Corrosion Rates

Materials Science and Engineering
Chapter 17
Introduction
Electrochemical considerations
Corrosion rates
Prediction of corrosion rates
Passivity
Environmental effects
Forms of corrosion
Corrosion environments
Corrosion prevention
Oxidation
Swelling and dissolution
Bond rupture
Weathering
魏茂國
1
魏茂國
Photograph of a 1936 Deluxe Ford Sedan having a body that is made entirely of unpainted stainless steel. Six of these cars were manufactured to provide an ultimate test as to the durability and corrosion resistance of stainless steels. Each automobile has logged hundreds miles of everyday driving. Whereas the surface finish on the stainless steel is essentially the same as when the car left the manufacture’s assembly line, other nonstainless components such as the engine, shock absorbers, brakers, springs, clutch, transmission, and gears have had to be replaced; for example, one car has gone through three engines.
2
魏茂國
By way of contrast, a classic automobile of the same vintage as the one above that is rusting away in a field in Bodie, California. Its body is made of a plain-carbon steel that at one time was painted. This paint offered limited protection for the steel, which is susceptible to corrosion in normal atmospheric environments.
3
魏茂國
With a knowledge of the types of and an understanding of the mechanisms and causes of corrosion and degradation, it is possible to take measures to prevent them from occurring. For example, we may change the nature of the environment, select a material that is relatively nonreactive, and/or protect the material from appreciable deterioration.
4
Materials Science and Engineering
Learning Objectives
魏茂國
1. Distinguish between oxidation and reduction electrochemical reactions.
2. Describe the following: galvanic couple, standard half-cell, and standard hydrogen electrode.
3. Compute the cell potential and write the spontaneous electrochemical reaction direction for two pure metals that are electrically connected and also submerged in solutions of their respective ions.
4. Determine metal oxidation rate given the reaction current density.
5. Name and briefly describe the two different types of polarization, and specify the conditions under which each is rate controlling.
6. For each of the eight forms of corrosion and hydrogen embrittlement, describe the nature of the deteriorative process, and then note the proposed mechanism.
7. List five measures that are commonly used to prevent corrosion.
8. Explain why ceramic materials are, in general, very resistant to corrosion.
9. For polymeric materials, discuss (a) two degradation processes that occur when they are exposed to liquid solvents and (b) the causes and consequences of molecular chain bond rupture.
5
Materials Science and Engineering
Deteriorative mechanisms
- Metals
Introduction
魏茂國
In metals, there is actual material loss either by dissolution (corrosion) or by the formation of nonmetallic scale or film (oxidation).
- Ceramics
Ceramic materials are relatively resistant to deterioration, which usually occurs at elevated temperatures or in rather extreme environments; the process is frequently called corrosion.
- Polymers
Polymers may dissolve when exposed to a liquid solvent, or they may absorb the solvent and swell; also, electromagnetic radiation (UV) and heat may cause alterations in their molecular structure. The processes are called degradation.
6
Materials Science and Engineering
Introduction
Corrosion of metals
Corrosion is defined as the destructive and unintentional attack of a metal.
魏茂國
- Corrosion is a chemical reaction in which there is transfer of electrons from one chemical species to another.
- Corrosion is electrochemical and ordinarily at the surface.
Importance of corrosion
Approximately 5% of an industrialized nation’s income is spent on corrosion prevention and the maintenance or replacement of products lost or contaminated as a result of corrosion reactions.
Advantage of corrosion
Etching procedures make use of the selective chemical reactivity of grain boundaries or various microstructural constituents.
- The current developed in dry-cell batteries is a result of corrosion processes.
7
Materials Science and Engineering
Electrochemical Considerations
魏茂國
Oxidation reaction
Metal atoms characteristically lose or give up electrons in what is called an oxidation reaction.
M
M n+ + ne n: valence (number of valence electrons)
(17.1)
- The site at which oxidation takes place is called the anode .
- Oxidation is sometimes called an anodic reaction .
- Examples
Fe
Fe 2+ + 2e -
Al
Al 3+ + 3e -
(17.2a)
(17.2b)
8
Materials Science and Engineering
Electrochemical Considerations
魏茂國
Reduction reaction
- The electrons generated from each metal atom that is oxidized must be transferred to and become a part of another chemical species in what is termed a reduction reaction .
- In acid solutions, which have a high concentration of hydrogen ions
2H + + 2e -
H
2
(17.3)
- For an acid solution having dissolved oxygen
O
2
+ 4H + + 4e -
2H
2
O (17.4)
- For a neutral or basic aqueous solution in which oxygen is dissolved
O
2
+ 2H
2
O + 4e -
4(OH ) (17.5)
- Any metal ions present in the solution may be reduced
M n+ + e -
M (n-1)+ or M n+ + ne -
M
(17.6)
(17.7)
- The location at which reduction occurs is called cathode .
- It is possible for two or more of the reduction reactions to occur simultaneous.
9
Materials Science and Engineering
Electrochemical Considerations
魏茂國
10
Materials Science and Engineering
Electrochemical Considerations
魏茂國
Overall electrochemical reaction
- An overall electrochemical reaction must consist of at least one oxidation and one reduction , and will be the sum of them.
- The individual oxidation and reduction reactions are termed half-reactions .
The total rate of oxidation must equal the total rate of reduction, or all electrons generated through oxidation must be consumed by reduction.
Zn
Zn 2+ + 2e -
2H + + 2e -
H
2
(gas)
(17.8)
(17.9) oxidation reduction
Zn + 2H +
Zn 2+ + H
2
(gas) (17.10) overall
Fig. 17.1
The electrochemical reactions associated with the corrosion of zinc in an acid solution.
11
Materials Science and Engineering
Electrochemical Considerations
魏茂國
- Another example is the oxidation or rusting of iron in water, which contains dissolved oxygen.
This process occurs in two steps; in the first, Fe is oxidized to Fe 2+ [as Fe(OH)
2
],
Fe + ½O
2
+ H
2
O
Fe 2+ + 2OH -
Fe(OH)
2
(17.11) in the second stage, to Fe 3+ [as Fe(OH)
3
].
2Fe(OH)
2
+ ½O
2
+ H
2
O
2Fe(OH)
3
(17.12)
12
Materials Science and Engineering
Electrochemical Considerations
魏茂國
Electrode potentials
- If the iron and copper electrodes are connected electrically, reduction will occur for copper at the expense of the oxidation of iron.
- Cu 2+ ions will deposit (electrodeposit) as metallic copper on the copper electrode, while iron dissolves (corrodes) on the other side of the cell and goes into solution as Fe 2+ ions.
Fe
Fe 2+ + 2e (oxidation)
Cu 2+ + 2e -
Cu (reduction)
(17.14a)
(17.14b)
Cu 2+ + Fe
Cu + Fe 2+ (overall reaction) (17.13)
- When a current passes through the external circuit, electrons generated from the oxidation of iron flow to the copper cell in order that Cu 2+ be reduced.
- There will be some net ion motion from each cell to the other across the membrane.
Fig. 17.2
An electrochemical cell consisting of iron and copper electrodes, each of which is immersed in a 1M solution of its ion. Iron corrodes while copper
13 electrodeposits.
Materials Science and Engineering
Electrochemical Considerations
魏茂國
- Galvanic couple
Two metals electrically connected in a liquid electrolyte wherein one metal becomes an anode and corrodes, while the other acts as a cathode.
- An electric potential or voltage will exist between the two cell halves, and its magnitude can be determined if a voltmeter is connected in the external circuit.
- Various electrode pairs have different voltages; the magnitude of such a voltage may be thought of as representing the driving force for the electrochemical oxidation-reduction reaction.
Cu 2+ + Fe
Cu + Fe 2+ (0.780V)
Fe 2+ + Zn
Fe + Zn 2+ (0.323V) (17.15)
Standard half-cell : a half-cell of a metal electrode immersed in a 1M solution of ions and at 25
C .
Fig. 17.3
An electrochemical cell consisting of iron and zinc electrodes, each of which is immersed in a 1M solution of its ion. The iron electrodeposits while the zinc
14 corrodes.
Materials Science and Engineering
Electrochemical Considerations
魏茂國
Standard hydrogen reference half cell
It consists of an inert platinum electrode in a 1M solution of H + ions, saturated with hydrogen gas that is bubbled through the solution at a pressure of 1 atm and a temperature of 25
C.
Standard electromotive force (emf) series
- It is generated by coupling to the standard hydrogen electrode, standard half-cells for various metals and ranking them according to measured voltage.
- Consider the generalized reactions involving the oxidation of metal M
1
M
1
M
1 n
and the reduction of metal M
ne
V
1
0 (17.16a)
2
M
2 n
ne
M
2
V
2
0 (17.16b) the overall cell potential
V 0
M
1
M n
2
M
1 n
M
2
(17.17) V
0
2
V
1
0
(17.18)
For this reaction to occur spontaneously,
V 0 must be positive.
15
Fig. 17.4
The standard hydrogen reference half-cell.
Materials Science and Engineering
Electrochemical Considerations
Table 17.1
The standard emf series.
(reduction)
Increasingly inert
(cathodic)
Increasingly active
(anodic)
(oxidation)
Electrode reaction
(reduction)
O
O
2
2
Au 3+
+ 4H
Pt 2+
+
Ag
+ 3e
+ 4e
+ 2e
+
-
+ e -
-
-
Au
2H
Pt
Ag
2
O
Fe
+ 2H
Cu
Pb
Sn
2
2H
3+ + e
O + 4e
2+
+
2+
2+
+ 2e
+ 2e
+ 2e
+ 2e
-
-
-
-
-
-
Fe 2+
4(OH )
Cu
H
Pb
2
Sn
Ni Ni
Co
Cd
Fe
Cr
Zn
Al
2+
Mg
2+
2+
2+
3+
2+
3+
2+
Na +
K
+ 2e -
+ 2e -
+ 2e -
+ 2e -
+ 3e -
+ 2e -
+ 3e -
+
+ 2e
+ e
+ e -
-
-
Co
Cd
Fe
Cr
Zn
Al
Mg
Na
K
Standard electrode potential, V 0 (V)
-0.250
-0.277
-0.403
-0.440
-0.744
-0.763
-1.662
-2.363
-2.714
-2.924
+1.420
+1.229
~+1.200
+0.800
+0.771
+0.401
+0.340
0.000
-0.126
-0.136
16
魏茂國
Materials Science and Engineering
Electrochemical Considerations
Influence of concentration & temperature on cell potential
- If M
1 and M
2 electrodes are pure metals.
M
1 n
ne
M
2 n
M
1 ne
M
1
M
2 n
M
1
M
1 n
M
M
1
2 n
ne
M
2
V
V
1
0
V
1
2
0
0
V
0
V
2
0
V
1
0
(17.18)
魏茂國
- Altering temperature or solution concentration or using alloy electrodes instead of pure metals will change the cell potential. According to the Nernst equation
V
1
V
2
V
1
0
V
2
0
RT nF
RT nF ln ln
1
2
V
V
V
2
V
1
V
2
0
V
1
0
RT nF ln
2
(17.19)
R : gas constant, 8.31 J/mol
K n : number of electrons participating in either of the half-cell reactions,
F : Faraday constant (96500 C/mol)
[ M
1 n
] & [ M
2 n
] : molar ion concentrations
17
Materials Science and Engineering
Electrochemical Considerations
- At 25
C
V
V
2
0
V
1
0
RT nF ln
2
V
V
V
V
2
0
V
1
0
V
2
0
V
1
0
V
2
0
V
1
0
J
8 .
31
298 K mol
K n
0 .
0592 n n
0 .
02566 ln log
C
96500 mol
2
2
J
C ln
2
(17.20) to give
V in volts.
For reaction spontaneously,
V must be positive.
魏茂國
18
Materials Science and Engineering
Example Problem 17.1
魏茂國
One-half of an electrochemical cell consists of a pure nickel electrode in a solution of
Ni 2+ ions; the other half is a cadmium electrode immersed in a Cd 2+ solution.
(a) If the cell is a standard one, write the spontaneous overall reaction and calculate the voltage that is generated.
0
( V
Cd
0 .
403 V , V
Ni
0
0 .
250 V )
(b) Compute the cell potential at 25
C if the Cd 2+ and Ni 2+ concentrations are 0.5 and
10 -3 M, respectively. Is the spontaneous reaction direction still the same as for the standard cell?
(a) From Table 17.1
Cd 2+ + 2e -
Cd
Ni 2+ + 2e -
Ni
V
V
0
0
= -0.403
= -0.250
Therefore
Cd
Cd 2+ + 2e -
Ni 2+ + 2e -
Ni
V 0 = +0.403
V 0 = -0.250
Ni 2+ + Cd
Ni + Cd 2+
V
V = 0.403 - 0.250 = 0.153
( V )
(b) Cd
Cd 2+ + 2e -
Ni 2+ + 2e -
Ni
V 0 = +0.403
V 0 = -0.250
Ni 2+ + Cd
Ni + Cd 2+
V
0
V
Cd
0
V
Ni
0 .
0592 n log
[ M 2
[ M
Cd
2
Ni
]
]
0 .
403
0 .
250
0 .
0592
2
log
10
3
0 .
5
0 .
073 ( V )
Materials Science and Engineering
Example Problem
魏茂國
A galvanic cell at 25
C consists of an electrode of zinc in a 0.10 M ZnSO
4 solution and another of nickel in a 0.05 M NiSO
4 solution. The two electrodes are separated by a porous wall and connected by an external wire. What is the emf of the cell when a switch between the two electrodes is just closed?
(For 1 M solutions, Zn 2+ + 2e -
Zn V 0 = -0.763 V; Ni 2+ + 2e -
Ni V 0 = -0.250 V)
Anode reaction:
V
V
0
0 .
0592 log C ion n
V
A
0 .
763
0 .
0592
2 log 0 .
10
0 .
793 ( V )
Cathode reaction:
Overall reaction:
V
C
V cell
0 .
250
0 .
0592 log 0 .
05
2
0 .
288 ( V )
E
A
E
C
0 .
793 V
0 .
288 V
0 .
505 V
20
Materials Science and Engineering
Example Problem
魏茂國
One end of an iron wire is immersed in an electrolyte of 0.02 M Fe 2+ ions and the other in an electrolyte of 0.005 M Fe 2+ ions. The two electrodes are separated by a porous wall. (a) Which end of the wire will corrode? (b) What will be the potential difference between the two ends of the wire when it is just immersed in the electrolytes?
(Fe 2+ + 2e -
Fe V 0 = -0.440V)
(a) The end of the wire that will corrode will be the one immersed in the more dilute electrolyte, which is the 0.005 M one. Thus, the wire end in the 0.005 M solution will be the anode.
(b)
For 0.005 M solution:
For 0.02 M solution:
V
Fe
2
V
0
0 .
0296 log C ion
V
A
0 .
440
0 .
0296 log 0 .
005
0 .
508 ( V )
V
C
0 .
440
0 .
0296 log 0 .
02
0 .
490 ( V )
V cell
V
A
V
C
0 .
508 V
0 .
490 V
0 .
018 V
21
Materials Science and Engineering
Electrochemical Considerations
Table 17.2
The galvanic series.
Increasingly inert
(cathodic)
Increasingly active
(anodic)
Platinum
Gold
Graphite
Titanium
Silver
316 Stainless steel (passive)
304 Stainless steel (passive)
Inconel (80Ni-13Cr-7Fe) (passive)
Nickel (passive)
Monel (70Ni-30Cu)
Copper-nickel alloys
Bronzes (Cu-Sn alloys)
Copper
Brasses (Cu-Zn alloys)
Inconel (active)
Nickel (active)
Tin
Lead
316 Stainless steel (active)
304 Stainless steel (active)
Cast iron
Iron and steel
Aluminum alloys
Cadmium
Commercially pure aluminum
Zinc
Magnesium and magnesium alloys
魏茂國
Galvanic series
- This represents the relative reactivities of a number of metals and commercial alloys in seawater .
- The alloys near the top are cathodic and unreactive, whereas those at the bottom are most anodic.
- Most metals and alloys are more stable in an ionic state than as metals. In thermodynamic term, there is a net decrease in free energy in going from metallic to oxidized states.
- Essentially all metals occur in nature as compounds. Two notable exceptions are the noble metals gold and platinum .
22
Materials Science and Engineering
Corrosion Rates
Corrosion rate
The rate of material removal as a consequence of the chemical action.
魏茂國
- Real corroding systems are not at equilibrium; there will be a net flow electrons from anode to cathode, which means that the half-cell potential parameters
(Table 17.1) cannot be applied.
Half-cell potentials : (1) the magnitude of a driving force, (2) determine spontaneous reaction directions, (3) provide no information as to corrosion rates.
Corrosion penetration rate ( CPR )
The thickness loss of material per unit of time.
CPR
KW
At
(17.23)
W : weight loss, t : exposure time,
: density, A : exposed specimen area, K : constant,
CPR : mils per year (mpy) or millimeters per year (mm/yr).
23
Materials Science and Engineering
Corrosion Rates
魏茂國
- There is an electric current associated with electrochemical corrosion reactions, we can express corrosion rate in terms of this current density .
r
i nF i : current density (C/m 2
s, A/m 2 ), n : number of electrons associated with the ionization of each atom,
F : Faraday constant, 96500 C/mol, r : mol/m 2
s.
(17.24)
24
Materials Science and Engineering
Corrosion Rates
魏茂國
Rate of uniform corrosion or electroplating of a metal in an aqueous solution
- The amount of metal uniformly corroded from an anode or electroplated on a cathode in an aqueous solution in a time period can be determined by using
Faraday’s equation of general chemistry.
Metal
Metal n+ + ne -
It nF
w
M w
ItM nF w : weight of metal (g) corroded or electroplated in an aqueous solution in time t (s),
I : current flow (A), M : atomic mass of the metal (g/mol), n : number of electrons/atom produced or consumed in the process,
F : faraday’s constant (96500 C/mol, 96500 A
s/mol).
- Sometimes the uniform aqueous corrosion of a metal is expressed in terms of a current density.
w
iAtM nF i : current density (A/cm 2 ), A : area (cm 2 ).
25
Materials Science and Engineering
Prediction of Corrosion Rates
魏茂國
Polarization & overvoltage
- The potentials of the two short-circuited electrodes are not at the values determined from the standard emf series because the system is a nonequilibrium one. The displacement of each electrode potential from its equilibrium value is termed polarization , and the magnitude of this displacement is the overvoltage (
).
- Overvoltage is expressed in terms of plus or minus volts relative to the equilibrium potential.
- For example, suppose that the zinc electrode has a potential of -0.621 V after it has been connected to the platinum electrode. The equilibrium potential is -0.763 V, therefore
= -0.621 - (-0.763) = +0.142 (V)
- Two types of polarization
Activation polarization
Concentration polarization
Fig. 17.5
Electrochemical cell consisting of standard zinc and hydrogen electrodes that
26 has been short-circuited.
Materials Science and Engineering
Prediction of Corrosion Rates
魏茂國
Activation polarization
- Activation polarization refers to the condition wherein the reaction rate is controlled by the one step in the series that occurs at the slowest rate .
- The term “activation” is applied to this type of polarization because an activation energy barrier is associated with this slowest, rate-limiting step.
The slowest of the steps determines 1. Adsorption of H + ions from the solution the rate of the overall reaction.
onto the zinc surface.
2. Electron transfer from the zinc to form a hydrogen atom.
H + + e -
H
3. Combining of two hydrogen atoms to form a molecule of hydrogen.
2H
H
2
4. The coalescence of many hydrogen molecules to form a bubble.
Fig. 17.6
Schematic representation of possible steps in the hydrogen reduction reaction, the rate
27 of which is controlled by activation polarization.
Materials Science and Engineering
Prediction of Corrosion Rates
魏茂國
- For activation polarization, the relationship between overvoltage and current density is
a
log i
0 i
(17.25)
a
: overvoltage,
: constant, i : current density, i
0
: exchange current density.
- For the standard hydrogen cell
2H + + 2e -
H
2
H
2
2H + + 2e rate: r rate: r red oxd
- At equilibrium, there is no net reaction.
r red
r oxid
i
0 nF
(17.26)
The value for i
0 is determined experimentally and will vary from system to system.
Fig. 17.7
For a hydrogen electrode, plot of activation polarization overvoltage versus logarithm of current density for
28 both oxidation and reduction reactions.
Materials Science and Engineering
Prediction of Corrosion Rates
魏茂國
- According to Equation 17.25, when overvoltage is plotted as a function of the logarithm of current density, straight-line segments results (Fig. 17.7).
a
log i
0 i
(17.25)
The line segment with a slope of +
corresponds to the oxidation half-reaction, whereas the line with a -
is for reduction.
- Also worth noting is that both line segments originates at i
0
(H
2
/H + ), the exchange current density, and at zero overvoltage, because at this point the system is at equilibrium and there is no net reaction.
29
Materials Science and Engineering
Prediction of Corrosion Rates
魏茂國
Concentration polarization
- Concentration polarization exists when the reaction rate is limited by diffusion in the solution .
- When the reaction is low and/or the concentration of H + is high, there is always an adequate supply of hydrogen ions available in the solution at the region near the electrode interface (Fig. 17.8a).
- At high rates and/or low H + concentrations, a depletion zone may be formed in the vicinity of the interface, inasmuch as the H + ions are not replenished at a rate sufficient to keep up with the reaction (Fig. 17.8b). Thus, diffusion of H + to the interface is rate controlling, and the system is concentration polarized.
- It generally occurs only for reduction reactions because for oxidation, there is virtually an unlimited supply of metal atoms at the corroding electrode interface.
- It may be noted that overvoltage is independent of current density until i approach i
L
(the limiting diffusion current density); at this point,
C abruptly in magnitude.
C
2 .
3 RT nF log
1
i i
L
(17.27) decreases
30
Materials Science and Engineering
Prediction of Corrosion Rates
魏茂國
Fig. 17.8
For hydrogen reduction, schematic representations of H + distribution in the vicinity of the cathode for (a) low reaction rates and/or high concentrations, and (b) high reaction rate and/or low concentrations wherein a depletion zone is formed that gives rise to concentration polarization.
31
Materials Science and Engineering
Prediction of Corrosion Rates
魏茂國
Both activation and concentration polarization
Both concentration and activation polarization are possible for reduction reactions.
The total overvoltage is just the sum of both overvoltage contributions (Fig. 17.9b).
Fig. 17.9
For reduction reactions, schematic plots of overvoltage versus logarithm of current density for (a) concentration polarization, and (b) combined activation-concentration polarization.
32
Materials Science and Engineering
Prediction of Corrosion Rates
Corrosion rates from activation polarization data
- In the first case, both oxidation and reduction reactions are rate limited by activation polarization (Fig. 17.1).
魏茂國
The potentials of the uncoupled hydrogen and zinc half-cells,
V (H + /H
2
) and V (Zn/Zn 2+ ), respectively, are indicated, along with their respective exchange current densities, i
0
(H + /H
2
) and i
0
(Zn/Zn 2+ ).
Upon immersion, both hydrogen and zinc experience activation polarization along their respective lines. Also, oxidation and reduction rates must be equal, which is only possible at the intersection of the two line segments; this intersection occurs at the corrosion potential , designated V
C
, and the corrosion current density i
C
. The corrosion rate of zinc (which also corresponds to the rate of hydrogen evolution) may thus be computed by insertion of this i
C value into Equation 17.24.
33
Materials Science and Engineering
Prediction of Corrosion Rates
V
H
V
( H
/ H
2
)
H log
i i
0
H
V
Zn
V
( Zn / Zn
2
)
Zn log i i
0
Zn
V
H
V
Zn r
i nF
魏茂國
Fig. 17.10
Electrode kinetic behavior of zinc in an acid solution; both oxidation and reduction reactions are rate limited by activation polarization.
34
Materials Science and Engineering
Prediction of Corrosion Rates
魏茂國
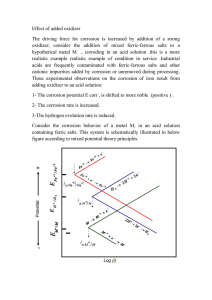
- In the second case, both concentration and activation polarization control the reduction reaction, whereas only activation polarization is important for oxidation.
Fig. 17.11 shows both polarization curves; corrosion potential and corrosion current density correspond to the point at which the oxidation and reduction lines intersect.
Fig. 17.11
Schematic electrode kinetic behavior for metal M; the reduction reaction is under combined activation-concentration polarization control.
35
Materials Science and Engineering
Example Problem
魏茂國
A copper electroplating process uses 15 A of current by chemically dissolving
(corroding) a copper anode and electroplating a copper cathode. If it is assumed that there are no side reactions, how long will it take to corrode 8.50 g of copper from the anode? (Atomic mass of Cu: 63.5 g/mol, F : 96500 A
s/mol.)
w
It nF
M
t
wnF
IM
Cu
Cu
2
2 e
n
2 t
8 .
5 g
2
96500
15 A
63 .
5 g
A
s
/ mol
/ mol
1722 s
28 .
7 min
36
Materials Science and Engineering
Example Problem
魏茂國
A mild steel cylindrical tank 1 m high and 50 cm in diameter contains aerated water to the 60 cm level and shows a loss in weight due to corrosion of 304 g after 6 weeks.
Calculate (a) the corrosion current and (b) the current density involved in the corrosion of the tank. Assume uniform corrosion on the tank’s inner surface and that the steel corrodes in the same manner as pure iron. ( M
Fe
: 55.85 g/mol, F : 96500 A
s/mol.)
(a)
(b)
50
w
ItM nF
I
wnF tM
40
60
Fe
Fe
2
2 e
n
2
I
6 week
7 days /
304 g
week
2
96500 A
24 h / day
s / mol
3600 s / h
63 .
5 g / mol
0 .
289 A i
I area area
Dh
r
2
50 cm
60 cm
50 cm / 2
2
11380 cm
2 i
0 .
289 A
11380 cm
2
2 .
53
10
5
A / cm
2
37
Materials Science and Engineering
Example Problem
魏茂國
The wall of a steel tank containing aerated water is corroding at a rate of 54.7 mdd.
How long will it take for the wall thickness to decrease by 0.50 mm? (
Fe
: 7.87 g/cm 3 ) mdd: milligram weight loss per square decimeter per day.
54 .
7 mdd
54 .
7
10
3 g
( 10 cm )
2 day
5 .
47
10
4 cm
2 g
day depth of corrosion per day =
5 .
47
10
4 g / cm
2 day
7 .
87 g / cm
3
6 .
95
10
5 cm / day t
0 .
50
10
1 cm
6 .
95
10
5 cm / day
719 days
38
Materials Science and Engineering
Example Problem
魏茂國
A sample of zinc corrodes uniformly with a current density of 4.27
10 -7 A/cm 2 in an aqueous solution. What is the corrosion rate of the zinc in milligrams per decimeter per day? The reaction for the oxidation of zinc is Zn
Zn 2+ + 2e .
Zn
Zn
2
2 e
n
2 w
ItM nF
i
area
tM nF w
4 .
27
10
7
A / cm
2
100 cm
2
24 h
3600
2
96500 A
s / mol s / h
65 .
38 g / mol
1 .
25
10
3 g
The corrosion rate is 1.25 mdd.
39
Materials Science and Engineering
Example Problem 17.2
魏茂國
Zinc experiences corrosion in an acid solution according to the reaction
Zn + 2H +
Zn 2+ + H
2
The rates of both oxidation and reduction half-reactions are controlled by activation polarization.
(a) Compute the rate of oxidation and reduction of Zn (mol/cm 2
s) given the following activation polarization data:
For Zn
V
(Zn/Zn2+)
= -0.763 i
0
= 10 -7 A/cm 2
= 0.09
V
For Hydrogen
V
(H+/H2) i
0
= 10 -10
= 0
= -0.08
V
A/cm 2
(b) Compute the value of the corrosion potential.
(a) For hydrogen reduction
V
H
V
( H
/ H
2
)
H log i i
0
H
For zinc oxidation
V
Zn
V
( Zn / Zn
2
)
Zn log i i
0
Zn
At equilibrium
V
H
V
Zn
40
Example Problem 17.2
魏茂國
Materials Science and Engineering
V
( H
/ H
2
)
V
( H
/ H
2
)
V
( H
/ H
2
)
log i
C
H
H log
i
C i
0
H log i
C
H
V
( Zn / Zn
2
) log i
0
H
Zn
V
( Zn / log
Zn
2
)
i
C i
0
Zn
Zn log i
C
V
( Zn / Zn
2
)
Zn
1
H
H log i
0
H
V
( H
/ H
2
)
Zn log i
0
Zn
V
( Zn / Zn
2
)
H
Zn log i
C log i
0
H
Zn log i
0
Zn
Zn
H log i
C log i
0
Zn
log i
C
0 .
09
1
(
0 .
08 )
0
(
0 .
763 )
(
0 .
08 )
log 10
10
0 .
09
log 10
7
3 .
924
i
C
10
3 .
924
1 .
19
10
4 (A/cm 2 ) r
i
C nF
1 .
19
10
4
2
96500
6 .
17
10
10 (mol/cm 2
s)
(b)
V
C
V
( H
/ H
2
)
V
C
H log
i
C i
0
H
0
(
0 .
08 )
1 .
19
log
10
10
10
4
0 .
486 (V)
41
Materials Science and Engineering
Passivity
魏茂國
Passivity
- Some normally active metals and alloys, under particular environmental conditions, lose their chemical reactivity and become extremely inert . This phenomenon is termed passivity .
- Passivity is displayed by Cr, Fe, Ni, Ti, and many of their alloys.
- This passive behavior results from the formation of a highly adherent and very thin oxide film on the metal surface , which serves as a protective barrier to further corrosion. If damaged, the protective film normally reforms very rapidly.
- Electrochemical potential vs current density
1. At low potential values, within the “active” region the behavior is like a normal metal.
2. With increasing potential, the current density suddenly decrease to a very low value that remains independent of potential; this is termed the “passive” region .
3. At even higher values, the current density again increases with potential in the
“transpassive” region .
42
Materials Science and Engineering
Passivity
魏茂國
Fig. 17.12
Schematic polarization curve for a metal that displays an active-passive transition.
43
Materials Science and Engineering
Passivity
魏茂國
Influence of corrosion environment
- Curve 1 intersects the oxidation polarization curve in the active region at point A, yielding a corrosion current density i
C
(A) .
- The intersection of curve 2 at point B is in the passive region and at current density i
C
(B) .
- The corrosion rate of metal M in solution
1 is greater than in solution 2 since i
C
(A) is greater than i
C
(B) and the rate is proportional to current density.
Fig. 17.13
Demonstration of how an activepassive metal can exhibit both active and passive corrosion behavior.
44
Materials Science and Engineering
Environmental Effects
魏茂國
Fluid velocity
In most instances, increasing fluid velocity enhances the rate of corrosion due to erosive effects.
Temperature
For the great majority of corrosion situations, the rates rise with increasing temperature.
Composition
- In many situations, increasing the concentration of the corrosive species produces a more rapid rate of corrosion.
- For materials capable of passivation, raising the corrosive content may result in an active-to-passive transition, with a considerable reduction in corrosion.
Cold work
A cold-worked metal is more susceptible to corrosion than the same material in an annealed state.
45
Materials Science and Engineering
Forms of Corrosion
Classification of metallic corrosion
- Uniform attack
- Galvanic corrosion
- Crevice corrosion
- Pitting corrosion
- Intergranular corrosion
- Selective leaching
- Erosion-corrosion
- Stress corrosion
- (Hydrogen embrittlement)
魏茂國
46
Materials Science and Engineering
Forms of Corrosion
魏茂國
Uniform attack (most common form of corrosion)
- Uniform attack is a form of electrochemical corrosion that occurs with equivalent intensity over the entire exposed surface and often leaves behind a scale or deposit.
In a microscopic sense, the oxidation and reduction reactions occur randomly over the surface.
- Example: general rusting of steel and iron, tarnishing of silverware.
47
Materials Science and Engineering
Forms of Corrosion
魏茂國
Galvanic corrosion
- It occurs when two metals or alloys having different compositions are electrically coupled while exposed to an electrolyte .
- The less noble or more reactive metal in the particular environment will experience corrosion; the more inert metal, the cathode, will be protected from corrosion.
- Example: steel screws corrode when in contact with brass in a marine environment.
- When two alloys are coupled in seawater, the one lower in the galvanic series (Table 17.2) will experience corrosion.
- The rate of galvanic attack depends on the relative anode-to-cathode surface areas that are exposed to the electrolyte, and is related directly to the cathode-anode area ratio . The reason is that corrosion rate depends on current density .
Fig. 17.14
Galvanic corrosion of a magnesium shell that was cast around a steel core.
48
Materials Science and Engineering
Forms of Corrosion
魏茂國
Anodic-cathodic behavior of steel with zinc and tin outside layers exposed to the atmosphere. (a) Zinc is anodic to steel and corrodes ( V 0 for Zn and Fe are -0.763 V and -0.440 V, respectively. (b) Steel is anodic to tin and corrodes (the tin layer was perforated before the corrosion began) ( V 0 for Sn is -0.136 V).
49
Materials Science and Engineering
Forms of Corrosion
魏茂國
Cu Steel
Steel Cu
Effect of area relationships between cathode and anode for copper-steel couples immersed in seawater. (a) Small cathode (copper rivets) and large anode (steel plates) cause only slight damage to steel. (b) Small anode (steel rivets) and large cathode (copper plates) cause severe corrosion of steel rivets.
50
Materials Science and Engineering
Forms of Corrosion
魏茂國
Reduction of galvanic corrosion
1. If coupling of dissimilar metals is necessary, choose two that are close together in the galvanic series.
2. Use an anode area as large as possible.
3. Electrically insulate dissimilar metals from each other.
4. Electrically connect a third, anodic metal to the other two; this is a form of cathodic protection.
51
Materials Science and Engineering
Forms of Corrosion
Crevice corrosion
- Electrochemical corrosion may occur as a consequence of concentration
魏茂國 differences of ions or dissolved gases in the electrolyte solution, and between two regions of the same metal piece. For such a concentration cell, corrosion occurs in the locale that has the lower concentration. Corrosion preferentially occurring at these positions is called crevice corrosion.
- The crevice must be wide enough for the solution to penetrate, yet narrow enough for stagnancy; usually the width is tenths of meters.
Fig. 17.15
On this plate, which was immersed in seawater, crevice corrosion has
52 occurred at the regions that were covered by washers.
Materials Science and Engineering
Forms of Corrosion
魏茂國
- After oxygen has been depleted within the crevice, oxidation of the metal occurs at the crevice (Fig. 17.16). Electrons from this electrochemical reaction are conducted through the metal to adjacent external regions, where they are consumed by reduction.
O
2
2 H
2
O
4 e
4
OH
Fig. 17.16
Schematic illustration of the mechanism of crevice corrosion between two riveted sheets.
53
Materials Science and Engineering
Forms of Corrosion
- In many aqueous environments, the solution of H + and Cl ions, which are especially corrosive.
魏茂國
Prevention of crevice corrosion
- Using welded instead of riveted or bolted joints.
- Using nonabsorbing gaskets when possible.
- Removing accumulated deposits frequently.
- Designing containment vessels to avoid stagnant areas and ensure complete drainage.
54
Materials Science and Engineering
Forms of Corrosion
Pitting
- A form of very localized corrosion attack in which small pits or holes form.
- The pits or holes ordinarily penetrate from the top of a horizontal surface downward in a nearly vertical direction.
魏茂國
Mechanism for pitting (next page)
It is probably the same as for crevice corrosion in that oxidation occurs within the pit itself, with complementary reduction at the surface. It is supposed that gravity causes the pits to grow downward, the solution at the pit tip becoming more concentrated and dense as pit growth progresses.
Improvement of pitting-resistance
- Specimens polished surfaces display a greater resistance to pitting corrosion.
- For stainless steel, alloying with about 2% molybdenum
Fig. 17.17
The pitting of a enhances their resistance significantly.
an acid-chloride solution.
Materials Science and Engineering
Forms of Corrosion
- The propagation of a pit is believed to involve the dissolution of the metal in the pit while maintaining a high degree of acidity at the bottom of the pit.
魏茂國
The anodic reaction of the metal at the bottom of the pit is M
M n+ + ne .
The cathodic reaction takes place at the metal surface surrounding the pit and is the reaction of oxygen with water and the electrons from the anodic reaction:
O
2
+ 2H
2
O + 4e -
4OH .
Thus, the metal surrounding the pit is cathodically protected.
The increased concentration of metal ions in the pit brings in chloride ions to maintain charge neutrality. The metal chloride then reacts with water to produce the metal hydroxide and free acid as
M
Cl
H
2
O
MOH
H
Cl
In this way a high acid concentration builds up at the bottom of the pit, which makes
56 the anodic reaction rate increase, and the whole process becomes autocatalyti c.
Materials Science and Engineering
Forms of Corrosion
Intergranular corrosion
- It occurs preferentially along grain boundaries for some alloys and in specific
魏茂國 environments. A macroscopic specimen disintegrates along its grain boundaries .
- When some stainless steel are heated to temperatures between 500 and 800
C for sufficiently long time periods, they become sensitized to intergranular attack.
Mechanism of intergranular corrosion
This heat treatment permits the formation of small precipitate particles of chromium carbide (Cr
23
C
6
). These particles form along the grain boundaries. Both the chromium and the carbon atom must diffuse to the grain boundaries to form the precipitates, which leaves a chromium-depleted zone adjacent to the grain boundary .
This grain boundary is now highly susceptible to corrosion.
Fig. 17.18
Schematic illustration of chromium carbide particles that have precipitated along grain boundaries in stainless steel, and
57 the attendant zones of chromium depletion.
Materials Science and Engineering
Forms of Corrosion
魏茂國
Weld decay
Intergranular corrosion is an especially severe problem in the welding of stainless steels, when it is often termed weld decay (Fig. 17.19).
Protection from intergranular corrosion
- Subjecting the sensitized material to a high-temperature heat treatment in which all the chromium carbide particles are redissolved.
- Lowering the carbon content below 0.03
wt% so that carbide formation is minimal.
- Alloying the stainless steel with another metal such as niobium or titanium, which has a greater tendency to form carbides than does chromium so that Cr remains in solid solution.
Fig. 17.19
Weld decay in a stainless steel.
The regions along which the grooves have
58 formed were sensitized as the weld cooled.
Materials Science and Engineering
Forms of Corrosion
魏茂國
Selective leaching
- Selective leaching is found in solid solution alloys and occurs when one element or constituent is preferentially removed as a consequence of corrosion processes.
- Example: dezincification of brass.
- The mechanical properties of the alloy are significantly impaired, because only a porous mass of copper remains in the region that has been dezincified.
59
Materials Science and Engineering
Forms of Corrosion
Erosion-corrosion
- Erosion-corrosion arises from the combined action of chemical attack and mechanical abrasion or wear as a consequence of fluid motion.
魏茂國
- It is especially harmful to alloys that passivate by forming a protective surface film; the abrasive action may erode away the film, leaving exposed a bare metal surface.
- Erosion-corrosion is commonly found in piping , especially at bends, elbows, and abrupt changes in pipe diameter-positions where the fluid changes direction or flow suddenly becomes turbulent.
- Increasing fluid velocity normally enhances the rate of corrosion.
- A solution is more erosive when bubbles and suspended particulate solids are present.
Fig. 17.20
Impingement failure of an elbow that was part of a steam condensate line.
60
Materials Science and Engineering
Forms of Corrosion
魏茂國
Erosion-corrosion wear pattern of silica slurry in mild-steel pipe.
Reduction of erosion-corrosion
- Changing the design to eliminate fluid turbulent and impingement effects.
- Utilizing of other materials that inherently resist erosion.
- Removal of particulates and bubbles from the solution.
61
Materials Science and Engineering
Forms of Corrosion
魏茂國
Stress corrosion (cracking)
- Stress corrosion results from the combined action of an applied tensile stress and a corrosive environment; both influence are necessary.
- Some materials that are virtually inert in a particular corrosive medium become susceptible to stress corrosion when a stress is applied. Small cracks form and then propagate in a direction to the stress.
- Failure behavior is characteristic of that for a brittle material, even though the metal alloy is intrinsically ductile.
- Cracks may form at relatively low stress levels, significantly below the tensile strength.
Fig. 17.21
Photomicrograph showing inter-
62 granular stress corrosion cracking in brass.
Materials Science and Engineering
Forms of Corrosion
魏茂國
- The stress that produces stress corrosion cracking need not be externally applied; it may be a residual one that results from rapid temperature changes and uneven contraction, or for a two-phase alloys in which each phase has a different coefficient of expansion.
Prevention from stress corrosion
- Reducing the stress.
- Annealing
Stress-corrosion cracks in a pipe.
63
Types of Corrosion
Materials Science and Engineering
Mechanism of stress-corrosion cracking
- Most SCC mechanisms involve crack initiation and propagation stages.
魏茂國
- In many cases the crack initiates at a pit of other discontinuity on the metal surface.
After the crack has been started, the tip can advance (Fig. 13.27).
A high stress builds up at the tip of the crack due to tensile stresses acting on the metal.
Anodic dissolution of the metal takes place by localized electrochemical corrosion at the tip of the crack as it advances. The crack grows in a plane perpendicular to the tensile stress until the metal fractures.
- If either the stress or the corrosion is stopped, the crack stops growing.
- Tensile stress is necessary for both the initiation and propagation of crack and is important in the rupturing of surface films.
64
Materials Science and Engineering
Forms of Corrosion
魏茂國
Hydrogen embrittlement (hydrogen-induced cracking)
- Some steels experience a significant reduction in ductility and tensile strength when atomic hydrogen (H) penetrates into the material. This phenomenon is hydrogen embrittlement.
- Hydrogen in its atomic form diffuses interstitially through the crystal lattice, and concentration as low as several parts per million can lead to cracking.
- Hydrogen-induced cracks are most often transgranular.
- In hydrogen embrittlement, a normally ductile metal experiences brittle fracture when exposed to both a tensile stress and a corrosive atmosphere.
- The presence of what are termed “poisons” such as sulfur (i.e., H
2
S) and arsenic compounds accelerates hydrogen embrittlement.
Reduction of hydrogen embrittlement
- Reducing the tensile strength of the alloy via a heat treatment.
- Removal of the source of hydrogen, “baking” the alloy at an elevated temperature to drive out any dissolved hydrogen.
65
- Substitution of a more embrittlement resistant alloy.
Materials Science and Engineering
Corrosion Environments
Corrosive environments
- Atmosphere: oxygen dissolved moisture
- Aqueous solutions: freshwater (oxygen), seawater (sodium chloride)
- Soils
- Acids
- Bases
- Inorganic solvents
- Molten salts
- Liquid metals
- Human body
Materials
- For freshwater use
Cast iron, aluminum, copper, brass, some stainless steels.
- For seawater use
Titanium, brass, some bronzes, copper-nickel alloys, nickel-chromium-
66 molybdenum alloys.
魏茂國
Materials Science and Engineering
Corrosion Prevention
Corrosion prevention
Material selection : the most common and easiest way.
魏茂國
Environmental alteration : lowering the fluid temperature and/or velocity, many times increasing or decreasing concentration of some species, adding inhibitors in relatively low concentration to the environment.
Design : easy washing and shutdown, provision for the exclusion of air.
Coatings
The coating must be nonreactive in the corrosive environment and resistant to mechanical damage that exposes the bare metal to the corrosive environment.
Cathodic protection
Inhibitor
- Substances that, when added in relatively low concentration to the environment, decrease its corossiveness.
- Inhibitors are normally used in closed systems such as automobile radiators and steam boilers.
67
Materials Science and Engineering
Corrosion Prevention
魏茂國
Cathodic protection
- Cathodic protection can be used for all 8 different forms of corrosion as discussed earlier and may, in some situations, completely stop corrosion.
- Cathodic protection involves supplying , from an external source, electrons to the metal to be protected , making it a cathode; the reaction is thus forced in the reduction direction.
Galvanic protection
The metal to be protected is electrically connected to another metal that is more reactive in the particular environment. The oxidized metal is often called a sacrificial anode , and magnesium and zinc are commonly used as such because they lie at the anodic end of the galvanic series (Fig. 17.22a).
Fig. 17.22a
Cathodic protection of an underground pipeline using a magnesium sacrificial anode.
68
Materials Science and Engineering
Corrosion Prevention
魏茂國
Impressed current
The source of electrons is an impressed current from an external dc power source
(Fig. 17.22b) for an underground tank. The negative terminal of the power source is connected to the structure to be protected. The other terminal is joined to an inert anode (often graphite), which is buried in the soil; high-conductivity backfill material provides good electrical contact between the anode and surrounding soil.
A current path exists between the cathode and anode through the intervening soil, completing the electrical circuit.
Fig. 17.22b
Cathodic protection of an underground tank using an impressed current.
69
Materials Science and Engineering
Corrosion Prevention
魏茂國
Galvanizing (Fig. 17.23)
The process of galvanizing is simply one in which a layer of zinc is applied to the surface of steel by hot dipping . In the atmosphere and most aqueous environments, zinc is anodic to and will cathodically protect the steel if there is any surface damage.
Any corrosion of the zinc coating will proceed at an extremely slow rate because the ratio of the anode-to-cathode surface area is quite large.
Fig. 17.23
Galvanic protection of steel as provided by a coating of zinc.
70
Materials Science and Engineering
Oxidation
魏茂國
Oxidation
Oxidation of metal alloys is possible in gaseous atmospheres, normally air, wherein an oxide layer or scale forms on the surface of the metal . This phenomenon is frequently termed scaling , tarnishing , or dry corrosion .
Mechanisms
- Oxidation half-reaction occurs at the metal-scale interface
M
M
2
2 e
(17.29)
- Reduction half-reaction occurs at the scale-gas interface
1
2
O
2
2 e
O
2
(17.31)
- For divalent metal, the process of oxide layer formation is an electrochemical one.
M
1
2
O
2
MO (17.28)
Fig. 17.15
Schematic representation of processes that are involved in gaseous
71 oxidation at a metal surface.
Materials Science and Engineering
Oxidation
魏茂國
- For the oxide layer to increase in thickness, it is necessary that electrons be conducted to the scale-gas interface; in addition, M 2+ ions must diffuse away from the metal-scale interface, and/or O 2ions must diffuse toward this same interface.
- The oxide scale serves both as an electrolyte through which ions diffuse and as an electrical circuit for the passage of electrons.
- The scale may protect the metal from rapid oxidation when it acts as a barrier to ionic diffusion and/or electrical conduction; most metal oxides are highly electrically insulative.
72
Materials Science and Engineering
Oxidation
魏茂國
Pilling-Bedworth ratio ( V
O
/V
M
)
- Rate of oxidation and the tendency of the film to protect the metal from further oxidation are related to the relative volumes of the oxide and metal (Pilling-
Bedworth ratio).
P-B ratio =
A
0
A
M
M
0
(17.32)
A
O
: molecular weight of the oxide, A
M
: atomic weight of the metal,
O
: oxide density,
M
: metal density.
- P-B ratio < 1, the oxide film tends to be porous and unprotective because it is insufficient to fully cover the metal surface.
- P-B ratio > 1, compressive stresses result in the film as it forms.
- P-B ratio > 2~3, the oxide coating may crack and flake off, continually exposing a fresh and unprotectived metal surface.
P-B ratio
1~2 , protective coatings normally form for metals .
73
Materials Science and Engineering
Oxidation
魏茂國
Factors for protective coatings
- P-B ratios: 1~2.
- High adherence between film and metal.
- Comparable thermal expansion coefficients for metal and oxide.
- A relatively high melting point.
- Good high-temperature plasticity.
Table 17.3
Pilling-Bedworth ratios for a number of metals.
Ce
Al
Pb
Ni
Be
Pd
Cu
Fe
Mn
Co
Cr
Si
Protective
1.16
1.28
1.40
1.52
1.59
1.60
1.68
1.77
1.79
1.99
1.99
2.27
Nonprotective
K
Li
Na
Cd
Ag
Ti
Ta
Sb
Nb
U
Mo
W
0.45
0.57
0.57
1.21
1.59
1.95
2.33
2.35
2.61
3.05
3.40
3.40
74
Materials Science and Engineering
Oxidation
魏茂國
Kinetics (Fig. 17.25)
- When the oxide that forms is nonporous and adheres to the metal surface, the rate of layer growth is controlled by ionic diffusion. A parabolic relationship exists between the weight per unit area W and the time t as follows:
W
2
K
1 t
K
2
(17.34)
K
1
& K
2
: time-independent constants at a given temperature.
- In the oxidation of metals for which the scale is porous or flakes off, the oxidation rate expression is linear:
W
K
3 t
(17.35)
K
3
: constant.
- For very thin oxide layers (< 100 nm) that form at relatively low temperatures, the dependence of weight gain on time is logarithmic:
W
2
K
4 log
K
5 t
K
6
(17.36)
K
4
& K
5
& K
6
: constants.
75
Materials Science and Engineering
Oxidation
魏茂國
Fig. 17.25
Oxidation film growth curves for linear, parabolic, and logarithmic rate laws.
76
Materials Science and Engineering
Corrosion of Ceramic Materials
魏茂國
Ceramic materials
- Ceramic materials, being compounds between metallic and nonmetallic elements, may be thought of as having already been corroded.
- Ceramic materials are frequently utilized because of their resistance to corrosion.
- Ceramic materials are much better suited to withstand most of severe environments for reasonable time periods than are metals.
Corrosion of ceramic materials
- Ceramic materials are exceedingly immune to corrosion by almost all environments, especially at room temperature.
- Corrosion of ceramic materials generally involves simple chemical dissolution, in contrast to the electrochemical processes found in metals.
77
Materials Science and Engineering
Degradation of Polymers
魏茂國
Degradation of polymers
- Polymeric degradation is physiochemical ; it involves physical as well as chemical phenomena.
- Polymers may deteriorate by swelling and dissolution .
Covalent bond rupture .
Chemical reactions .
Radiation .
78
Materials Science and Engineering
Swelling & Dissolution
魏茂國
Swelling
- With swelling, the liquid or solute diffuses into and is absorbed within the polymer ; the small solute molecules fit into and occupy positions among the polymer molecules. Thus the macromolecules are forced apart such that specimen expands.
- This increase in chain separation results in a reduction of the secondary intermolecular bonding forces; as a consequence, the material becomes softer and more ductile .
- The liquid solute lowers the glass transition temperature of polymers.
- Swelling may be considered to be a partial dissolution process in which there is only limited solubility of the polymer in the solvent.
Dissolution
- Dissolution, which occurs when the polymer is completely soluble , may be thought of as just a continuation of swelling.
- The greater the similarity of chemical structure between the solvent and polymer, the greater is the likelihood of swelling and/or dissolution.
79
Materials Science and Engineering
Swelling & Dissolution
魏茂國
Swelling & dissolution
- In general, increasing molecular weight, increasing degree of crosslinking and crystallinity, and decreasing temperature result in a reduction of deteriorative processes.
- In general, polymers are much more resistant to attack by acidic and alkaline solutions than are metals.
Table 17.5
Resistance to degradation by various environments for selected elastomeric materials.
80
Materials Science and Engineering
Swelling & Dissolution
魏茂國
Table 17.4
Resistance to degradation by various environments for selected plastic materials.
81
Materials Science and Engineering
Bond Rupture
Scission
Scission is the severence or rupture of molecular chain bonds . This causes a
魏茂國 separation of chain segments at the point of scission and a reduction in the molecular weight.
- Bond rupture may result from exposure to radiation or to heat, and from chemical reaction.
Radiation effects
- One reaction is ionization , in which the radiation removes an orbital electron from a specific atom, converting that atom into a positively charged ion. As a consequence, one of the covalent bonds associated with the specific atom is broken , and there is a rearrangement of atoms or groups of atoms at that point.
- This bond breaking leads to either scission or crosslinking at the ionization site, depending on the chemical structure of the polymer and also on the dose of radiation.
Stabilizers may be added to protect polymers from ultraviolet damage.
82
Materials Science and Engineering
Bond Rupture
魏茂國
Chemical reaction effects
- Oxygen, ozone, and other substances can cause or accelerate chain scission as a result of chemical reaction.
Thermal effects on bond rupture
Thermal degradation corresponds to the scission of molecular chains at elevated temperature; as a consequence, some polymers undergo chemical reactions in which gaseous species are produced.
83
Materials Science and Engineering
Weathering
魏茂國
Weathering
- Many polymeric materials serve in applications that require exposure to outdoor conditions. Any resultant degradation is termed weathering.
- The deterioration is primarily a result of oxidation, which is initiated by ultraviolet radiation from the sun.
- The fluorocarbons are virtually inert under these conditions.
84