Week 10
advertisement

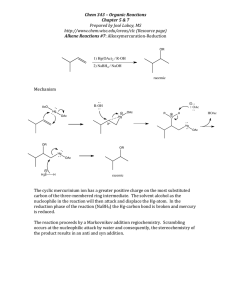
M.C. White, Chem 153 Nu attack on Olefins -327- Week of November 25, 2002 Olefin functionalization via metal promoted Nu attack C Recall that the balance of electron flow in olefin-metal bonding can be shifted predominantly in one direction depending on the electronic properties of the metal. If the metal is electron withdrawing, M-L σ-bonding predominates and withdraws electron density from the π-bond of the olefin (see Structure and Bonding, pg. 39). This results in the induction of a δ+ charge on the olefin that activates it towards nucleophilic attack. M C Dewar-ChattDuncanson Model C-H σ-donation to the electrophilic metal activates the metal alkyl towards β-hydride elimination. olefin σ-donation to the electrophilic metal activates it towards Nu attack R Nu δ+ X Pd X L Nu X PdII X II L Nu R R X PdII L R Nu X H Pd II L H recall that the equilibrium for late metals lies towards the olefin form which is stabilized via π-backbonding. σ donation>> π-backbonding Catalyst regeneration stoichiometric oxidants H H2O2 L O 2/ 2 HX Pd II O L X L Pd L II RE Pd II O O L H L Pd L II 0 2 Cu X2 L H L O I 2 Cu X HX O L H+ O OH OH X PdII X PdIILn Pd 0Ln O HO 2 HX Pd IILn O O O H+ OH M.C. White, Chem 153 Nu attack on Olefins -328- Week of November 25, 2002 Wacker Oxidation PdCl2 (cat) CuCl2 (cat) O2, H2O, HCl R O 2 mechanistic possibilities for hydroxypalladation: R OH The Wacker oxidation is used industrially to produce ~ 4 million tons of acetaldehyde/year. H2O Cl PdII OH H2O Cl Pd II Cl Cl 2 CuCl H 2O 2 CuCl2 Pd R Cl II H2O OH 2 PdII Cl O PdII Cl H2O Cl R Pd II Pd II H H2O Cl D R OH H OH 2 LnPdII H H β-hydride elimination D H LnPd II H+ D OH H D CO OH HO Commericial production of acetaldehyde H D H DH D O · Binding specificity: terminal olefins · Regioselectivity: 2o carbon · Remote functionality tolerated OH Deuterium labeling study indicates that hydroxypalladation proceeds via palladium-nucleophile anti-addition. R R Pd Cl II OH 2 R OH 2+ HCl H2O Cl Pd anti hydroxypalladation II Cl R LnPd(0) H H2O Cl HCl H2O R R Cl H2O Pd II Cl H 2O hydroxypalladation 1/2 O2 + 2 HCl H2O R R H2O syn hydroxypalladation O Stille JOMC 1979 (169) 239. Pd 0Ln O HD OH Pd IILn M.C. White, Chem 153 Nu attack on Olefins -329- Week of November 25, 2002 Oxidation of terminal olefins Standard Wacker conditions: Selective oxidation of terminal olefins O PdCl2 (cat.) CuCl2 (cat.)/O2 DMF/H2O O O 70% Chadha J. Chem. Soc. Perkin I 1979 2346. Cuprous chloride (CuCl)/O2 as the oxidant system leads to faster reactions with no chlorinated biproducts. H PdCl2 (30 mol%) CuCl (1.6 eq)/O2 DMF/H2O (10:1.2) O H H H OTHP H PdCl2 (20 mol%) CuCl (10 mol%)/O2 DMF/H2O (9:1) O H 77% O O OTHP O O O 91% OTBS OTBS O Ikegami Tetrahedron 1981 (37) 4411. Money Tetrahedron 1996 (52) 6307. Benzoquinone can be used as a stoichiometric oxidant: Cu(OAc)2/O2 oxidant system: O O H O O PdCl2 (10 mol%) Cu(OAc)2 (20 mol%)/O2 DMF: H 2O (7:1) O Pd(OAc)2 (10 mol%) HClO4 (0.3M), CH 3CN H O O H H O O 85% Smith JACS 1999 (121) 10468. O O Santelli TL 1994 (35) 6481. O H H H H M.C. White,M.S. Taylor Chem 153 Olefin Nu attack -330- Week of November 25, 2002 Palladium (II)-mediated [3,3]-sigmatropic rearrangements PdCl2(MeCN)2 (10 mol%) OAc BnO OBn PdCl2(MeCN)2 (10 mol%) BnO OBn THF, 23°C OAc OBn THF, 23°C OAc OAc OAc BnO 82% BnOH2C Cl2Pd R OAc BnOH2C O Cl2Pd O BnOH2C O R O O Cl2Pd CH3 CH3 R CH3 Saito TL 1988 (29) 1157. The vinyl ether 1 is unreactive to Pd(II) catalysis. However, the similar substrate 2 shows good reactivity: Et O O PdCl2(MeCN)2 (10 mol%) THF, 23°C 1 Et Recovered starting material O PdCl2(MeCN)2 (10 mol%) THF, 23°C Me 2 Et O Me 71% Bickelhaupt TL 1986 (27) 6267. The authors speculate that when reacting with 1, Pd(II) binds preferentially to the electron-rich vinyl ether olefin over the terminal olefin. Such binding does not lead to a productive reaction. When the steric bulk of the vinyl ether is increased, as in 2, binding to the vinyl ether is less favourable. Pd(II) coordination to the terminal allylic olefin occurs resulting in catalysis of the Claisen rearrrangement. Note that these structural requirements limit the scope of this reaction. M.C. White, Chem 153 Nu attack on Olefins -331- Week of November 25, 2002 Cyclic ether formation Pd(OAc)2 (20 mol%) Cu(OAc)2 (50 mol%)/O2 MeOH/H2O OH Pd(OAc)2 O 54% H LnPdII Pd(OAc)2 O OAc Cycle A OAc 2 Cu(OAc) 1/2 O2 + 2 HOAc 2 Cu(OAc)2 H 2O Cycle B Pd II O H PdII O OH 5-exo-trig O HOAc O HOAc LnPd0 Hosokawa Bull. Chem. Soc. Jpn. 1975 (48) 1533. Asymmetric version: 5 membered ring formation Pd(OCOCF3) 2 (10 mol%) (S,S)-boxax (10 mol%) MeOH O OH O O (4 eq) 5-exo-trig 71 % yield 93 % ee 6 membered ring formation O Pd(OCOCF3) 2 (10 mol%) (S,S)-boxax (10 mol%) MeOH R N PdII * O 6-exo-trig Hayashi JACS 1997 (119) 5063. O OCOCF3 N O OH R (4 eq) 61 % yield 97 % ee O OCOCF3 M.C. White, M.S. Taylor Chem 15 Nu attack on Olefins -332- Week of November 25, 2002 Application of an intramolecular Wacker oxidation in the synthesis of Garsubellin A O O O O O O O Na2P dCl4 (40 mol%) t-BuOOH O O HO O O O O HO AcOH / H2O 69% – HCl AcOH / H2 O Pd 0 Garsubellin A O O O reductive elimination of HCl HClPdII O t-BuOOH HCl HO OH β-hydride elimination O O O O HO HO OH H O HO Shibasaki OL 2002 (4) 859. O O HO PdIICl2 Cl2PdII O Pd IICl2 O O O – HCl PdIICl O O O M.C. White, Chem 153 Nu attack on Olefins -333- Week of November 25, 2002 Tandem oxopalladation/Heck-type cyclization H H i-Pr i-Pr i-Pr i-Pr N O Pd II OCOCF3 20 mol% F3 COCO N O H OBz O HO OBz 5%, 45% ee + o O (4 eq) CH 2Cl2, 0 C O 68%, 95% ee (single diastereomer) OBz O 27%, 60% ee Sasai JACS 2001 (123) 2907. * N Proposed mechanism: N OCOCF3 II Pd OCOCF3 OH HO 6-endo-trig O OH N Pd II OCOCF3 OCOCF3 H OBz 2 HOCOCF3 + N Pd II N N Pd O OCOCF3 H N OBz O OCOCF3 H OBz O N Pd N N II OBz O H OCOCF3 HOCOCF3 H N N Pd 0 O OBz N Pd II N O OBz O OBz H N O OBz OCOCF3 Pd II N N O OBz HOCOCF3 Pd II N O N H N OBz Pd II H OCOCF3 M.C. White, Chem 153 Nu attack on Olefins -334- Week of November 25, 2002 Tandem sequences Oxo-palladation/intermolecular Heck AcO Pd(OAc)2 (10 mol%) CuCl (1 eq)/O2, DMF OH CO2Me CO2Me PdII O O CO2Me β-hydride elimination is not an option for this intermediate (or Ph) 89% Semmelhack TL 1993 (34) 7205. Oxo-palladation/carbonylation L PdII Pd(Cl)2 (10 mol%) CuCl2 (3 eq), MeOH R CO OH R O Cl O CO Pd(Cl)Ln R O O HOMe R Semmelhack JACS 1984 (106) 1496. OMe O Pr OH Pr OMe O O Pd(Cl) 2 (10 mol%) CuCl2 (3 eq), MeOH CO O + 30% of the furan product O 70% note: O2 is not necessary to re-oxidize Pd if supra-stoichiometric amounts of Cu(II) salts are used OMe O OMe O CO PdII L Cl Pr OH O O O 70% (trans:cis ,3:1) Pr O CO2Me O CO2Me Semmelhack JACS 1982 (104) 5850. M.C. White, Chem 153 Nu attack on Olefins -335- Week of November 25, 2002 Nitrogen nucleophiles Conversion of o-allylanilines to indoles: Disubstituted olefins The excess quinone and the Cl are thought to alter the regioselectivity of aminopalladation by coordinating to the Pd. PdCl2(CH3CN)2 (10 mol%) THF N H R O N R O (1 eq) N R R= H, 86% R = Me, 89% R= C(O)CH3, 71% Both benzoquinone and Cu(II) salts are effective stoichiometric oxidants for Pd(0) in the presence of readily oxidized o-allylanilines and indoles. NH2 standard cat. conditions Hydrogenation of "trapped" Pd-alkyl intermediate leads to formal hydroamination product PdCl2(CH3CN)2 (10 mol%) LiCl (10 eq) O H2 PdCl2(CH3CN) (1 eq) NEt3, THF NH2 N H no reaction occurs in the absence of NEt3 + N H O (2 eq) PdClLn NH2 N H 79% N mj. product Hegedus JACS 1978 (100) 5800. Proposed mechanism: Asymmetric synthesis of 5-membered nitrogen heterocycles O NHTs 5 mol% pre-cat*/AgOCOCF3 CH2Cl 2:MeNO 2 (1:1) rt (10-20 h) OAc O X pre-catalyst: SiMe3 O t-Bu N I Pd II X NTs H X = O, 96% (91% ee) NH, 96% (90% ee) CH2, 95% (90% ee) C N Ts NH O OCOCF3 Pd II O R O or Fe N Pd C II HOCOCF3 O X Ts N * X N Ts N C O X Pd O R O Ts N * X O C O Pd II N O OCOR Overman JACS 2001 (124) 12. M.C. White, Chem 153 Nu attack on Olefins -336- Week of November 25, 2002 Nitrogen nucleophiles/O2 as a stoichiometric oxidant Pd(OAc)2 (10 mol%) Ts N pyridine (10 mol%) O2, toluene, 80oC NHR Ts N R = Ts, 87% Ns, 87% Cbz, 76% Ts N 91% 60% Proposed mechanism: 2 AcOH N N PdII O H2O2 N O PdII OAc Pd II OAc N O N NHR O N O2 OAc N N Pd0 N PdII Ph Ph OAc N NHR AcOH N Pd N II OAc statistically and sterically prefered site of elimination H N H AcOH OAc N Pd II N PdII OAc N Ts N NTs Stahl ACIEE 2002 (41) 164. NTs Stahl JACS 2001 (129) 7188. M.C. White, Chem 153 Nu attack on Olefins -337- Week of November 25, 2002 Hydroamination Ph Ph P OTf Pd II OTf P PhPh 2 mol% Fe NH 2 Original mechanism proposed: Ar NHAr NHPh P H H OTf Pd II P OTf toluene, 100oC 97% Ph 2 P P Pd II P Ph 2 NH 2 OTf NHPh OTf PdII OTf P OTf P P OTf OTf PdII NH2Ar ArH2N 10 mol% Ar Ar toluene, 45oC, 36 h >99% yield, 64% ee NH2Ar Hartwig JACS 2000 (122) 9546. Revised mechanism based on "isolation of a catalytic intermediate" P Pd P NAr OTf Ph 2 P Pd II P Ph 2 OTf II OTf NH2 Ar based on crystal P ArNH2 -H + P Pd P 0 H Pd II P OTf Ar P Pd inversion OTf P H Pd II II P P Ar OTf Me (S) Pd H P P H2NAr major diastereomer isolated P Pd II NHPh Ar H Hartwig claims the η3-arylethyl complex is chemically and kinetically competent to be a reactive intermediate. When heated in the presence of excess aniline at 75oC for 2h, hydroaminated products were formed in 61-83% yields (note, no direct comparison with the catalytic system under identical conditions is made). (S) H Me observed in catalytic reaction OTf P Ar retention NHAr (R) Me H observed NHAr NH 2Ar Hartwig claims that minor diastereomer (not isolated) reacts faster (3.5 x) based on a comparison of rates of the mj. vs. the mixture of η3-aryl complexes. Note that he never makes a reaction rate comparison with the catalytic reaction. Hartwig JACS 2002 (124) 1166. M.C. White, Chem 153 Nu attack on Olefins -338- Week of November 25, 2002 C nucleophiles Enol ether nucleophilic attack/ β-hydride elimination CO2Et O Pd(OAc)2 (1 eq) CH3CN SiMe3 O Ln(OAc)Pd CO2Et PdIILn SiMe3 H CO2Et O O O (OAc)HPd II A catalytic process for the addition of C nucleophiles to Pd(II) activated olefins is precluded in this case (as in most) by the competative oxidation of the carbon nucleophile by the stoichiometric oxidant. CO2Et O O 58% Quadrone Kende JACS 1982 (104) 5808. Hydroalkylation H O O O OH O O O R R R PdCl2(CH3CN) 2 5-10 mol% dioxane, rt Cl Pd Cl R PdCl2(CH3CN)2 II NCCH3 protonolysis O Cl PdII Cl NCCH3 H It's unclear why protonolysis rather than β-hydride elimination occurs in this system. Widenhoefer JACS 2001 (123) 11290. M.C. White, Chem 153 Q&A -339- Week of November 25, 2002 Question 1: Intermolecular [5+2] Propose a mechanism for the following transformation: O O O CH3 O H [Rh(CO) 2Cl]2 (2.5 mol%) C(O)CH3 CO (1-2 atm), dioxane, 60oC; H3O+ + OH Et Et O transannular closure O O C(O)CH 3 H O (CO) nClRh Et H OH O C(O)CH3 O O reductive elimination O O Et O O (CO)nClRh O O C(O)CH3 Et O H (CO) nClRh CO insertion C(O)CH 3 CO oxidative cyclization O O OH Et (CO)nClRh H3O+ work up Et (CO) nClRh O O C(O)CH3 O H (CO) nClRh C(O)CH 3 OH alkyne insertion O Et Et Et Wender JACS 2002 (124) 2876 O O CH3 CH3 O M.C. White, Q. Chen Chem 153 Q&A -340- Week of November 25, 2002 Question 2: Hydrozirconation 1) Et2Zn (1.1 eq.) -60 °C Cp 2Zr(H)Cl (1.1 eq.) TBDPSO CH2Cl2, 0 °C TBDPSO TBDPSO Zr(Cl)Cp 2 2) OH 94% over 3 steps H o O 0 C, 6 h The authors noted no loss of diene geometry upon hydrozirconation of this triene. Cp 2Zr(H)Cl (1.0 eq.) TBDPSO Zr(Cl)Cp 2 TBDPSO CH2Cl2, rt 1) n-BuNC, 0 °C to rt, 4h 2) 3M HCl, -78 °C to 0 °C OMe H TBDPSO O N S 54% over 3 steps (+)-curacin A Wipf JOC 1996 6556. M.C. White,M.S. Taylor Chem 153 Q&A -341- Week of November 25, 2002 Question 3: oxidative cyclization/hydrosilylation Provide a mechanism for the following transformation SiPh3 Ph3SiD MeO2C Pd 2(dba)3 (5 mol%) THF, 25°C, 2 hours MeO2C MeO2C MeO2C CH2D MeO2C + MeO2C Ph3Si DH2C A B 6:1 A:B SiPh3 CH2D MeO2C MeO2C MeO2C MeO2C Pd(0)L n DH2C SiPh3 B A MeO2C MeO2C D PdLn SiPh3 PdLn MeO2C MeO2C PdLn MeO2C MeO2C MeO2C MeO2C Ph 3Si DH2C MeO2C PdLn MeO2C Ph3SiD σ-bond metathesis σ-bond metathesis D MeO2C Takacs OM 1990 (9) 2877. MeO2C PdLn SiPh3 M.C. White,Q. Chen Chem 153 Question -341- Week of November 25, 2002 Question 1 An interesting synthesis of cyclopropanes has been recently reported by Grigg. Propose a mechanism for this transformation. I Pd 2(dba)3, TFP NHMe + NMe K2CO 3, DMF O O 68% O P TFP = 3 M.C. White,Q. Chen Chem 153 Question -342- Week of November 25, 2002 Question 2 Murai has described the synthesis of butanolides from a three component coupling shown below. Propose a mechanism for this process. O ethylene (3 atm) CO (5 atm) O S Me N Ru 3CO12 (2.5 mol%) tol., 140 °C, 20h O S N Me M.W. Kanan/M.C. White, Chem 15 Questions -section- Week of November 25, 2002 Section question 1: Cycloaddition Disconnections Provide a substrate or pair of substrates for the indicated cycloaddition reactions O H Rh(I) TsN H Rh(I) MeO2C MeO2C + Rh(I) O H Rh(I) O H Q. Chen /M.C. White,Chem 153 Question -section- Week of November 25, 2002 Section Question 2 Provide a mechanism for the following transformation: PdCl2 (10 mol%) CuCl2 (3.0 equiv.) NH NHCH3 O CO (1 atm) NaOAc (3.0 equiv.) HOAc, 23°C O N NMe O 95%