The Freezing Point Depression of t
advertisement
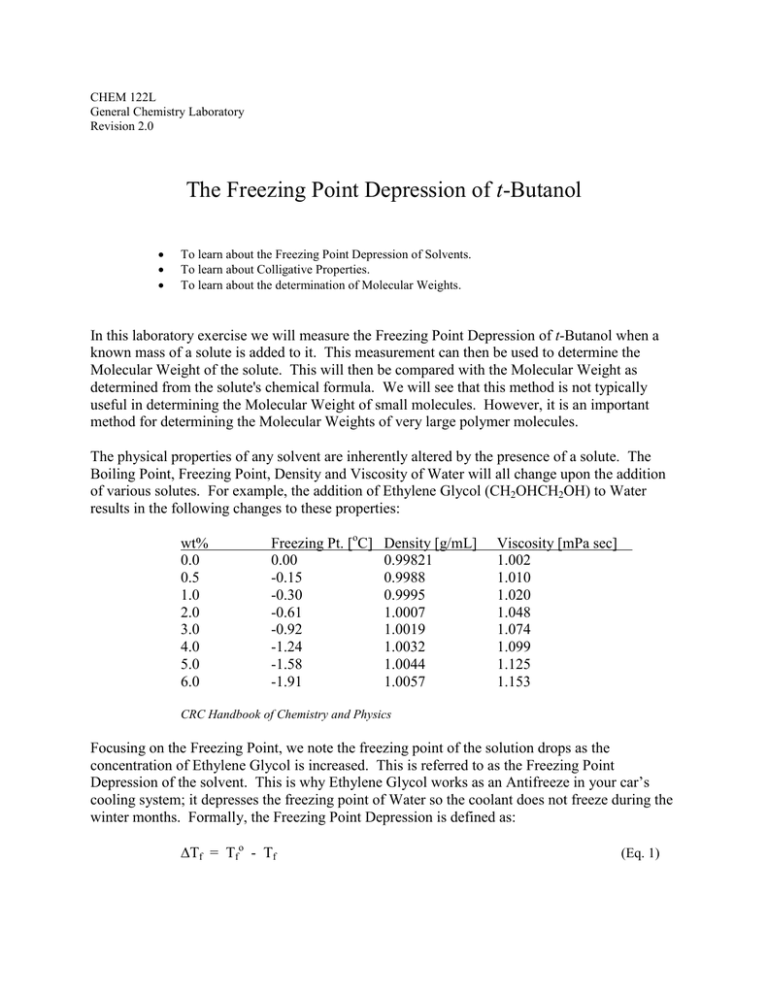
CHEM 122L General Chemistry Laboratory Revision 2.0 The Freezing Point Depression of t-Butanol To learn about the Freezing Point Depression of Solvents. To learn about Colligative Properties. To learn about the determination of Molecular Weights. In this laboratory exercise we will measure the Freezing Point Depression of t-Butanol when a known mass of a solute is added to it. This measurement can then be used to determine the Molecular Weight of the solute. This will then be compared with the Molecular Weight as determined from the solute's chemical formula. We will see that this method is not typically useful in determining the Molecular Weight of small molecules. However, it is an important method for determining the Molecular Weights of very large polymer molecules. The physical properties of any solvent are inherently altered by the presence of a solute. The Boiling Point, Freezing Point, Density and Viscosity of Water will all change upon the addition of various solutes. For example, the addition of Ethylene Glycol (CH2OHCH2OH) to Water results in the following changes to these properties: wt% 0.0 0.5 1.0 2.0 3.0 4.0 5.0 6.0 Freezing Pt. [oC] 0.00 -0.15 -0.30 -0.61 -0.92 -1.24 -1.58 -1.91 Density [g/mL] 0.99821 0.9988 0.9995 1.0007 1.0019 1.0032 1.0044 1.0057 Viscosity [mPa sec] 1.002 1.010 1.020 1.048 1.074 1.099 1.125 1.153 CRC Handbook of Chemistry and Physics Focusing on the Freezing Point, we note the freezing point of the solution drops as the concentration of Ethylene Glycol is increased. This is referred to as the Freezing Point Depression of the solvent. This is why Ethylene Glycol works as an Antifreeze in your car’s cooling system; it depresses the freezing point of Water so the coolant does not freeze during the winter months. Formally, the Freezing Point Depression is defined as: Tf = Tfo - Tf (Eq. 1) P age |2 where Tf is the freezing point of the solution and Tfo is the freezing point of the pure solvent. If we convert the Concentration Scale from wt% to molality, the above data presents itself as: molality [mol/kg] 0.081 0.163 0.329 0.498 0.671 0.848 1.028 Tf [oC] 0.15 0.30 0.61 0.92 1.24 1.58 1.91 The conversion of concentration units is straight forward. For example, consider the conversion of 6 wt% to molality Ethylene Glycol in Water. First assume we have 100g of solution. At 6%, the solution is composed of 6g Ethylene Glycol and 94g of Water. Converting the mass Ethylene Glycol to number of moles, we have: # mole Eth. Gly. = 6 g / (62.07 g/mole) = 0.09667 mole Eth. Gly. Converting the mass Water to kilograms, we have: # kg Water = 94 g x (1 kg / 1000 g) = 0.094 kg So, the molality of the solution is: molality = = = 1.028 m If we plot the Freezing Point Depression data in this guise, we find there exists a linear relationship between FP Depression and the solution's molality. P age |3 In other words, the Freezing Point Depression is proportional to the solution’s molality: Tf ~ m (Eq. 2) The proportionality constant (slope of the line) is called the Freezing Point Depression Constant, Kf. Thus, (Eq. 2) becomes: Tf = Kf m (Eq. 3) with Kf = 1.86oC/m for the Aqueous Ethylene Glycol case. Further, if we plot similar data for a series of different solutes, for example Methanol (CH3OH) and Urea( NH2CONH2), we find they all fall along the same line; with slope = 1.86 oC/m. P age |4 This means the Freezing Point Depression Constant Kf does not depend on the nature of the solute. This observation concerning the Freezing Point Depression is generally true for many dilute solutions. The value of Kf does not depend on the nature of the solute, but it does depend on the nature of the solvent. Experimentally determined values of Kf for various solvents are reported below: Solvent Water Naphthalene Benzene Camphor p-Dichlorobenzene t-Butanol Kf [oC/m] 1.86 6.9 4.90 37.7 7.10 9.1 Physical properties of a solution, like Freezing Point Depression, that depend on the relative amount of solute present, but not on the nature of the solute, are collectively referred to as Colligative Properties; Colligative (from the Latin co-, together, and ligare, to bind) because they are bound together in their common origin in the Laws of Thermodynamics. Other common Colligative Properties are the Vapor Pressure Lowering (P), the Boiling Point Elevation (Tb) and the Osmotic Pressure (). It should be noted the data presented above is for relatively dilute solutions; concentrations below ~1.0 molal. As a solution becomes more concentrated, not only is the Freezing Point Depression no longer proportional to the molality, but it will begin to depend on the nature of the P age |5 solute. The above data extended to ~30 molal demonstrate this deviation for Ethylene Glycol and Urea. CRC Handbook of Chemistry and Physics Thus, the Freezing Point Depression relationship of (Eq. 3) is a limiting law; true only in the limit of zero concentration: Tf = Kf m (Eq. 4) Physically, the solute acts to disrupt the formation of the solvent’s crystal structure, causing the freezing point to decrease. At sufficiently low concentrations, it doesn’t matter what the nature of the solute is so long as it can disrupt crystal formation. As the solute’s concentration increases, crystallization of the solvent becomes more difficult and the freezing point of the solution decreases further. However, if the solution becomes sufficiently concentrated, solute molecules begin to interact with each other, as well as with the solvent molecules, in ways that cause the freezing point to deviate from the ideal prediction of (Eq. 3). In fact, Colligative Property data is useful in measuring these solution non-idealities. In addition to being useful for measuring non-idealities in solutions, Colligative Property data can also be useful in determining the Molecular Weight of a solute. If we measure the Freezing Point Depression of the solvent due to the presence of a known mass of a solute, we can determine the molality of the solute. And, if we know the mass of the solvent used to prepare the solution, we can determine the number of moles solute present. Finally, if we know the mass of the solute used, we can calculate the solute’s Molecular Weight: P age |6 Molecular Weight = (Eq. 7) As an example, consider a solution prepared from 1.00g of an unknown solute dissolved in 50.0g of the solvent Benzene. The Freezing Point Depression is measured to be 1.81oC. As mentioned above, we can then directly determine the molality of the solute using (Eq. 3): m = Tf / Kf = 1.81oC /( 4.90oC/m) = 0.369 m This then allows us to calculate the number of moles solute: # moles = molality x #kg solvent = 0.369m x 0.0500kg = 0.0185 mole which allows for an application of (Eq. 7) to determine the Molecular Weight: MW = 1.00g / 0.0185 mole = 54.0 g/mole For small molecules, Colligative Property data is usually not sufficiently accurate to be useful for Molecular Weight determinations. (A Mass Spectrometer offers a very, very accurate and precise alternative for determining molecular weights.) However, for high molecular weight polymers (proteins, nucleic acids, carbohydrates, synthetic organic polymers), Colligative Property data can be useful in making Molecular Weight determinations. Finally, as a practical point, the Freezing Point of a system can be determined by measuring its Cooling Curve; the temperature of the system followed over time when the system is immersed in a cold bath. For the pure solvent, the cooling of the liquid will be relatively linear. Following a brief period of supercooling, which occurs because the system is cooling faster than the molecules can lock into position in a crystalline lattice, the temperature of the system will remain relatively constant while the freezing process occurs. The temperature of the system will again decline as the solid cools. The Freezing Point Tfo is the temperature at which the constancy occurs. This process is modified slightly in the case of solutions. In the case of a solution, as crystals of the solvent begin to form, the remaining liquid solution becomes more concentrated and the freezing point is depressed further. Hence, the region of the Cooling Curve representing the P age |7 freezing of the solvent will not be constant, but instead will fall with time. The Freezing Point Tf is graphically extrapolated from the cooling curve data as illustrated below. In this laboratory exercise, we will measure the Freezing Point Depression of a t-Butanol solution: when one of the following solutes is added: Ethanol (CH3CH2OH) Propanol (CH3CH2CH2OH) Isoamyl Alcohol (CH3CH(CH3)CH2CH2OH) Each of these solutes, as well as the solvent t-Butanol, is an Organic Alcohol; an organic compound containing the –OH functionality. t-Butanol is industrially important for its solvent power. It is also used in the extraction of drugs, the manufacture of perfumes (particularly as an important raw material in the preparation of artificial musk), and the recrystallization of chemicals and as a chemical intermediate in the manufacture of t-butyl chloride or t-butyl phenol. It is an authorized denaturant for ethyl alcohol. It has also been patented for use as a gasoline antiknock agent. We will use our Freezing Point Depression data to directly determine the Molecular Weight of a given solute. This will then be compared with the Molecular Weight as determined from the chemical formula. This will allow us to judge the usefulness of this methodology in determining the Molecular Weight of small compounds. We must always keep in mind that just because a measurement is theoretically possible, it does not mean it is experimentally practical. Further, P age |8 just because a measurement is not useful for one type of calculation, it does not mean it is not useful for another. P age |9 Pre-Lab Safety Questions 1. Do a short internet search for an NFPA 704 Fire Diamond for t-Butnaol. What is the Degree of Hazard for t-Butnaol's flammability? 2. Do a short internet search for and MSDS for t-Butanol. Examine "Section 4 - First Aid Measures" of the MSDS. Determine what to do if t-Butnaol is ingested. 3. Examine "Section 5 - Fire and Explosion Data" of the above MSDS. If a small fire were to break out because the t-Butanol a student is working with ignites, would it be wise to use a Water based Fire Extinguisher to put it out? A Carbon Dioxide base Fire Extinguisher? P a g e | 10 Procedure 1. Assemble the freezing point apparatus as pictured below: The Water bath, an 800 mL beaker, should be cooled with a small amount of Ice. Make sure the Ice-Water bath is cooled to very close to 0oC. (The beaker is suspended on a ring in order to ensure that the cooling bath does not warm asymmetrically by absorbing heat from the counter-top.) 2. Use a clean, dry 150 mL beaker to support the large test tube. P a g e | 11 Weigh this assembly on a Top-Loading Balance to a precision of 0.01g. Add about 25g (~31 mL) of t-Butyl Alcohol, and reweigh the assembly. (The container of t-Butanol is located in the fume hood where it is being warmed in a Water bath to liquify the alcohol. When obtaining your sample be sure the outside of the container is thoroughly dry as even minute amounts of Water in the t-Butanol will contaminate the alcohol such that it is unusable.) !Good Practice – Always use the same balance for repeat weighings! 3. Separately fill another 800 mL beaker with Water, place it on a Hot Plate, place the solvent-containing test tube, with the looped stirrer and thermometer, in the Water bath and heat the system until all the solvent has melted and has been heated to ~40oC. 4. Remove the test tube from the hot Water bath and place it in the Ice-Water bath. The IceBath should be stirred occasionally during the course of your measurements. 5. Measure the temperature every 10 sec until solidification is complete. During this time the solvent should be continuously stirred using the looped stirrer. Do not dispose of the solvent when you are done obtaining the Cooling Curve. You will use this same solvent for the next part of the experiment. 6. Obtain a clean, dry 10 mL graduated cylinder with cork. Add ~1.5g of a solute chosen from those listed in the discussion to the graduated cylinder and cork the cylinder. Weigh the graduated cylinder with solute on a Top-Loading Balance to a precision of 0.01g. 7. Again, heat the solvent in the large test tube until it is melted and again at ~40oC. 8. Quantitatively transfer the solute from the graduated cylinder to the large test tube containing the warmed solvent. Be certain none of the solute adheres to the walls of the test tube. If some does, roll the test tube so that the solvent contacts and dissolves the solute. Immediately re-cork the “empty” cylinder. Re-weigh the graduated cylinder. The difference in weights of the “full” and “empty” cylinders gives the mass of the solute used. 9. Stir the solvent-solute mixture in the large test tube and repeat the determination of the Cooling Curve. 10. In order to obtain a second determination of the Freezing Point Depression of the solvent, re-heat the solution and repeat the measurement of the Cooling Curve. 11. Repeat the determination of the Cooling Curve for a third trial. P a g e | 12 Data Analysis 1. Prepare Cooling Curves for the Solvent (t-Butanol) and the Solution; you should have four curves. Use the same procedure at that used in Problem #1 of the Pre-Lab assignment to determine the freezing points for each case. Lastly, report Tfo for t-Butanol and the Average Tf for the Solution. 2. Determine the Freezing Point Depression. 3. Calculate the molality of the Solution. 4. Determine the Molecular Weight of the Solute from the Freezing Point Depression data. 5. Compare this to the Molecular Weight determined from the Chemical Formula of the Solute. Calculate the Percentage Error in your FP MW determination. 6. Comment on which measurement or procedure has the largest error. How might you improve this measurement? P a g e | 13 Post Lab Questions 1. Calculate the Freezing Point Depression which will result when 0.50g of each of the following compounds is dissolved in 30.00g of Water: Cmpd 1: Cmpd 2: MW = 100.0 g/mol MW = 105.0 g/mol Comment on the advisability of using the Freezing Point Depression measurement in determining the Molecular Weight of each of these compounds. 2. In our discussion, we considered the Freezing Point Depression of Ethylene Glycol (CH2OHCH2OH) in Water. Draw the Lewis Structure of Ethylene Glycol and explain why it will form a solution with Water. 3. When adding Ethylene Glycol to your cooling system, it is usually mixed 50:50 by volume. This will prepare a 52.7 wt% solution. Convert this concentration to molality and predict the Freezing Point Depression based on (Eq. 3). The measured value, as reported by Dennis R. Cordray, Lisa R. Kaplan, Peter M. Woyciesjes and Theodore F. Kozak in Fluid Phase Equilibria 117 (1996) 146 is 38.3oC. What is the Percentage Error introduced by assuming the solution is dilute? P a g e | 14 Addendum Solutions versus Colloidal Systems The above procedure for Molecular Weight determination relies on the fact that the solute forms a true solution when dissolved in the solvent. This is not always the case. In many instances, the solute will instead combine with the solvent to form a colloidal system. Colloids are systems in which one phase is dispersed into another. Examples of colloids include fog, milk, shaving cream, and Jello. At the macroscopic level a colloid, such as milk, appears homogenous. However, upon microscopic examination, milk will reveal itself to be extremely small droplets of liquid fat dispersed in liquid Water. Most colloids, such as milk, are opaque or cloudy. However, in some cases the system appears transparent to the naked eye and therefore appears as though it is a solution. But because the dispersed colloidal particles are at least as large as the wavelengths of visible light, a beam of light passing through the system is scattered. This scattering phenomenon is known as the Tyndall Effect. The Tyndall Effect is what is observed when a beam of light passes through dust or fog dispersed in Air. This effect is one method for distinguishing between true solutions, where the solutes are dissolved as individual molecules, and which do not exhibit a Tyndall Effect, and colloidal systems which merely appear as though they are solutions. Because Colligative Properties depend only on the number of “particles” dissolved in the solvent, molecular weights derived from Freezing Point Depression data of colloidal systems are really the average molar masses of the colloidal particles comprising the systems. In this short exercise, we will examine two apparent “solutions”, one of which is a true solution and the other of which is a colloid. We will identify which exhibits a Tyndall Effect, and is therefore a colloid, and which does not. P a g e | 15 Procedure 1. Examine the two “solutions” provided by your laboratory instructor. One is an aqueous mixture of Ferric Hydroxide; Fe(OH)3. The other is an aqueous mixture of organic dyes. Can you tell which the true solution is and which is the colloid? 2. In a darkened room, pass a beam of light from a laser “pointer” through each system. Which exhibits a Tyndall Effect and which does not? Identify the colloid.






