Chap 18 Learn Obj

CHAPTER 17
CORROSION AND DEGRADATION OF MATERIALS
LEARNING OBJECTIVES
1. Define corrosion .
2. (a) Distinguish between oxidation and reduction reactions.
(b) State which reaction occurs at the anode and which at the cathode.
3. List five possible reduction reactions that can occur in aqueous solutions.
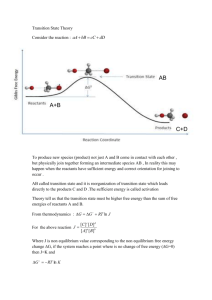
4. Given an oxidation reaction and a reduction reaction specify the overall electrochemical reaction.
5. Describe the following:
(a) galvanic couple,
(b) standard half-cell, and
(c) standard hydrogen electrode.
6. For two pure metals that are electrically connected and submerged in solutions of their respective ions, given the molar concentrations of these solutions, the standard emf series, and temperature,
(a) compute the cell potential, and
(b) write the spontaneous direction for the electrochemical reaction.
7. (a) Describe the galvanic series, (b) the conditions under which it was generated, and (c) its utility.
8. Given the weight loss over some time period for a piece of metal of specified density and area, compute the corrosion penetration rate in both mils per year and millimeters per year.
9. For some metal oxidation reaction, given the current density, determine the oxidation rate in units of moles per meters squared per second.
10. Define (a) polarization , and (b) overvoltage .
11. (a) Name and briefly describe the two different types of polarization.
(b) Specify the conditions under which each type is rate controlling.
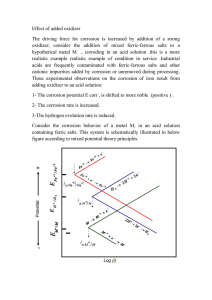
(c) Make a plot of overvoltage versus the logarithm of current density for both types of polarization.
12. Define exchange current density .
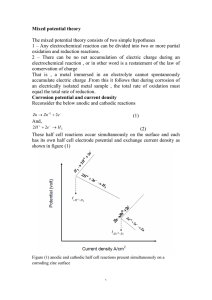
13. For some electrochemical reaction the rate of which is controlled by activation polarization, determine:
(a) the rate of oxidation, and
(b) the value of the corrosion potential, given appropriate activation polarization data for both oxidation and reduction half-reactions.
14. Define passivity .
15. (a) For a metal that exhibits passive behavior, make a schematic polarization plot of electrochemical potential versus the logarithm of current density.
(b) Using such a plot demonstrate how a metal may display both active and passive corrosion behaviors.
16. (a) List four environmental factors that can influence corrosion.
(b) Describe how each of these factors normally affects the corrosion rate.
17. For each of the eight forms of corrosion, and, in addition, hydrogen embrittlement, do the following:
(a) Describe the nature of the deteriorative process;
(b) With all but uniform corrosion, cite the corrosion mechanism; and
(c) Note at least one measure that may be taken to reduce or eliminate the likelihood of corrosion.
18. In addition to cathodic protection, list four general measures that are commonly used to prevent corrosion.
19. (a) Describe the mechanism of cathodic protection.
(b) Describe each of the three cathodic protection techniques.
20. Briefly describe the mechanism by which an oxide layer forms on the surface of a metal.
21. (a) Given the atomic weight of a metal and the molecular weight of its oxide, as well as values for their densities, compute the Pilling-Bedworth ratio.
(b) On the basis of this ratio, predict whether or not the oxide coating will act as a protective barrier for the metal.
22. (a) Write equations for weight gain per unit area as functions of time for linear, parabolic, and logarithmic oxidation rates.
(b) Given weight gain-time data for the oxidation of some metal or alloy, determine whether the kinetics are linear, parabolic, or logarithmic.
23. Explain why ceramic materials are, in general, very resistant to corrosion.
24. For polymeric materials discuss (a) two degradation processes that occur when they are exposed to liquid solvents, and (b) the causes and consequences of molecular chain bond rupture.