Sugandha Rajput, Sadie L. Hebert, and Linda K. McLoon
advertisement

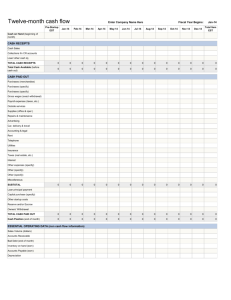
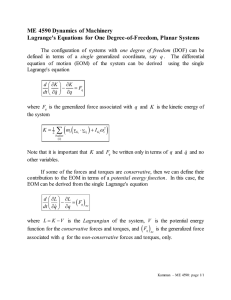
Sparing of Extraocular Muscles in Muscular Dystrophies: A Role for Retinoic Acid Signaling Sugandha Rajput, Sadie L. Hebert, and Linda K. McLoon University of Minnesota, Department of Ophthalmology Introduction Duchenne muscular dystrophy (DMD) is a muscular degenerative disease that is characterized by an age-related loss of muscle mass and function (Blake et al. 2012). It is caused by a mutation in the dystrophin gene. This mutation results in the absence of the dystrophin protein - a cytoskeletal protein that plays an important role in muscle function (Blake et al. 2012). DMD patients experience decreased lower limb muscle strength and joint contractures and most often die at an early age of respiratory complications due to intercostal muscle weakness (Blake et al. 2012). Interestingly, the extraocular muscles (EOM) that function in eye movement, primary gaze position and motor fusion are functionally and morphologically spared in DMD patients (McLoon EOM chapter, Kaminski et al. 1992, Karpati et al. 1988 and Kallestad et al. 2011). No studies have been able to find why EOM are spared while limb skeletal muscles degenerate in DMD patients. We hypothesize that two differences between EOM and limb muscle contribute to the sparing of EOM in DMD. First, unlike limb muscles, EOM undergo continuous myofiber remodelling(McLoon and Wirtschafter 2002). Second, EOM and limb muscles have different requirements for their development. The transcription factor Pitx2 is required for the development of EOM but not for the development of limb muscle (Diehl et al. 2006 and Zhou et al. 2011). Retinoic acid, a derivative of vitamin A, controls the expression of Pitx2 during development. A loss of retinoic acid signaling results in absence of EOM as well (Matt et al. 2008 and Duester 2012). Conclusion: Immunostaining for RARα in EOM and TA was successful. However, we can not make a count of RARα per The aim of this project was to find the best optimized condition for myofiber in these tissue samples as the outline of each staining for RARα and dystrophin. myofiber is not specified. Next, we doubled stained for RARα and dystrophin (myofiber Method membrane cytoskeleton) using flourescent 1. All tissue samples were prepared by following the same protocol. immunohistrochemistry. The mouse globes with the EOM attached or tibialis anterior from the limb were removed, embedded in tragacanth gum, and frozen in Fluorescent IHC for RAR α and dystrophin methylbutane chilled to slurry on liquid nitrogen. 2. The tissue samples were then subjected to different immunostaining 1. The EOM sections were incubated in primary antibodies against dystrophin and RARα at a concentration of 1:500 conditions to find the best one. and 1:100-1:1000, respectively. Immunostaining for RARα in EOM 2. The sections were reacted with secondary antibodies, anti-rabbit AlexaFluor 488 and Elite ABC reagents. 1. The EOM sections were incubated in primary antibody against 3. The peroxidase was developed using substrate Amplex RARα at a concentration of 1:100. UltraRed. The staining for dystrophin was red, and the 2. The sections were reacted with secondary antibody, Elite ABC RARα should be green. reagents. Objective 3. The stained sections were developed using DAB (3,3’ diaminobenzidine tetrahydrochloride). The staining for the RARα was black. Figure 3: Myofiber from EOM double stained for RARα (picture not shown) and dystrophin (red). Dystrophin staining defined the outline of each myofiber,. Staining for RARα didn’t work. Figure 1: Myofiber from EOM immunostained for RARα (black). No primary section was subjected to the same procedure without primary antibody. Conclusion: DAB staining was able to locate RARα in EOM. Next, we stained for RARα in EOM and TA. Conclusion: Fluorescent IHC gave no staining for RARα in the subsequent staining procedures too. We tried to double stain for Aldehyde dehydrogenase (ALDH, an enzyme involved in converting retinol to retinoic acid), however, it wasn’t successful either. Summary Immunostaining for RARα in EOM and TA 1. Similar procedure were followed for immunostaining as before, using primary antibody concentration of 1:250. One way to study the role of retinoic acid signaling in the sparing of EOM could be to investigate the number of the retinoic acid receptor alpha (RARα) positive nuclei per myofiber in EOM and tibialis anterior (TA) limb muscle . We predict that EOM Figure 2: Myofiber from EOM and TA Immunostained for RAR α (black). would possess more RARα than limb muscle, thereby indicating that retinoic acid signaling is I wish to express my gratitude to Dr. Linda McLoon, Sadie L. Hebert, the McLoon lab and involved in the sparing of EOM in DMD. the UROP department for the overall success of this project. Immunostaining for RARα using DAB gave no information about the number of RARα-positive nuclei per myofiber, which is critical for understanding the role of RA signaling in the sparing of EOM in DMD. Unfortunately, double staining for RARα (or ALDH) and dystrophin using IHC was not successful either. In the future, try different double staining conditions, such as varied antibody concentrations or varied incubation times to find out the best condition. Blake, Derek J et al. “Function and Genetics of Dystrophin and Dystrophin-Related Proteins in Muscle.” Physiological Reviews 82.2 (2002): 291–329. Web. 14 Mar. 2012. Diehl, Adam G et al. “Extraocular Muscle Morphogenesis and Gene Expression Are Regulated by Pitx2 Gene Dose.” Investigative Ophthalmology & Visual Science 47.5 (2006): 1785– 1793. Duester, Gregg. “Retinoic Acid Synthesis and Signaling During Early Organogenesis.” Cell 134.6 (2008): 921–931. Web. 30 Mar. 2012. Kallestad, Kristen M. et al. “Sparing of Extraocular Muscle in Aging and Muscular Dystrophies: A Myogenic Precursor Cell Hypothesis.” Experimental Cell Research 317.6 (2011): 873– 885. Kaminski, H J et al. “Extraocular Muscles Are Spared in Advanced Duchenne Dystrophy.” Annals of Neurology 32.4 (1992): 586–588. Karpati, G et al. "Small-caliber skeletal muscle fibers do not suffer necrosis in mdx mouse dystrophy." Muscle Nerve 11 (1988) 795–803. Matt, Nicolas et al. “Impairing Retinoic Acid Signalling in the Neural Crest Cells Is Sufficient to Alter Entire Eye Morphogenesis.” Developmental Biology 320.1 (2008): 140–148. McLoon LK. The Extraocular Muscles, Chapter 8, In: Adler’s Physiology of the Eye, 11th edition, Eds: Kaufman P, Alm A, Levin LA, Nilsson S, Ver Hoeve J, Wu SM. Mosby Press. In press, 2010. McLoon, Linda K, and Jonathan Wirtschafter. “Activated Satellite Cells Are Present in Uninjured Extraocular Muscles of Mature Mice.” Transactions of the American Ophthalmological Society 100 (2002): 119–123; discussion 123–124. Print. Zhou, Yuefang, Dan Liu, and Henry J Kaminski. “Pitx2 Regulates Myosin Heavy Chain Isoform Expression and Multi-innervation in Extraocular Muscle.” The Journal of Physiology 589.Pt 18 (2011): 4601–4614. Web.