trends in the periodic table - Atomic Theory and Periodic Table
advertisement
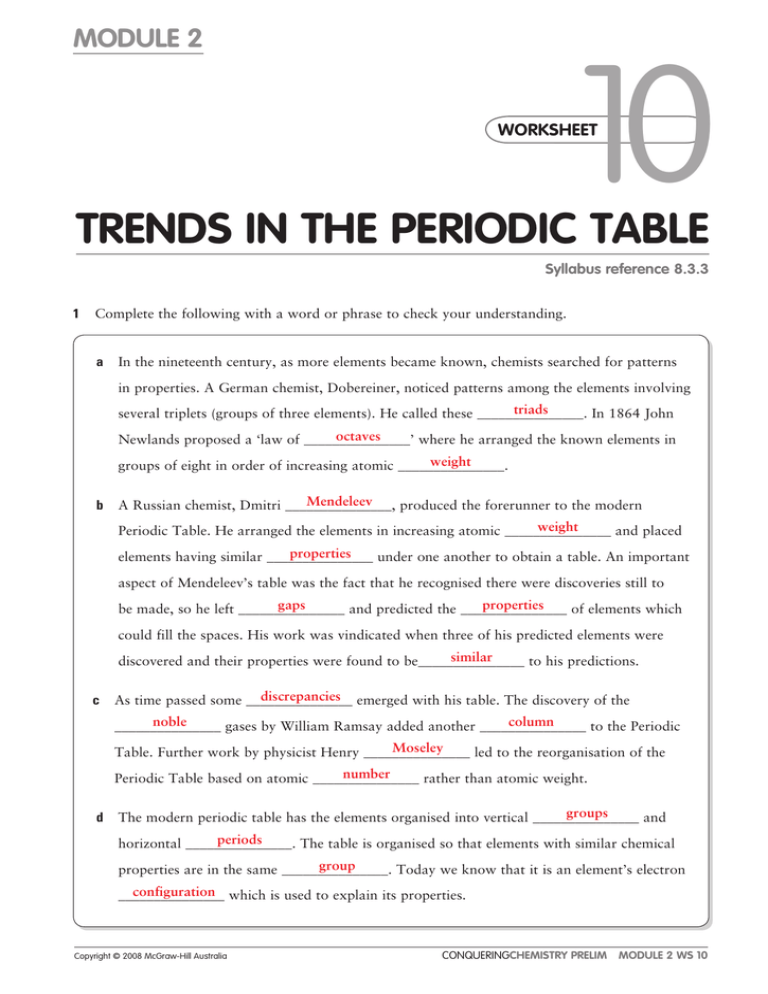
MODULE 2 10 WORKSHEET WORKSHEET TRENDS IN THE PERIODIC TABLE Syllabus reference 8.3.3 1 Complete the following with a word or phrase to check your understanding. a In the nineteenth century, as more elements became known, chemists searched for patterns in properties. A German chemist, Dobereiner, noticed patterns among the elements involving triads several triplets (groups of three elements). He called these _______________. In 1864 John octaves Newlands proposed a ‘law of _______________’ where he arranged the known elements in weight groups of eight in order of increasing atomic _______________. b Mendeleev A Russian chemist, Dmitri _______________, produced the forerunner to the modern weight Periodic Table. He arranged the elements in increasing atomic _______________ and placed properties elements having similar _______________ under one another to obtain a table. An important aspect of Mendeleev’s table was the fact that he recognised there were discoveries still to gaps properties be made, so he left _______________ and predicted the _______________ of elements which could fill the spaces. His work was vindicated when three of his predicted elements were similar discovered and their properties were found to be_______________ to his predictions. c discrepancies emerged with his table. The discovery of the As time passed some _______________ noble column _______________ gases by William Ramsay added another _______________ to the Periodic Moseley Table. Further work by physicist Henry _______________ led to the reorganisation of the number Periodic Table based on atomic _______________ rather than atomic weight. d groups The modern periodic table has the elements organised into vertical _______________ and periods horizontal _______________. The table is organised so that elements with similar chemical group properties are in the same _______________. Today we know that it is an element’s electron configuration which is used to explain its properties. _______________ Copyright © 2008 McGraw-Hill Australia CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 10 e decrease Atomic radii generally _______________ as you go from left to right across a period. Atomic radii increase electrons generally _______________ as you move down a group because there are more _______________. f ionisation The energy required to remove an electron from an atom is called the _______________ increases energy. This generally _______________ as you move from left to right across a period decreases highest and _______________ as you move down a group. The noble gas has the _______________ ionisation energy within each period. g increases As you remove successive electrons the ionisation energy _______________ but not always uniformly. The differences between successive ionisation energies shows that atoms tend to noble lose electrons to attain the electron configuration of the _______________ gases. 2 a Complete the table below by inserting the values for atomic number, group number and period. b Use the table below to make generalisations about the electrical conductivity and boiling point across a period and down a group. ELEMENT SYMBOL ATOMIC NUMBER GROUP NUMBER PERIOD ELECTRICAL CONDUCITIVITY (MS m1) BOILING POINT (ºC) Hydrogen H 1 1 1 — 253 Helium He 2 8 1 — 269 Lithium Li 3 1 2 10.6 1342 Beryllium Be 4 2 2 27 2970 Boron B 5 3 2 1010 3660 Carbon C 6 4 2 0.07 4830 Nitrogen N 7 5 2 — 196 Oxygen O 8 6 2 — 183 Fluorine F 9 7 2 — 188 Neon Ne 10 8 2 — 246 Sodium Na 11 1 3 21 883 Magnesium Mg 12 2 3 22 1110 Aluminium Al 13 3 3 37 2450 3 103 3267 3 1015 280 445 Silicon Phosphorous Si P 14 15 4 5 Sulfur S 16 6 3 1021 Chlorine Cl 17 7 3 — 35 Argon Ar 18 8 3 — 189 Potassium K 19 1 4 14 760 Calcium Ca 20 2 4 29 1484 Rubidium Rb 37 1 5 7.6 39 Cesium Cs 55 1 6 5.0 28 Francium Fr 87 1 7 — 27 Copyright © 2008 McGraw-Hill Australia CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 10 i Identify any trend in electrical conductivity. Generally electrical conductivity increases then decreases from left to right across a period. Generally electrical conductivity decreases from top to bottom down a group. ii Identify any trend in boiling point. Boiling point generally increases then decreases from left to right across a period and decreases from top to bottom down a group. iii Use the graph paper to plot a graph of boiling water vs atomic number for elements in Group 1. iv Use the same graph paper and a different colour to plot a graph of boiling point vs atomic number for Period 2. ���� ���� ������������������ ���� ���� ���� ���� �������� ���� ���� ���� ��� ������� � ���� � � �� � �� � �� � �� � �� � �� � �� � �� � �� �� �� �� �� �� �� �� �� �� �� �� �� �� �� �� ������������� Copyright © 2008 McGraw-Hill Australia CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 10 �� �� 3 Classify each of these statements as true or false. Rewrite the false statements so they are true. a In his periodic table Mendeleev arranged the elements according to increasing atomic number. FALSE In his periodic table Mendeleev arranged the elements according to increasing atomic WEIGHT. b The element in Group IV Period 4 is gallium. FALSE c The element in Group IV Period 4 is GERMANIUM. Removing one electron from an atom results in an ion with a charge of 1+. TRUE d The metallic character increases as you move from left to right and decreases as you move from top to bottom. FALSE The metallic character DECREASES as you move from left to right and INCREASES as you move from top to bottom. e Moving across a period the bonding changes from metallic to covalent. TRUE f The melting points generally rise to the element in Group V then decrease. FALSE 4 Element W forms the chloride WCl2 and the oxide WO. Element Y is in the same group of the Periodic Table as W. What would you expect for the formula of the hydroxide and bromide of element Y? Y(OH)2 5 The melting points generally rise to the element in Group IV then decrease. YBr2 a In the Periodic Table how does the atomic radius vary: b i across a period from left to right? decreases ii down a group from top to bottom? increases In each of the following sets place the elements in order of increasing atomic radius: i potassium, calcium, magnesium magnesium, calcium, potassium ii chlorine, argon, silicon argon, chlorine, silicon iii gallium, phosphorus, aluminium phosphorus, aluminium, gallium iv chlorine, argon, arsenic, bromine argon, chlorine, bromine, arsenic Copyright © 2008 McGraw-Hill Australia CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 10 6 The first ionisation energies for sodium, silicon and sulfur are 1.01, 0.50 and 0.79 MJ/mol but not necessarily in that order. Assign the values to the elements and explain your reasoning. Ionisation energy increases from left to right so sodium would be 0.50 MJ/mol, silicon would be 0.79 MJ/mol and sulfur would be the highest at 1.01 MJ/mol. 7 The following table lists successive ionisation energies (in kJ/mol) for three elements A, B and C. a A B C First ionisation energy 580 502 744 Second ionisation energy 1820 4570 1460 Third ionisation energy 2750 6920 7740 Fourth ionisation energy 11600 9550 10500 Why is the second ionisation energy always greater than the first? It requires more energy to remove the second electron because this involves removing a negative electron from a positive ion due to extra electrostatic attraction. b Why is there a big jump in value between the second and third energies for C and between the third and fourth for A? The jump would mean an electron is being removed from a filled electron shell. c What would you expect to be the charges on the ions formed by each of A, B and C? A3, B1, C2 8 The following diagram summarises some ways in which properties vary across and down the Periodic Table. Insert the word increases or decreases in the space provided. decreases atomic radius increases ionisation energy reactivity of metals electronegativity increases decreases metallic character decreases increases decreases increases decreases increases Copyright © 2008 McGraw-Hill Australia CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 10 9 The electronegativity of an element is a measure of its ability to attract electrons. The higher the electronegativity of an element, the more strongly it attracts electrons. An element’s ability to attract electrons will have a bearing on whether it forms positive ions, covalent bonds or negative ions. The shared pair of electrons in a covalent bond are rarely shared equally between the two atoms involved. One atom usually has a higher electronegativity than the other and thus attracts the electrons more strongly. This atom has therefore acquired an extra share of negative charge and begins to resemble a negative ion. The other atom correspondingly begins to resemble a positive ion. The extent to which this sharing of an electron pair is unequal is indicated by the percentage ionic character of a simple bond. The table below gives the electronegativity of atoms of selected elements. It is arranged in the same way as a Periodic Table from which the noble gases (helium, neon, argon and krypton) and the transition elements (scandium to zinc) have also been omitted. TABLE OF ELECTRONEGATIVITY OF SELECTED ELEMENTS H 2.1 Li 1.0 Be 1.5 B 2.0 C 2.5 N 3.0 O 3.5 F 4.0 Na 0.9 Mg 1.2 Al 1.5 Si 1.8 P 2.1 S 2.5 Cl 3.0 K 0.8 Ca 1.0 Ga 1.6 Ge 1.8 As 2.0 Se 2.4 Br 2.8 ������������������������ The graph below shows how the per cent ionic character of a single bond varies according to the difference in electronegativity between the two elements forming the bond. 100 90 80 70 60 50 40 30 20 10 0 0 1 2 3 ���������������������������� Answer the following questions using the table and graph above. a What is the relationship between the per cent ionic character of single bonds and the electronegativity difference between their elements? The greater the electronegativity difference the greater the per cent ionic character. b What electronegativity difference will result in a bond with a 50% ionic character? Approximately a 1.5 difference. c Estimate the percentage ionic character of bonds formed between: Difference is 1 so 20–24% ionic character. i nitrogen and fluorine ii sodium and oxygen Copyright © 2008 McGraw-Hill Australia Difference is 2.6 so 90–92% ionic character. CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 10