Chapter 2 Notes (Day 2)
advertisement

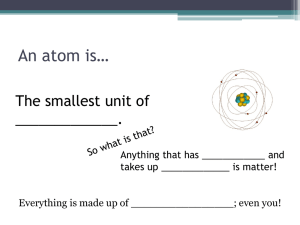
Objective: Draw a model of the atom illustrating all of the subatomic particles and energy levels. http://www.ndt-ed.org/EducationResources/HighSchool/Electricity/Graphics/atom.jpg Atomic Structure All things are made up of matter. Matter is made up of atoms. Atoms are the smallest unit of matter that can not be broken down by chemical means. Subatomic Particles Proton: positive charge, located in the nucleus Neutron: no charge, located in the nucleus Electron: - charge, located outside the nucleus. 1 Electrons revolve around the nucleus in different orbits. The closer the orbit is to the nucleus, the fewer the electrons that can fit in that particular orbit or energy level. Energy Level (Orbit) 1st 2nd 3rd # of Electrons 2 8 18 Electrons move vary fast around the nucleus, forming a hazy cloud-like appearance to the atom. Therefore, the location of the electrons is often called an electron cloud. 2 Elements and Such Elements: substance made up of only one type of atom. Example: Carbon Compound: Substance made of joined atoms from 2 or more different elements. Example: Carbon Dioxide (CO2) = 1 carbon + 2 oxygen Molecule: A group of different atoms held together by covalent bonds Example: H2O and O2 Chemical Bonds It is important to remember that compounds are the most stable or happy when their outer energy level is filled with electrons. 1.Covalent Bonds: forms when 2 or more atoms share electrons. Neither atom truly has possession of the electron Example: H2O Hydrogen Bonds: Very weak bonds, easy to break. They form between a positively charged Hydrogen atom from one covalently bonded molecule to a negatively charged portion of another covalently bonded molecule. * Polar molecules: have a positive and negative charge. Example: H2O * Hydrogen side of molecule has a + charge Oxygen side of molecule has – charge. Ionic Bonds: Created by oppositely charged ions. * Ion: Occasionally atoms or molecules will gain or lose an electron to fill its outer energy level. The atom or molecule becomes + or – charged. This is an ion. Oppositely charged ions can bond to form an ionic bond. Example: NaCl (salt) 3