TNF-α and IL-10 Modulate CK and CK
advertisement

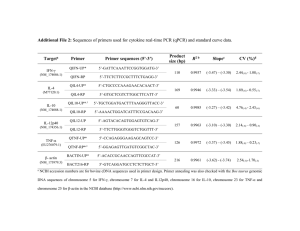
Acta Scientiae Veterinariae, 2014. 42: 1247. RESEARCH ARTICLE ISSN 1679-9216 Pub. 1247 TNF-α and IL-10 Modulate CK and CK-MB Activities in Naturally Infected Dogs by Leishmania infantum José Cláudio Carneiro de Freitas1, Adam Leal Lima1, Carla Tamára de Araújo Rodriguês1, Gian Karlo Gama de Almeida1, Glauco Jonas Lemos Santos1, Bruno Mendes Roatt2, Alexandre Barbosa Reis2 & Diana Célia Sousa Nunes-Pinheiro1 ABSTRACT Background: Canine visceral leishmaniasis (CVL) is a multisystem inflammatory disease caused by the Leishmania genus protozoa, characterized by increased production of pro-inflammatory mediators. The parasite presence has been reported in various organs and tissues, such as bone marrow, lymph nodes, spleen, liver and skin, in addition the cardiac muscle. Cardiac muscle lesions provide the substances release, among which we can mention the creatine kinase (CK), its MB subunit (CK-MB) and troponin. The changes that cause these injuries depend not only of the parasite presence, but also the increased concentrations of proinflammatory cytokines. Although most studies focus on the proinflammatory cytokines action, regulatory cytokines of the inflammatory process has gained evidence in the immune response to the Leishmania infantum and can be directly associated with the cardiac damage pathogenesis. Therefore, this study aimed to evaluate the TNF-α and IL-10 serum levels in naturally infected dogs by L. infantum and relates them to cardiac biomarkers, in the different clinical forms of disease. Materials, Methods & Results: A total of 30 adult dogs were used, being 10 negative (ND) and 20 positive for CVL. All seropositive animals were subjected to clinical examination, observing the presence of characteristic clinical signs of disease, being divided into two groups: asymptomatic (AD, n = 10) and symptomatic (SD, n = 10) dogs. Blood samples from all animals were collected to obtain serum for subsequent measurement of TNF-α, IL-10, CK and CK-MB. Data were analyzed by Kruskal-Wallys test, followed by Dunn’s test. The correlation and influence of TNF-α, IL-10 and IL-10/ TNF-α on CK, CK-MB and CK-MB/CK, were determined by Spearman correlation test and linear regression. To evaluate the relationship between the clinical signs onset and the TNF-α, IL-10, IL-10/TNF-α, CK, CK-MB and CK-MB/CK serum levels, the unpaired t-test was used. TNF-α and IL-10, in the different groups, showed no significant differences. Already IL-10/TNF-α ratio was significantly increased in AD, when compared to ND. CK was significantly increased in infected animals, when compared to ND. Already the CK-MB was significantly increased in SD, when compared to ND. CK-MB/ CK ratio, in the different groups, showed no significant differences. TNF-α and IL-10 showed a moderate correlation and weak negative and positive influence on CK and CK-MB, in AD and SD. The IL-10/TNF-α ratio presented a weak correlation and moderate negative influence in AD, and moderate correlation and strong positive influence, in SD, on CK and CK-MB. It was also observed weak and moderate correlations, besides the weak and strong positive influences, of the IL-10/TNF-α ratio on CK-MB/CK ratio, in AD and SD dogs. Discussion: The elevation of CK and CK-MB serum levels, in asymptomatic and symptomatic animals, is directly related to the impairment of skeletal muscles, due to the increased production of proinflammatory cytokines, since the analysis of a cardiac marker more specific, such as troponin I, excluded the possibility of heart damage. Furthermore, our results suggest that the L. infantum infection can trigger cardiovascular disorders, regardless of their relationship with the increased production of TNF-α. However, CK and CK-MB, separately evaluated, were not presented as good markers of cardiac damage in naturally infected dogs by L. infantum. Keywords: canine visceral leishmaniasis, cardiac biomarkers, TNF-α, IL-10. Received: 2 August 2014 Accepted: 28 November 2014 Published: 24 December 2014 Laboratório de Imunologia e Bioquímica Animal, Programa de Pós-graduação em Ciências Veterinárias, Faculdade de Veterinária, Universidade Estadual do Ceará (UECE), Fortaleza, CE, Brazil. 2Laboratório de Imunopatologia, Núcleo de Pesquisa em Ciências Biológicas (NUPEB), Instituto de Ciências Exatas e Biológicas (ICEB II), Universidade Federal de Ouro Preto (UFOP), Ouro Preto, MG, Brazil. CORRESPONDENCE: J.C.C. Freitas [joseclaudiocarneiro@yahoo.com.br - Fax: +55 (85) 3101-9860]. Avenida Dr Silas Munguba n. 1700, Campus do Itaperi, Serrinha. CEP 60740-903 Fortaleza, CE, Brazil. 1 1 J.C.C. Freitas, A.L. Lima, C.T.A. Rodriguês, et al. 2014. TNF-α and IL-10 Modulate CK and CK-MB Activities in Naturally Infected Dogs by Leishmania infantum. Acta Scientiae Veterinariae. 42: 1247. INTRODUCTION heartworm excluding, a chromatographic immunoassay2 (Anigen Rapid Heartworm Ag Test) was used for the qualitative detection of Dirofilaria immitis antigen according to manufacturer’s instructions. Thirty animals were divided into three groups (n = 10), according to the serology and the clinical signs onset: negative dogs (ND), composed of serologically negative dogs for CVL; asymptomatic dogs (AD), consisting of seropositive dogs for CVL, and who had no clinical signs characteristic of the disease and symptomatic dogs (SD), composed of seropositive dogs for CVL, and had at least three characteristic clinical signs of disease. AD and SD were from the CCZ, as those belonging to ND were from private kennels. All clustered animals were serologically negative for canine heartworm disease. Animals were immobilized and blood samples were collected with sterile syringe by jugular venipuncture, being distributed in tubes without anticoagulant. Furthermore, the blood samples were centrifuged at 4000 g, for 10 min, to obtain the serum which were stored at frozen (-20ºC) until use. Canine visceral leishmaniasis (CVL) is a multisystem inflammatory disease caused by the Leishmania genus protozoa, characterized by increased production of pro-inflammatory mediators, generated by excessive immune response and culminating in tissue damages [18]. The presence of the parasite has been reported in various organs and tissues, such as bone marrow [15], lymph nodes [2], spleen [33], liver [16] and skin [28], in addition the cardiac muscle [25,27]. Cardiac muscle lesions provide substances releasing, among which we can mention the kinases, creatine kinase (CK) and its MB subunit (CK-MB), and troponin I [14,32]. It has been reported that the changes causing these injuries depend not only of the parasite presence, but also the proinflammatory cytokines increased concentrations, such as TNF-α, INF-γ, IL-6 and IL-12 [3,39]. These cytokines can directly contribute to the cardiac injury progression in the muscle fiber, as well as the CVL clinical manifestations onset, particularly those related to reduced muscle mass [17,33]. Although most studies focus on the proinflammatory cytokines action, regulatory cytokines of the inflammatory process, such as IL-10, has gained evidence in the immune response to the L. infantum and can be directly associated with the cardiac damage pathogenesis [29,37]. Furthermore, studies have shown that IL-10/TNF-α ratio is more accurate in detecting heart function loss [19,23]. Based on this, the aim of this study was to evaluate the TNF-α and IL-10 serum levels in naturally infected dogs by L. infantum, and relate them to the cardiac enzyme biomarkers, CK and CK-MB, in different clinical forms of the disease. Semi-quantitative analysis of troponin I serum levels Troponin I serum levels were determined by immunochromatographic test3 (Troponina I Test K085) according to the manufacturer’s instructions. The animals considered positive have troponin I serum levels ≥ 0.5 ng/mL. CK and CK-MB serum levels determination CK and CK-MB activities were determined by using a commercial kit4 (CK and CK-MB Test), at automatic biochemical. For the measurement of CKMB isoenzyme, previously sera underwent to heating in water bath at 45°C, for 20 min, before the samples are taken to the dosing automatic biochemical. The values obtained were compared with reference values for the canine species, providing essential data to characterize the animals. MATERIALS AND METHODS Animals TNF-α and IL-10 serum levels determination Dogs of different ages, irrespective weight, breed and sex, from the Zoonosis Control Center of Fortaleza city (CCZ) and private kennels were used. All animals underwent a complete clinical examination, performed by appropriately trained professionals, and serological screening test for chromatographic immunoassay1 and confirmatory enzyme linked immunosorbent assay (ELISA)1 for CVL were performed according to manufacturer’s instructions. For canine Cytokines levels were determined by enzymelinked immunosorbent assay (ELISA), according to the manufacturer’s instructions. DuoSet ELISA5 (Immunoassay - R&D Systems) was used for analysis of anti-canine TNF-α and IL-10. Minimum sensitivity was 63 ρg/mL for TNF-α. All experiments were performed using 96-well plates. The reading was performed using the microplate automatic reader, at a wavelength of 450 nm. 2 J.C.C. Freitas, A.L. Lima, C.T.A. Rodriguês, et al. 2014. TNF-α and IL-10 Modulate CK and CK-MB Activities in Naturally Infected Dogs by Leishmania infantum. Acta Scientiae Veterinariae. 42: 1247. different clinical forms, were determined by Spearman correlation test and linear regression, respectively. To evaluate the relationship between the onset of clinical signs and the TNF-α, IL-10, IL-10/TNF-α ratio, CK, CK-MB and CK-MB/CK ratio serum levels (P value) the unpaired t-test was used. All analyzes were performed using the statistical program GraphPad Prism 5.0, with a significance level of 5%. Statistical Analysis TNF-α, IL-10, IL-10/TNF-α ratio, CK, CK-MB and CK-MB/CK ratio serum levels were expressed as mean and standard deviation and analyzed by KruskalWallys test, followed by Dunn’s test, for comparison of three groups (ND, AD and SD). The correlation and influence of TNF-α, IL-10 and IL-10/TNF-α ratio serum levels on CK, CK-MB and CK-MB/CK ratio, in the Table 1. Mean of TNF-α, IL-10, IL-10/TNF-α ratio, CK, CK-MB and CK-MB/CK ratio serum levels in naturally infected dogs by L. infantum and their respective reference values for canine species. Parameter TNF-α (ρg/mL) IL-10 (ρg/mL) IL-10/TNF-α Ratio CK (U/L x100) CK-MB (U/L x100) CK-MB/CK Ratio ND 39.36±3.14 17.57±0.86 0.46±0.17 0.82±0.04 0.55±0.12 0.68±0.04 AD 35.43±2.15 25.62±1.02 0.71±0.30 1.58±0.81 1.36±0.22 0.61±0.13 SD 37.25±3.04 24.93±1.78 0.64±0.15 1.86±0.06 1.38±0.29 0.59±0.11 Reference* a ≤10.0 b ≤10.0 -------------c 0.24 - 1.70 c 0.11 - 0.39 c 0.19 - 0.69 * aAccording to Resende et al. [34]. bAccording to Boggiatto et al. [8]. cAccording to Lopes et al. [24]. significantly increased in asymptomatic animals, when compared to the negative animals (P < 0.05). SD showed no significant difference, when compared to ND and AD (P < 0.05) (Figure 1). The correlation and influence of TNF-α, IL-10 and IL-10/TNF-α ratio serum levels on CK, CK-MB and CK-MB/CK ratio, in AD and SD groups, were expressed in Figures 2, 3, 4 and 5. TNF-α serum levels showed a moderate correlation (ρ = -0.55 and ρ = 0.38) and weak negative (y = 4.79 - 0.09x) and positive influence (y = 0.16 + 0.04x) on CK serum levels, in AD and SD, respectively (Figure 2). The same way occurs with respect to CKMB serum levels, in AD (ρ = -0.2 and y = 2.05 - 0.02x) and SD (ρ = 0.5 and y = 0.84 + 0.04x), respectively (Figure 3). IL-10 serum levels also showed moderate correlation (ρ = -0.2, ρ = 0.5, ρ = -0.05 and ρ = 0.85) and weak negative influence (y = 2.05 - 0.02x and y = 1.82 - 0.02x), in AD, and positive (y = 0.84 + 0.04x and y = -0.02 + 0.06x), in SD, both as compared to CK and CK-MB, respectively (Figures 2 and 3). IL-10/TNF-α ratio presented a weak correlation (ρ = -0.4 and ρ = 0.05), and moderate negative influence (y = 2.05 - 0.67x and y = 1.68 - 0.44x), in AD, and moderate correlation (ρ = 0.54 and ρ = 0.78) and strong positive influence (y = -0.2 + 3.35x and y = -1.77 + 4.91x), in SD, on CK and CK-MB serum levels, respectively (Figure 4). RESULTS The mean values of TNF-α (ρg/mL), IL-10 (ρg/mL) IL-10/TNF-α ratio, CK (U/L x100), CK-MB (U/L x100) and CK-MB/CK ratio serum levels, in all groups (ND, AD and SD), were shown in Table 1 and Figure 1. The relationship between the clinical signs onset and serum levels of TNF-α, IL-10, IL-10/TNF-α ratio, CK, CK-MB and CK-MB/CK ratio (P value) were shown in Table 2. CK serum levels were significantly increased in infected animals (AD and SD), when compared to negative animals (ND). Already the CK-MB serum levels were significantly increased in symptomatic animals (SD), when compared to negative animals (ND). However, the asymptomatic animals (AD) showed no significant differences, in CK-MB serum levels, when compared to animals belonging to ND and SD. CK-MB/CK ratio, in the different groups, showed no significant differences (P < 0.05) [Figure 1]. CK, CKMB and CK-MB/CK ratio serum levels did not present relation with the characteristic clinical signs onset (P < 0.05) [Table 2]. Additionally, all animals (ND, AD and SD) were negative to troponin I test, ie, with serum levels <0.5 ng/mL. Evaluation of TNF-α and IL-10 serum levels, in the different groups, showed no significant differences (P < 0.05). Already IL-10/TNF-α ratio was 3 J.C.C. Freitas, A.L. Lima, C.T.A. Rodriguês, et al. 2014. TNF-α and IL-10 Modulate CK and CK-MB Activities in Naturally Infected Dogs by Leishmania infantum. Acta Scientiae Veterinariae. 42: 1247. It was also observed weak and moderate correlations (ρ = 0.08 and ρ = 0.59), besides the weak and strong positive influences, of the IL-10/TNF-α ratio on CK-MB/CK ratio (y = 0.78 + 0.03x and y = -0.21 + 1.58x), in AD and SD, respectively (Figure 5). Furthermore, it was observed that the TNF-α, IL-10, IL-10/TNF-α ratio serum levels did not present relation with the characteristic clinical signs onset (P < 0.05) [Table 2]. Table 2. P value of TNF-α, IL-10, IL-10/TNF-α ratio, CK, CK-MB and CK-MB/CK ratio serum levels in naturally infected dogs by Leishmania infantum. Where P compares the differences in serum levels from asymptomatic (AD) and symptomatic (SD) dogs. Parameter (AD x SD) P value TNF-α 0.55 IL-10 0.90 IL-10/TNF-α Ratio 0.50 CK 0.51 CK-MB 0.97 CK-MB/CK Ratio 0.99 Figure 1. TNF-α, IL-10, IL-10/TNF-α ratio, CK, CK-MB and CK-MB/CK ratio in the different clinical forms of naturally infected dogs by Leishmania infantum. Different letters represent differences at a significance level of 5%. 4 J.C.C. Freitas, A.L. Lima, C.T.A. Rodriguês, et al. 2014. TNF-α and IL-10 Modulate CK and CK-MB Activities in Naturally Infected Dogs by Leishmania infantum. Acta Scientiae Veterinariae. 42: 1247. Figure 2. Correlation and influence of TNF-α and IL-10 on CK serum levels, in the different clinical forms (AD and SD), in naturally infected dogs by Leishmania infantum. Figure 3. Correlation and influence of TNF-α and IL-10 on CK-MB serum levels, in the different clinical forms (AD and SD), in naturally infected dogs by Leishmania infantum. 5 J.C.C. Freitas, A.L. Lima, C.T.A. Rodriguês, et al. 2014. TNF-α and IL-10 Modulate CK and CK-MB Activities in Naturally Infected Dogs by Leishmania infantum. Acta Scientiae Veterinariae. 42: 1247. Figure 4. Correlation and influence of IL-10/TNF-α ratio on CK and CK-MB serum levels, in the different clinical forms (AD and SD), in naturally infected dogs by Leishmania infantum. Figure 5. Correlation and influence of IL-10/TNF-α ratio on CK-MB/CK ratio, in the different clinical forms (AD and SD), in naturally infected dogs by Leishmania infantum. disease development [2,9,20], being associated with the parasites persistence in the lesions [1,7]. In the present study, TNF-α and IL-10 serum levels (Figure 1), in naturally infected dogs by L. infantum, both symptomatic (SD) and asymptomatic (AD), showed no significant differences when compared to non-infected animals (ND). Already when evaluating IL-10/TNF-α ratio, significant increase was observed in AD, when compared to SD and to ND (Figure 1), showing the IL-10 predominance, in these animals, in relation to TNF-α. However, it has been reported that elevated IL- DISCUSSION TNF-α is an important cytokine that is related to proinflammatory activity; on the other hand, IL-10 is a cytokine associated to immunoregulatory activity. TNF-α has been associated to the infections control caused by intracellular parasites, through the Th1-mediated response activation [2,6] while IL-10 modulate the Th1-mediated response and it prevents host damages caused by exacerbated inflammatory responses [4,29]. In CVL, IL-10 inhibits the macrophages and dendritic cells response, facilitating the 6 J.C.C. Freitas, A.L. Lima, C.T.A. Rodriguês, et al. 2014. TNF-α and IL-10 Modulate CK and CK-MB Activities in Naturally Infected Dogs by Leishmania infantum. Acta Scientiae Veterinariae. 42: 1247. 10 and TNF-α serum levels, in naturally infected dogs by L. infantum, are associated, respectively, to the onset or not of disease clinical signs [6]. Here, we can also observe that there was no relationship of IL-10 and TNF-α serum levels with the clinical signs onset of the disease (Table 2). It has been demonstrated that the affected animals by visceral leishmaniasis presented cardiac lesions [25,27]. To evaluate the parasite influence on biomarkers related to heart diseases, the confirmation of doubtful cases, to establish prognosis and deciding on a specific treatment [26,30], it is necessary to evaluate the clinical signs onset. In this work it was used the measurement of troponin I, creatine kinase (CK), and its MB subunit (CK-MB) serum levels and its correlation with IL-10 and TNF-α serum levels. CK is identified as a highly sensitive and specific biomarker of muscle injury, which may be expressed in skeletal and cardiac muscle tissue [13]. As CK is an extravasation enzyme, in cases of reversible muscle damage or necrosis, may be an increase in their serum levels in animals [5,12]. In this study, CK serum levels were significantly increased in AD and SD, regardless of their clinical presentation, compared to ND (Figure 2). However, the mean values, of ND and AD, were within of the reference values for the canine species and increased, in SD, when compared to reference values (0.24 - 1.70 U/L x100) [24] (Table 1). Significant increase in CK-MB serum levels in SD was observed in the present study, when compared to ND (Figure 2). A similar result was described in dogs with and myocardial damage secondary to parvovirus, heartworm, endocarditis, hypertrophic cardiomyopathy and CVL [10,27,31,36], being CK-MB described as a sensitive and specific cardiac marker, can be used for detection of myocardial injury in these diseases. When compared to the reference values of the canine species (0.11 - 0.39 U/L x100) [24], the CK-MB serum levels, in this study, were significantly increased (Table 1). CK-MB serum levels are pointed as biomarker of cardiac and muscular injury [5,40]. However, it has been reported that elevated CK-MB serum levels cannot be directly associated with myocardial impairment, since it can also be found in other tissues, even in smaller amount, thus lowering their specificity [13]. Furthermore, macromolecules can be formed (macro CK 1 and 2), altering the results of the measurement, inducing false-positive data [22]. To eliminate this interference in our study the samples were heated. The assessment of troponin I has high sensitivity and specificity in the detection of cardiac damage in dogs [35]. Troponin I blood release occurs by dissociation of the contractile apparatus and relate to irreversible myocardial cell injury [32]. Here, it can be related that none of the dogs, both symptomatic and asymptomatic, presented troponin I serum levels above 0.5 ng/mL, suggesting the absence of heart muscle damage. Similar values were obtained in a study performed by Santos et al. [35] in animals demonstrably without myocardial damage. These data could indicate that the increase of CK and CK-MB serum levels is directly associated with impairment of the skeletal muscle integrity [5]. The muscular and cardiac changes can be directly related to the action of inflammatory mediators, such as TNF-α, IL-1β and IL-6, which may act directly on the functionality and structure of cardiac and skeletal muscle [17,23,39]. The increased production of proinflammatory cytokines, in CVL, has been described [11,21,28]. In our work, TNF-α and IL-10 serum levels did not differ significantly among the groups (ND, AD and SD) [Figure 1]. However, both cytokines showed increased serum concentrations, when compared to the reference values (< 10 ρg/mL), according to Resende et al. [34], for TNF-α, and Boggiatto et al. [8], for IL-10 (Table 1). However, it have been reported significantly increased TNF-α serum levels in AD when compared to SD [28]. In this study, TNF-α showed a slight negative influence on CK (Figure 3) and CK-MB (Figure 4) in AD. However, slight positive influence on the same biomarkers, in symptomatic dogs was observed. IL-10 behaved similarly to TNF-α, regardless of the animal clinical status, in relation to the cardiac biomarkers (CK and CK-MB). Our findings demonstrate the predominance of IL-10 in AD, and TNF-α in SD. These results may be associated to increase of others pro-inflammatory cytokines production, which can potentiate the TNF-α action and promote degenerative effect on cardiac and skeletal muscle. Similar results were observed for in humans and rats, where also there was verified the remodeling effect of heart muscles associated with the action of TNF-α and consequent production of metalloproteinases [37,38]. IL-10/TNF-α ratio evaluation on CK and CKMB serum levels (Figure 5) corroborate with greater 7 J.C.C. Freitas, A.L. Lima, C.T.A. Rodriguês, et al. 2014. TNF-α and IL-10 Modulate CK and CK-MB Activities in Naturally Infected Dogs by Leishmania infantum. Acta Scientiae Veterinariae. 42: 1247. emphasis on what has been described previously, where it was observed a slight negative influence, in AD, highlighting the predominance of the IL-10 action, and a strong positive influence in SD, suggesting the participation of other pro-inflammatory cytokines, with increased production in CVL, in addition to the effects of TNF-α. Furthermore, we may report that, in the present study, none of the biomarkers assessed has influenced the characteristic clinical signs onset (Table 2). This reinforces the hypothesis of the involvement of other cytokines that can potentiate this effect. Therefore, in summary, the increase of CK and CK-MB serum levels in AD and SD is directly related to the impairment of skeletal muscles, due to the elevated production of proinflammatory cytokines, since the analysis of a cardiac marker more specific, such as troponin I, excludes the possibility of heart damage. Furthermore, our results suggest that the L. infantum infection can trigger cardiovascular disorders, regardless of their relationship with the increased production of TNF-α. However, CK and CK-MB, individually evaluated, were not presented as good markers of cardiac damage in naturally infected dogs by L. infantum. More studies are needed for the understanding of the molecular mechanisms that modulate the production, as well as, the participation of other cytokines that may be involved in the process. MANUFACTURERS Instituto de Tecnologia em Imunobiológicos (Bio-Manguinhos). Rio 1 de Janeiro, RJ, Brazil. Bioeasy Diagnóstica Ltda. São Paulo, SP, Brazil. 2 Bioclin Inc. Brussels, Belgium. 3 Wiener Lab Group. Rosario, Argentina. 4 R&D Systems. Minneapolis, MN, USA. 5 Funding. This work was financial supported by Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES) and Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP). Ethical approval. The experimental procedures were performed in accordance with the ethical principles of animal experimentation, adopted by the Brazilian College of Animal Experimentation (COBEA) and was approved by the Ethics Committee for Animal Use of the State University of Ceará, Protocol No. 12783812-0. Declaration of interest. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. REFERENCES 1 Alexander J. & Bryson K. 2005. T helper (h)1/Th2 and Leishmania: paradox rather than paradigm. Immunology Letters. 99(1): 17-23. 2 Alves C.F., Amorim I.F.G., Moura E.P., Ribeiro R.R. Alves C.F., Michalick M.S., Kalapothakis E., Bruna-Romero O., Tafuri W.L., Teixeira M.M. & Melo M.N. 2009. Expression of IFN-gamma, TNF-alpha, IL-10 and TGF-beta in lymph nodes associates with parasite load and clinical form of disease in dogs naturally infected with Leishmania (Leishmania) chagasi. Veterinary Immunology and Immunopathology. 128(4): 349-358. 3 Anker S.D. & von Haehling S. 2004. Inflammatory mediators in chronic heart failure: an overview. Heart. 90(4): 464470. 4Asadullah K., Sterry W. & Volk H.D. 2003. Interleukin 10 therapy - Review of a new approach. Pharmacological Reviews. 55(2): 241-269. 5 Baird M.F., Graham S.M., Baker J.S. & Bickerstaff G.F. 2012. Creatine-kinase and exercise related muscle damage implications for muscle performance and recovery. Journal of Nutrition and Metabolism. 2012: 1-13. 6Barbiéri C.L. 2006. Immunology of canine leishmaniasis. Parasite Immunology. 28(7): 329-337. 7Belkaid Y., Hoffmann K.F., Mendez S., Kamhawi S., Udey M.C., Wynn T.A. & Sacks D.L. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. The Journal of Experimental Medicine. 194(10): 1497-1506. 8Boggiatto P.M., Ramer-Tait A.E., Metz K., Kramer E.E., Gibson-Corley K., Mullin K., Hostetter J.M., Gallup J.M., Jones D.E. & Petersen C.A. 2010. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clinical and Vaccine Immunology. 17(2): 267-273. 9 Caldas A., Favali C., Aquino D., Vinhas V., van Weyenbergh J., Brodskyn C., Costa J., Barral-Neto M. & Barral A. 2005. Balance of IL-10 and interferon-gamma plasma levels in human visceral leishmaniasis: implications in the pathogenesis. BMC Infectious Diseases. 5: 113. 8 J.C.C. Freitas, A.L. Lima, C.T.A. Rodriguês, et al. 2014. TNF-α and IL-10 Modulate CK and CK-MB Activities in Naturally Infected Dogs by Leishmania infantum. Acta Scientiae Veterinariae. 42: 1247. 10Carreton E., Morchon R., Gonzalez-Miguel J., Juste M.C., Simon F. & Montoya-Alonso J.A. 2013. Utility of cardiac biomarkers during adulticide treatment of heartworm disease (Dirofilaria immitis) in dogs. Veterinary Parasitology. 197(1 - 2): 244-250. 11 Carrillo E. & Moreno J. 2009. Cytokine profiles in canine visceral leishmaniasis. Veterinary Immunology and Immunopathology. 128(1 - 3): 67-70. 12 Costa A.C.F. & Saraiva J.F.K. 2005. Pequenas elevações nos níveis de creatinina plasmática como marcador prognóstico na insuficiência cardíaca. Revista Ciências Médicas. 14(3): 267-271. 13 Ferreira F.S., Silveira L.L., Costa A.C., Albernaz A.P., Carvalho C.B. & Oliveira A.L.A. 2010. Estudo do comportamento da creatino quinase (CK) e creatino quinase-mb (CK-MB) sérica de cães submetidos à oxigenação por membrana extracorpórea (ECMO) durante um período de três horas. Ciência Animal Brasileira. 11(3): 705-712. 14 Fonfara S., Loureiro J., Swift S., James R., Cripps P. & Dukes-McEwan J. 2010. Cardiac troponin I as a marker for severity and prognosis of cardiac disease in dogs. Veterinary Journal. 184(3): 334-339. 15 Freitas J.C.C., Nunes-Pinheiro D.C.S., Lopes-Neto B.E., Santos G.J.L., Abreu C.R.A., Braga R.R., Campos R.M. & Oliveira L.F. 2012. Clinical and laboratory alterations in dogs naturally infected by Leishmania chagasi. Revista da Sociedade Brasileira de Medicina Tropical. 45(1): 24-29. 16 Freitas J.C.C., Ferreira F.V.A., Oliveira E.S. & Nunes-Pinheiro D.C.S. 2013. Canine visceral leishmaniasis: structural and immune-inflammatory changes in lymphoid organs of naturally infected dogs. Acta Scientiae Veterinariae. 41: 1165. 17 Gielen S., Adams V., Linke A., Erbs S., Mobius-Winkler S., Schubert A., Schuler G. & Hambrecht R. 2005. Exercise training in chronic heart failure: correlation between reduced local inflammation and improved oxidative capacity in the skeletal muscle. European Journal of Cardiovascular Prevention & Rehabilitation. 12(4): 393-400. 18 Gregory D.J., Godbout M., Contreras I., Forget G. & Olivier M. 2008. A novel form of NF-κB is induced by Leishmania infection: Involvement in macrophage gene expression. European Journal of Immunology. 38(4): 1071-1081. 19 Kaur K., Sharma A. & Singal P. 2006. Significance of changes in TNF-(alpha) and IL-10 levels in the progression of heart failure subsequent to myocardial infarction. American Journal Physiology Heart and Circulatory Physiology. 291(1): H106-13. 20 Leavy O. 2007. T helper cells: Self-control by TH1 cells. Nature Reviews Immunology. 7(3): 171. 21 Lima V.M., Peiro J.R. & Oliveira-Vasconcelos R. 2007. IL-6 and TNF-alpha production during active canine visceral leishmaniasis. Veterinary Immunology and Immunopathology. 115(1-2): 189-193. 22 Liu C.Y., Lai Y.C., Wu Y.C., Tzeng C.H. & Lee S.D. 2010. Macroenzyme creatine kinase in the era of modern laboratory medicine. Journal of the Chinese Medical Association. 73(1): 35-39. 23 Lopes R.D., Batista-Junior M.L., Rosa J.C., Lira F.S., Martins-Junior E., Shimura A.Y., Brum P.C., LanchaJunior A.H., Seelaender M.C.L. & Lopes A.C. 2010. Changes in the production of IL-10 and TNF-α in skeletal muscle of rats with heart failure secondary to acute myocardial infarction. Arquivo Brasileiro de Cardiologia. 94(3): 293-300. 24 Lopes S.T.A., Franciscato C., Teixeira L.V., Oliveira T.G.M., Germatz B.C., Veiga A.P.M. & Mazzanti A. 2005. Determinação da creatina quinase em cães. Revista da FZVA. 12(1): 116-122. 25 Lopez-Peña M., Alemañ N., Muñoz F., Fondevila D., Suaréz M.L., Goicoa A. & Nieto J.M. 2009. Visceral leishmaniasis with cardiac involvement in a dog: a case report. Acta Veterinaria Scandinavica. 51(1): 20-22. 26 Lozovoy M.A.B., Priesnitz J.C. & Silva S.A. 2008. Infarto agudo do miocárdio: Aspectos clínicos e laboratoriais. Interbio. 2(1): 4-10. 27 Mendes R.S., Gurjão T.A., Oliveira L.M., Santana V.L., Tafuri W.L., Santos J.R.S., Dantas A.F.M. & Souza A.P. 2014. Miocardite crônica em um cão naturalmente infectado com Leishmania (Leishmania) infantum chagasi: aspectos clínicos e patológicos. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 66(1): 79-84. 28 Menezes-Souza D., Correa-Oliveira R., Guerra-Sá R., Giunchetti R.C., Teixeira-Carvalho A., Martins-Filho O.A., Oliveira G.C. & Reis A.B. 2011. Cytokine and transcription factor profiles in the skin of dogs naturally infected by Leishmania (Leishmania) chagasi presenting distinct cutaneous parasite density and clinical status. Veterinary Parasitology. 177(1-2): 39-49. 29 Ouyang W., Rutz S., Crellin N.K., Valdez P.A. & Hymowitz S.G. 2011. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual Review of Immunology. 29: 71-109. 30 Oyama M.A. & Sisson D.D. 2004. Cardiac troponin-I concentration in dogs with cardiac disease. Journal of Veterinary Internal Medicine. 18(6): 831-839. 9 J.C.C. Freitas, A.L. Lima, C.T.A. Rodriguês, et al. 2014. TNF-α and IL-10 Modulate CK and CK-MB Activities in Naturally Infected Dogs by Leishmania infantum. Acta Scientiae Veterinariae. 42: 1247. 31 Pino O., Li O., Alvarado A., Fernández V., Dávila R. & Gavidia C. 2008. Determinación de los niveles séricos de enzimas cardíacas en perros adultos con enfermedad cardiovascular. Revista de Investigación Veterinária del Peru. 19(2): 144-147. 32 Procajlo A., Zbanyszek M., Sobiech P., Stopyra A. & Rajski K. 2005. Troponin: a new marker in the diagnostics of muscle diseases in animals. Journal of Veterinary Science. 6(4): 297-309. 33 Reis A.B., Teixeira-Carvalho A., Vale A.M., Marques M.J. & Giunchetti R.C. 2006. Isotype patterns of immunoglobulins: hallmarks for clinical status and tissue parasite density in Brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Veterinary Immunology and Immunopathology. 112(3-4): 102-116. 34 Resende L.A., Roatt B.M., Aguiar-Soares R.D.O., Viana K.F., Mendonça L.Z., Lanna M.F., Silveira-Lemos D., Correa-Oliveira R., Martins-Filho O.A., Fujiwara R.T., Carneiro C.M., Reis A.B. & Giunchetti R.C. 2013. Cytokine and nitric oxide patterns in dogs immunized with LBSap vaccine, before and after experimental challenge with Leishmania chagasi plus saliva of Lutzomyia longipalpis. Veterinary Parasitology. 198(3-4): 371-381. 35Santos A.L.F., Larsson M.H.M.A., Pereira G.G., Santos M.M. & Gutierrez V.C.R. 2011. Dosagem sérica de troponina I em cães com desnível do segmento ST utilizando quimioluminescência. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 63(6): 1330-1336. 36 Souza A.I., Paulino-Junior D., Sousa M.G. & Camacho A.A. 2008. Aspectos clínico-laboratoriais da infecção natural por Trypanosoma cruzi em cães de Mato Grosso do Sul. Ciência Rural. 38(5): 1351-1356. 37 Stumpf C., Lehner C., Yilmaz A., Daniel W.G. & Garlichs C.D. 2003. Decrease of serum levels of the anti-inflammatory cytokine interleukin-10 in patients with advanced chronic heart failure. Clinical Science. 105(1): 45-50. 38 Stumpf C., Seybold K., Petzi S., Wasmeier G., Raaz D., Yilmaz A., Anger T., Daniel W.G. & Garlichs C.D. 2008. Interleukin-10 improves left ventricular function in rats with heart failure subsequent to myocardial infarction. The European Journal of Heart Failure. 10(8): 733-739. 39 Toth M.J., Ades P.A., Tischler M.D., Tracy R.P. & LeWinter M.M. 2006. Immune activation is associated with reduced skeletal muscle mass and physical function in chronic heart failure. International Journal of Cardiology. 109(2): 179-87. 40 Yonezawa L.A., Silveira V.F., Machado L.P. & Kahayagawa A. 2009. Marcadores cardíacos na medicina veterinária. Ciência Rural. 40(1): 222-230. www.ufrgs.br/actavet 10 1247