the grade 9 chemistry vocabulary list!
advertisement
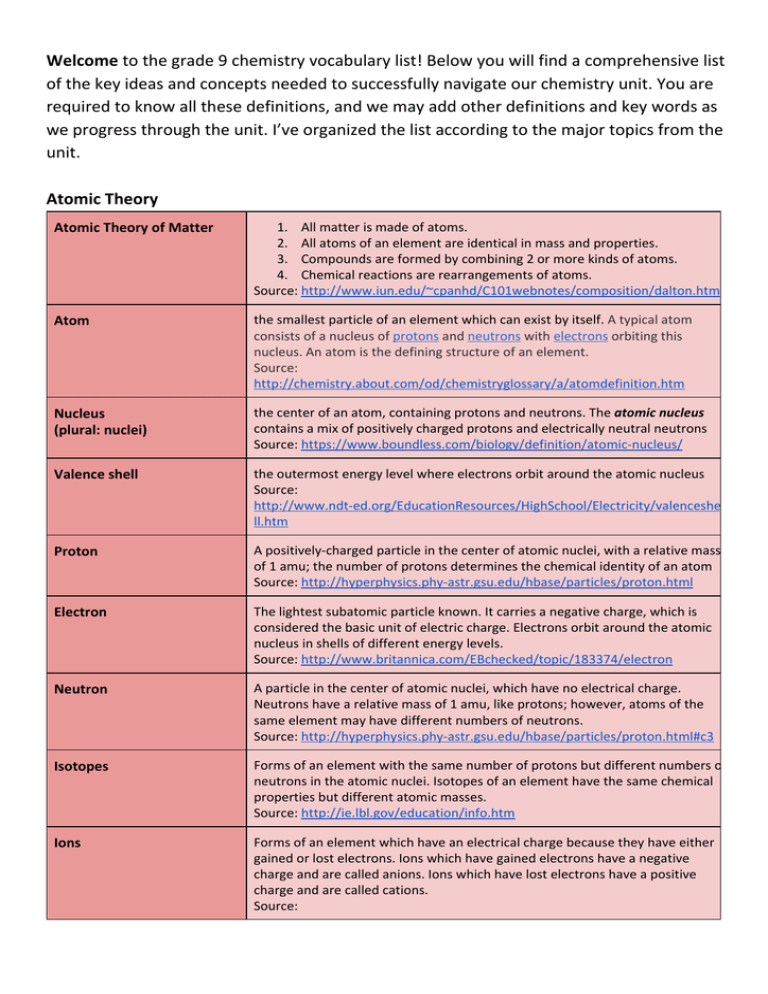
Welcome to the grade 9 chemistry vocabulary list! Below you will find a comprehensive list of the key ideas and concepts needed to successfully navigate our chemistry unit. You are required to know all these definitions, and we may add other definitions and key words as we progress through the unit. I’ve organized the list according to the major topics from the unit. Atomic Theory Atomic Theory of Matter 1. All matter is made of atoms. 2. All atoms of an element are identical in mass and properties. 3. Compounds are formed by combining 2 or more kinds of atoms. 4. Chemical reactions are rearrangements of atoms. Source: http://www.iun.edu/~cpanhd/C101webnotes/composition/dalton.html Atom the smallest particle of an element which can exist by itself. A typical atom consists of a nucleus of protons and neutrons with electrons orbiting this nucleus. An atom is the defining structure of an element. Source: http://chemistry.about.com/od/chemistryglossary/a/atomdefinition.htm Nucleus (plural: nuclei) the center of an atom, containing protons and neutrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons Source: https://www.boundless.com/biology/definition/atomic-nucleus/ Valence shell the outermost energy level where electrons orbit around the atomic nucleus Source: http://www.ndt-ed.org/EducationResources/HighSchool/Electricity/valenceshe ll.htm Proton A positively-charged particle in the center of atomic nuclei, with a relative mass of 1 amu; the number of protons determines the chemical identity of an atom Source: http://hyperphysics.phy-astr.gsu.edu/hbase/particles/proton.html Electron The lightest subatomic particle known. It carries a negative charge, which is considered the basic unit of electric charge. Electrons orbit around the atomic nucleus in shells of different energy levels. Source: http://www.britannica.com/EBchecked/topic/183374/electron Neutron A particle in the center of atomic nuclei, which have no electrical charge. Neutrons have a relative mass of 1 amu, like protons; however, atoms of the same element may have different numbers of neutrons. Source: http://hyperphysics.phy-astr.gsu.edu/hbase/particles/proton.html#c3 Isotopes Forms of an element with the same number of protons but different numbers of neutrons in the atomic nuclei. Isotopes of an element have the same chemical properties but different atomic masses. Source: http://ie.lbl.gov/education/info.htm Ions Forms of an element which have an electrical charge because they have either gained or lost electrons. Ions which have gained electrons have a negative charge and are called anions. Ions which have lost electrons have a positive charge and are called cations. Source: http://www.bbc.co.uk/schools/gcsebitesize/science/add_edexcel/ionic_comp ounds/ionicrev1.shtml Anion A negatively charged ion, produced when one or more electrons are added to an atom. Source: http://chemistry.about.com/od/chemistryglossary/a/Aniondefinition.htm Cation A positively charged ion, produced when one or more electrons are lost from an atom. Source: http://chemistry.about.com/cs/glossary/g/cationdef.htm Octet Rule The tendency of atoms to gain or lose electrons in order to have 8 electrons in their valence shell. The number of electrons gained or lost determines the charge of the resulting ion. Source: http://dl.clackamas.cc.or.us/ch104-08/octet.htm Atomic symbol A one-letter or two-letter abbreviation for a specific element. The first letter of symbols is always capitalized, but the second letter of 2-letter symbols is never capitalized. Source: http://www.wisc-online.com/Objects/ViewObject.aspx?ID=GCH3404 Atomic number (Z) the number of protons in one atom of a particular element, abbreviated as the letter ‘Z’ Source: http://www.colorado.edu/physics/2000/periodic_table/atomic_number.html Atomic mass (A) The combined number of neutrons and protons in the atomic nuclei of an element, abbreviated by the letter ‘A’. Source: http://www.ndt-ed.org/EducationResources/HighSchool/Radiography/atomic massnumber.htm Atomic Mass Unit (AMU) The unit of measurement for subatomic particles. It is based on the average mass of a proton or neutron, so that the mass of 1 proton = 1 amu. Source: http://www.colorado.edu/physics/2000/periodic_table/atomic_mass.html Electron configuration The way that electrons are arranged in different energy levels around the nucleus of an atom. The energy levels correspond with electron shells in the Bohr model of atoms. Source: Armstrong, Rick, Kevin Gaylor, and Jenny Sharwood. Chemistry 4/5 for the International Student. South Melbourne, Vic.: Cengage Learning, 2010. Print. Electron shell Electrons are arranged in shells at different distances around the nucleus.Each shell represents a different energy level, with the lowest-energy shell closest to the atomic nucleus. Each shell may hold a maximum number of electrons, arranged in pairs as often as possible. Source: http://www.bbc.co.uk/schools/gcsebitesize/science/add_ocr_pre_2011/perio dic_table/electronsrev1.shtml Periodicity and the Periodic Table Periodicity Repeating trends that are seen in elements’ properties, and which can be predicted based on an element’s position in the periodic table. The major properties we will study are electronegativity, electron affinity, atomic radius, and ionization energy. Source: http://chemistry.about.com/od/periodicproperties/a/What-Is-Periodicity-On-T he-Periodic-Table.htm Group A column of elements in the periodic table, sometimes also called a family of elements. Each group has common chemical and physical characteristics based on the elements’ electron arrangements. Source: http://chemistry.about.com/od/chemistryglossary/a/groupdefinition.htm Period A row of elements in the periodic table. Periods are based on the number of energy levels where electrons orbit atomic nuclei. Source: http://chemistry.about.com/od/periodicproperties/f/What-Is-The-DifferenceBetween-An-Element-Group-And-Period.htm Metal a substance (such as gold, tin, or copper) that usually has a shiny appearance, is a good conductor of electricity and heat, can be melted, and is usually capable of being shaped. Source: http://www.merriam-webster.com/dictionary/metal Nonmetal Elements which do not exhibit metallic properties, generally located in the upper righthand corner of the Periodic Table. Nonmetals usually gain electrons in ionic bonds, or they may share electrons with other nonmetals in covalent bonds. Source: http://chemistry.about.com/od/chemistryglossary/a/nonmetaldef.htm Metalloid Metalloids are the elements found along the stair-step line that distinguishes metals from nonmetals. Metalloids have properties of both metals and nonmetals. Some are semi-conductors. Source: http://www.chemicalelements.com/groups/metalloids.html Alkali metals The elements in the first group (Group 1) on the left side of the periodic table. Properties of Alkali metals are: ● highly reactive ● always found bonded with other elements ● 1 valence electron can be easily donated - become +1 cations ● form ionic bonds with nonmetals ● malleable and ductile ● extremely soft ● silvery color ● good conductors ● low boiling and melting points ● explosive reactions with water Source: http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/Main _Group_Elements/Group__1%3A_The_Alkali_Metals Alkaline Earth metals The elements in Group 2 of the periodic table. Properties of Alkaline Earth metals: ● reactive, but less so than Alkali metals ● malleable ● soft ● silver color ● conductors ● 2 valence electrons are lost to become +2 cations ● form ionic bonds with nonmetals ● never found alone in nature Source: http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/Main _Group_Elements/Group__2%3A_The_Alkaline_Earth_Metals Transition metals The elements in the center of the periodic table, from Group 3 - Group 12. They tend to lose electrons to form cations with charges from +1 to +7, and many transition metals will form several different cations (Mn, for example, may be Mn2+, Mn3+, Mn4+, Mn6+, or Mn7+. Properties of transition metals: ● form coloured compounds ● good conductors of heat and electricity ● hammered or bent into shape easily ● less reactive than alkali metals such ● high melting points - but mercury is a liquid at room temperature ● usually hard and tough ● high densities Source: http://www.bbc.co.uk/schools/gcsebitesize/science/edexcel_pre_2011/patte rns/transitionmetalsrev1.shtml Noble Gases The elements in Group 18 (the far right column) of the periodic table. They are all gases, and they are almost totally non-reactive with other elements. They have a full valence shell, so that they don’t gain or lose electrons. Source: http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/Main _Group_Elements/Group_18%3A_The_Noble_Gases Halogens The elements in Group 17 of the periodic table, the halogens all have 7 valence electrons. This means they all gain 1 electron in order to fulfil the octet rule. Properties of halogens: ● form anions with a 1- charge ● extremely reactive ● diatomic molecules ● poisonous ● react with metals to form salts Source: http://hyperphysics.phy-astr.gsu.edu/hbase/pertab/halog.html Diatomic elements Atoms of these elements will combine with atoms of the same element in order to form stable molecules of the element. The molecules are formed when atoms share one or more pairs of electrons. Diatomic elements are not diatomic when they bond with different elements!The diatomic elements you have to know are: ● H2 ● N2 ● O2 ● F2 ● Cl2 ● Br2 ● I2 Source: http://ths.talawanda.net/~BrambleN/classroom/Chemistry/Notes/Section%20 1B/DiatomicElements.htm Oxygen group All the elements underneath oxygen in the periodic table, which have 6 valence electrons. They are less reactive than the halogens, but because they gain 2 electrons, are still fairly reactive. Nitrogen group All the elements under nitrogen in the periodic table (group 15), which have 5 valence electrons. They may form 3 covalent bonds, which are the strongest type of covalent bond. Nitrogen and phosphorus are important for the growth of producers. Source: http://hyperphysics.phy-astr.gsu.edu/hbase/pertab/ng.html Lanthanides The top row of the Rare Earth metals, which are the two rows separated from the rest of the periodic table at the bottom. They dissolve in acids and tarnish easily when exposed to air. They also react with water, but slowly. Source: http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/Trans ition_Metals_and_Coordination_Complexes/The_Lanthanides#Properties_and_ Chemical_Reactions Actinides The bottom row of the Rare Earth metals. All are radioactive (this is where uranium is found in the periodic table!) because their nuclei are unstable, and all are considered toxic. Most have been made synthetically and do not occur much naturally. Source: http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/Trans ition_Metals_and_Coordination_Complexes/The_Actinides#Common_Propertie s Atomic radius The distance from the nucleus of an atom to the valence shell, where the outermost electrons orbit. One of the important trends we study in chemistry because it relates to the strength with which atoms hold onto electrons when bonding with other atoms. Atoms become smaller as we move to the right across periods because the electrons are more strongly attracted to the greater number of protons in the atomic nuclei. Atoms become larger as we move down the groups because additional electrons are added in shells further from the nuclei. Source: http://www.princeton.edu/~achaney/tmve/wiki100k/docs/Atomic_radius.html Melting point The temperature at which a substance changes from a solid to a liquid. In theory, this is the same temperature at which the liquid will turn into a solid, called the freezing point. Source: http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch14/melting.php Electronegativity A measure of how strongly an atom holds electrons in a covalent bond. This is important in determining polarity of substances. Source: http://www.files.chem.vt.edu/RVGS/ACT/notes/electronegativity.html Reactivity A measure of how easily an element will bond with other elements to form compounds. The trends are different for metals and nonmetals. Source: http://edtech2.boisestate.edu/lindabennett1/502/Periodic%20Table%20e%2 0config/PTable_trends%20around%20table.html Bonding and Naming Compounds Ionic bond bond in which one or more electrons from one atom are removed and attached to another atom, resulting in positive and negative ions which attract each other. Source: http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/bond.html Covalent bond bond in which one or more pairs of electrons are shared by two atoms. One shared pair makes a single bond, two shared pairs make a double bond, and three shared pairs make a triple bond. Source: http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/bond.html Element A substance made of atoms with the same number of protons in their nuclei. They are chemically the simplest substances and hence cannot be broken down using chemical methods. Source: http://www.chemicool.com/definition/element.html Compound A substance composed of 2 or more elements that are chemically combined together. It can be split up into its original elements by running an electrical current through it or by heating it. Source: Armstrong, Rick, Kevin Gaylor, and Jenny Sharwood. Chemistry 4/5 for the International Student. South Melbourne, Vic.: Cengage Learning, 2010. Print. Mixture A substance composed of 2 or more elements or compounds that are NOT chemically combined. They can be separated by physical means such as filtration or distillation. Source: Armstrong, Rick, Kevin Gaylor, and Jenny Sharwood. Chemistry 4/5 for the International Student. South Melbourne, Vic.: Cengage Learning, 2010. Print. Diatomic element elements which cannot exist as single atoms. They form molecules of 2 atoms of the same element if they are isolated from other elements. There are 8 diatomic elements you must memorize for this unit: ● hydrogen (H2) ● nitrogen (N2) ● oxygen (O2) ● fluorine (F2) ● chlorine (Cl2) ● bromine (Br2) ● iodine (I2) ● astatine (At2) Source: http://ths.talawanda.net/~BrambleN/classroom/Chemistry/Notes/Section%20 1B/DiatomicElements.htm Molecule Atoms of 2 or more elements, which are covalently bonded together. The atoms share one, two, or three pairs of electrons to form single, double, or triple bonds respectively. Source: http://education.jlab.org/qa/compound.html Formula unit The simplest repeating unit of a substance, showing all the elements within that substance. It usually - but not always - refers to ionic compounds, in which oppositely-charged ions are electrically attracted to one another. Source: http://www.chemicool.com/definition/formula_unit.html Balancing Equations Law of Conservation of Matter All the atoms present at the start of a chemical reaction are still present at the end of the reaction. They have simply been rearranged in new ways. Source: http://employees.oneonta.edu/viningwj/modules/CI_law_of_conservation_of_ matter_4_3.html Reactant One of the starting substances in a chemical reaction, which may be changed during the course of the reaction. They are written on the left side of chemical equations. Source: http://chemistry.about.com/od/chemistryglossary/a/reactantdef.htm Product One of the substances made during a chemical reaction. It is assembled from the atoms which make up the initial ingredients of the reaction. They are written on the right side of chemical equations. Source: http://www.chemprofessor.com/outline7b.htm Reagent a substance that is used to test for the presence of another substance by causing a chemical reaction with it Source: http://www.merriam-webster.com/dictionary/reagent Yield The amount of material produced in a chemical reaction, which depends on the quantities of chemicals used and whether the reaction goes to completion or not. Source: http://www.wisc-online.com/objects/ViewObject.aspx?ID=GCH7504 Coefficient The large number in front of a chemical symbol in reaction equations. It shows the number of moles of a substance. Source: http://chemistry.about.com/od/chemistryquickreview/a/balanceequation.htm Subscript The small number after an individual element’s symbol in a chemical formula. It shows how many atoms of that element are present in the compound. Source: http://crescentok.com/staff/jaskew/isr/chemistry/class12.htm Mole The primary unit for measuring the amount of a substance present in chemical reactions. Since atoms are so small, this makes it easier to calculate yields in chemistry. It is based on the number of carbon atoms in 12g of pure carbon. Source: http://www.files.chem.vt.edu/RVGS/ACT/notes/The_Mole.html Avogadro’s number The number of particles in one mole of any substance. Through experimental observation it has been fixed at 6.02e23 (6.02 x 1023) particles per mole. Source: http://www.files.chem.vt.edu/RVGS/ACT/notes/The_Mole.html Molar mass The mass (in grams) of 6.02e23 particles of a substance. It may be calculated by adding the atomic masses of all elements in a given compound. Source: http://www.princeton.edu/~achaney/tmve/wiki100k/docs/Molar_mass.html Mole ratio The relative number of moles, molecules, or formula units of substances involved in chemical reactions. It is important in determining yields from the reactions. Source: http://www.occc.edu/kmbailey/Chem1115Tutorials/Molar_Ratios.htm Mole-mass problem Conversion between the number of particles and the number of grams of various substances in chemical reactions. Source: http://www.chemteam.info/Mole/Moles-to-Grams.html Mass-mass problem A type of conversion or problem which uses the mass and molar mass of one substance to determine how many grams of another substance are produced by a chemical reaction. Source: https://www.boundless.com/chemistry/mass-relationships-and-chemical-equat ions/reaction-stoichiometry/mass-to-mass-conversions/ Types of Reactions Synthesis A type of reaction in which two or more substances combine to form a new and chemically different substance. The general format is A + X → AX. Source: http://www.files.chem.vt.edu/RVGS/ACT/notes/Types_of_Equations.html Decomposition A type of reaction in which a single compound breaks down into simpler parts. The general format is AX → A + X. Source: http://www.files.chem.vt.edu/RVGS/ACT/notes/Types_of_Equations.html Single replacement A type of reaction in which a more chemically reactive element takes the place of another element in a compound and sets the less-reactive element free. The general format is A + BX → AX + B or AX + Y → AY + X. Source: http://www.files.chem.vt.edu/RVGS/ACT/notes/Types_of_Equations.html Double replacement A type of reaction in which two ionic compounds ‘swap’ the partner ions with each other. The general format is AX + BY → AY + BX. Source: http://www.files.chem.vt.edu/RVGS/ACT/notes/Types_of_Equations.html Combustion A type of reaction in which hydrocarbons react with oxygen gas to produce carbon dioxide and water. The products always include CO2 and H2O. Source: http://www.files.chem.vt.edu/RVGS/ACT/notes/Types_of_Equations.html





