Left Ventricular Scar in Atrial Fibrillation: Cause or Effect?∗
advertisement

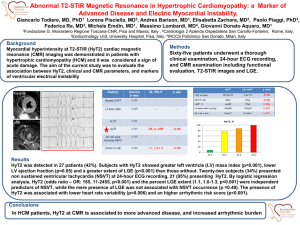
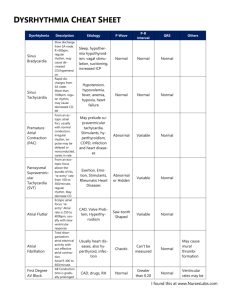
Journal of the American College of Cardiology Ó 2013 by the American College of Cardiology Foundation Published by Elsevier Inc. EDITORIAL COMMENT Left Ventricular Scar in Atrial Fibrillation Cause or Effect?* Zhiyu Ling, MD, Harikrishna Tandri, MD Baltimore, Maryland Atrial fibrillation (AF) is the most common clinical arrhythmia and is associated with increased risk for stroke, heart failure, and all-cause mortality (1–3). The mortality rate of patients with AF is about double that of patients in normal sinus rhythm, and data from the Framingham Heart Study show an AF-associated mortality risk even after adjusting for preexisting cardiovascular conditions (3). Although some of the mortality risk, such as stroke-related death (4) and worsening heart failure, could be traced back to AF, the fact remains that in most cases, AF is viewed merely an association (5–8). See page 2205 In this issue of the Journal, Neilan et al. (9) provide intriguing data that attempt to connect the dots between AF and all-cause mortality. In this study, pre-ablation late gadolinium enhancement (LGE) cardiac magnetic resonance (CMR) images of 664 consecutive patients with AF were analyzed for the presence of left ventricular (LV) fibrosis. None of the patients had a history of myocardial infarction. In this otherwise low-risk cohort, 88 patients (13%) had LV LGE on CMR. Furthermore, not only was this unanticipated finding associated with increased mortality, but the investigators also found a “dose effect”: the larger the LGE, the worse the mortality. Two obvious questions arise from this study: (1) What comes first, AF or LV fibrosis? and (2) Are they independent variables, or does one cause the other? Although no direct evidence exists, circumstantial evidence suggests that AF might be both the cause and subsequently the effect. AF has been shown to affect LV systolic function by causing a raterelated cardiomyopathy. In animal models, AF results in interstitial fibrosis with adverse LV remodeling (10). Although *Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology. From the Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital, Baltimore, Maryland. Both authors have reported that they have no relationships relevant to the contents of this paper to disclose. Downloaded From: https://content.onlinejacc.org/ on 10/01/2016 Vol. 62, No. 23, 2013 ISSN 0735-1097/$36.00 http://dx.doi.org/10.1016/j.jacc.2013.08.692 it is uncommon to find LV LGE in patients with paroxysmal AF who are referred for catheter ablation, this statement is not true especially in older patients with LV hypertrophy. Arterial stiffness and LV diastolic dysfunction are often encountered with older age and metabolic syndrome, which has been associated with both AF and LV fibrosis. In a longitudinal follow-up of older patients with pacemakers who had LV diastolic dysfunction and fibrosis detected by echocardiographic acoustic densitometry, the incidence of new AF was correlated with the severity of LV fibrosis, suggesting that LV scar comes first (11). In contrast, using T1 mapping, Ling et al. (12) showed diffuse LV myocardial fibrosis in older patients with AF, suggesting abnormal LV remodeling in AF. Focal LGE was also seen in 5 of 67 subjects (7%) in this study. Although mild interstitial fibrosis is common in older subjects, the onset of AF might accelerate the remodeling process, which may in turn perpetrate AF. This vicious cycle eventually will culminate in atrial and ventricular dysfunction, with or without focal or macroscopic LGE (13). The propensity to develop LGE might be multifactorial and might depend upon coronary perfusion, hypertension, diabetes, renal failure, and, last but not least, genetic factors that regulate injury and repair. Whatever the reason, the very finding of LGE on CMR appears to be not trivial and has important consequences. The presence of LV myocardial fibrosis identified by LGE CMR imaging is an independent predictor of adverse outcomes in various cardiomyopathies (14,15). In this study by Neilan et al. (9), patients with LGE were on average older and more likely to have heart failure and sleep apnea. Patients with LGE were also more likely to have lower glomerular filtration rates, lower LV ejection fractions, and increased LV mass and/or left atrial dimensions. Many of these comorbidities have been individually linked to both AF and to myocardial fibrosis. Unrecognized coronary disease was also prevalent in this cohort. Because of the confounders, which are well known to promote and maintain AF, and at the same time to cause adverse LV remodeling, the causal link between AF and LV fibrosis is difficult to ascertain. Yet another interesting question this study raises is the optimal imaging modality to image the left atrium before AF ablation. Noninvasive cardiac imaging plays an important role in pre-procedural planning and guidance of the procedures in many electrophysiology centers worldwide (16). Multiple different techniques exist for anatomical imaging, including angiography, computed tomography (CT), ultrasound, and CMR imaging. There are currently no guidelines, and the choice of imaging modality usually depends on local expertise and available equipment. Both CT and CMR imaging can provide accurate and detailed pulmonary vein anatomy. LGE CMR imaging can provide additional myocardial fibrosis information, but CMR has less availability, lower spatial resolution, and standard contraindications. Cardiac CT is widely available and may also provide additive 2216 Ling and Tandri LV Scar in Atrial Fibrillation: Cause or Effect? information beyond pulmonary vein anatomy. But CT is associated with radiation exposure and has less evidence for the identification of myocardial fibrosis. Neilan et al. (9) provide a valuable reference for choosing CMR as the preferred image modality before catheter ablation in certain groups of patients with AF, especially in those who are older, have heart failure or sleep apnea, have lower glomerular filtration rates or LV ejection fractions, and have increased LV mass or left atrial dimensions. The finding of LGE in the left ventricle might identify patients with occult coronary disease or cardiomyopathy or trigger aggressive risk factor management of modifiable risks such as sleep apnea and hypertension. Whether this strategy will better risk-stratify patients at risk for mortality and thus change the observed outcomes needs to be tested in prospective studies designed specifically to answer this question. Patients who undergo pulmonary vein isolation are just the tip of the AF iceberg, and those patients who are screened and are thought not to be good candidates for pulmonary vein isolation because of persistent AF with severe comorbidities are very likely to harbor LV fibrosis. The logical next step would be to investigate both the prevalence of LV LGE and its association with cardiovascular mortality in this enriched group. Further studies are needed to establish whether LGE is a major independent predictive factor of cardiovascular mortality in patients with AF. Reprint requests and correspondence: Dr. Harikrishna Tandri, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, 600 N Wolfe Street, Carnegie 565D, Baltimore, Maryland 21287. E-mail: htandri1@jhmi.edu. JACC Vol. 62, No. 23, 2013 December 10, 2013:2215–6 2. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation 2009;119: 2516–25. 3. Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–52. 4. Saposnik G, Gladstone D, Raptis R, et al. Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke 2013;44:99–104. 5. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840–4. 6. Psaty BM, Furberg CD, Kuller LH, et al. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the Cardiovascular Health Study. JAMA 1992;268:1287–91. 7. Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004;292:2471–7. 8. Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107:2589–94. 9. Neilan TG, Shah RV, Abbasi SA, et al. The incidence, pattern, and prognostic value of left ventricular myocardial scar by late gadolinium enhancement in patients with atrial fibrillation. J Am Coll Cardiol 2013;62:2205–14. 10. Dosdall DJ, Ranjan R, Higuchi K, et al. Chronic atrial fibrillation causes left ventricular dysfunction in dogs but not goats: experience with dogs, goats, and pigs. Am J Physiol Heart Circ Physiol 2013;305: H725–31. 11. Shantsila E, Shantsila A, Blann AD, et al. Left ventricular fibrosis in atrial fibrillation. Am J Cardiol 2013;111:996–1001. 12. Ling LH, Kistler PM, Ellims AH, et al. Diffuse ventricular fibrosis in atrial fibrillation: non-invasive evaluation and relationships with aging and systolic dysfunction. J Am Coll Cardiol 2012;60:2402–8. 13. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–5. 14. Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol 2008;51: 2414–21. 15. Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013;309:896–908. 16. Wazni OM, Tsao HM, Chen SA, et al. Cardiovascular imaging in the management of atrial fibrillation. J Am Coll Cardiol 2006;48:2077–84. REFERENCES 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–8. Downloaded From: https://content.onlinejacc.org/ on 10/01/2016 Key Words: atrial fibrillation - cardiac magnetic resonance late gadolinium enhancement. -