.6 4521.7 4521.8 4521.9 4521.10 0 1 5 1 5 7 0 6 2 7 4 25 2 5 113 618 273 371 182 331 486 234 691 183 411 288 478 9 213 1147 278 563 219 464 708 320 1298 332 1184 14 320 1573 347 821 322 691 868 507 2058 394 2144 712 21 306 1927 414 994 369 953 1073 582 2130 513 2234 1031 28 390 2163 505 1085 367 1313 1410 1207 2190 940 2218 1348 Mean CO2 Evolved Days Incubation 4521R 4521.1 4521.2 4521.3 4521.4 4521.5 4521.6 4521.7 4521.8 4521.9 4521.10 0 0.19 0.04 0.17 0.32 0.00 0.22 0.08 0.24 0.17 1.03 5 20.09 5.94 9.51 3.38 7.94 13.95 4.40 19.96 3.55 13.25 6.84 9 37.03 2.46 12.82 0.58 9.15 18.47 3.89 37.25 5.89 40.94 10.32 14 49.61 1.06 18.29 0.42 13.53 20.40 6.72 59.48 3.72 76.00 15.19 21 64.12 4.05 25.04 3.12 23.59 28.52 9.90 62.44 10.13 80.26 28.03 28 70.12 4.27 25.30 -0.74 33.61 37.82 29.09 61.59 26.60 76.15 37.02 Mean Percent Biodegradation Stevens Ecology Project Report 4521 rev.1 Page 20 0.09