Gene 221 (1998) 35–43
Cloning vectors for the expression of green fluorescent protein fusion
proteins in transgenic plants
A.G. von Arnim a,*, X.-W. Deng b, M.G. Stacey a
a Department of Botany, HBB 437, The University of Tennessee, Knoxville, TN 37996-1100, USA
b Department of Molecular, Cellular and Developmental Biology, Yale University, 165 Prospect Street, PO Box 208104,
New Haven, CT 06520-8104, USA
Received 10 May 1998; received in revised form 8 August 1998; accepted 10 August 1998; Received by W. Martin
Abstract
A series of versatile cloning vectors has been constructed that facilitate the expression of protein fusions to the Aequorea victoria
green fluorescent protein (GFP) in plant cells. Amino-terminal- and carboxy-terminal protein fusions have been created and
visualized by epifluorescence microscopy, both in transgenic Arabidopsis thaliana and after transient expression in onion epidermal
cells. Using tandem dimers and other protein fusions to GFP, we found that the previously described localization of wild-type
GFP to the cell nucleus is most likely due to diffusion of GFP across the nuclear envelope rather than to a cryptic nuclear
localization signal. A fluorescence-based, quantitative assay for nuclear localization signals is described. In addition, we have
employed the previously characterized mutants GFP–S65T and GFP–Y66H in order to allow for the expression of red-shifted
and blue fluorescent proteins, respectively, which are suitable for double-labeling studies. Expression of GFP-fusions was controlled
by a cauliflower mosaic virus 35S promoter. Using the Arabidopsis COP1 protein as a model, we confirmed a close similarity in
the subcellular localization of native COP1 and the GFP-tagged COP1 protein. We demonstrated that COP1 was localized to
discrete subnuclear particles and further confirmed that fusion to GFP did not compromise the activity of the wild-type COP1
protein. © 1998 Elsevier Science B.V. All rights reserved.
Keywords: GFP; Nuclear targeting; Subnuclear architecture; COP1
1. Introduction
Fusions between the green fluorescent protein (GFP)
from Aequorea victoria and specific passenger proteins
have found a wide acceptance in protein targeting
research (e.g. Wang and Hazelrigg, 1994; Olson et al.,
1995). In plants, subcellular targeting has been investigated primarily by two techniques. On the one hand,
recombinant b-glucuronidase (GUS )-fusion proteins
have been expressed in transient assays (e.g. Restrepo
et al., 1990; Varagona et al., 1992) or in transgenic
plants (e.g. von Arnim and Deng, 1994). Typically, the
GUS fusion proteins are visualized in situ by histochemi* Corresponding author. Tel: +1 423 974 6206; Fax: +1 423 974 0978;
e-mail: vonarnim@utk.edu
Abbreviations: CaMV, cauliflower mosaic virus; CCD, charge coupled
device; FRET, fluorescence resonance energy tranfer; GFP, green
fluorescent protein; gfp, gene encoding GFP; GUS, b-glucuronidase;
NLS, nuclear localization signal; TEV, tobacco etch virus; UV, ultraviolet light; wt, wild type.
0378-1119/98/$19.00 © 1998 Elsevier Science B.V. All rights reserved.
PII: S0 3 7 8 -1 1 1 9 ( 9 8 ) 0 0 43 3 - 8
cal staining and light microscopy, and the subcellular
resolution of this assay is sufficient for distinguishing
nuclear from non-nuclear localization. On the other
hand, indirect immunofluorescence detection provides a
high spatial resolution, but relies on specific antibodies,
and requires fixation combined with either tissue sectioning or digestion of the cell wall to facilitate access of
the antibody to the antigen. Compared with the aforementioned techniques, GFP-based detection has the
advantage that living cells can be examined, allowing
real-time imaging, and avoiding fixation artifacts.
Moreover, many plant tissues are easily transformable
and exhibit little green autofluorescence under ultraviolet
( UV ) excitation. Therefore, wild-type GFP as well as a
mutant derivative of GFP with enhanced fluorescence,
both of which emit green light upon UV excitation, are
detectable with high sensitivity (Haseloff et al., 1997).
Additional spectral variants of GFP, most notably the
red-shifted S65T mutant and the blue fluorescent Y66H
mutant, have been shown to fluoresce in plant cells with
characteristics similar to those described in non-plant
36
A.G. von Arnim et al. / Gene 221 (1998) 35–43
cells (Heim et al., 1994, 1995; Davis and Vierstra, 1998).
Specifically in plant cells, targeting of GFP-fusions to
chloroplasts ( Köhler et al., 1997a), mitochondria
( Köhler et al., 1997b), cytoskeletal structures (Heinlein
et al., 1995) and the nucleus (Grebenok et al., 1997)
has provided new insights into diverse cellular processes.
However, no versatile cloning vectors for the expression
of GFP-fusion proteins have become available.
Moreover, the adoption of GFP as a tag for nuclear
targeting assays has been impeded by the spontaneous
uptake of unfused GFP by the nucleus (Grebenok et al.,
1997). It has also remained open as to whether
transgenic plants expressing GFP-fusion proteins are
viable, and whether fusion of GFP to a passenger
protein necessarily interferes with the functionality of
the passenger protein in transgenic plants.
Here, we have created a series of GFP-fusion cloning
vectors and established GFP as a reporter for nuclear
protein localization assays. A fusion gene between gfp
and CONSTITUTIVELY PHOTOMORPHOGENIC 1
(COP1) complements a lethal cop1 loss-of-function allele
in Arabidopsis, suggesting that fusion to GFP does not
necessarily compromise the function of the fusion partner. Our data further indicate that GFP fusions can
provide a reliable means of tagging diverse proteins in
live plant cells.
carried out with high-fidelity DNA polymerases. The
DNA sequence of pAVA319 is available under GenBank
Accession No. AF078810.
2.2. Transient expression assays
Transient expression assays in onion epidermal cells
using particle bombardment (Bio-Rad, Richmond, CA)
were carried out as described previously (von Arnim
and Deng, 1994). Typically, 2 mg of wild-type GFP or
GFP-S65T plasmids or 5 mg of BFP plasmid were used
per assay. Tissue was examined on a Zeiss Axiovert 135
inverted microscope with a 100-W mercury arc lamp
and the following filter cubes: I, 365-nm excitation;
395-nm beam splitter; 420-nm long-pass emission (Zeiss
cube 02); II, Chroma FITC-cube CZ917 with an additional block of infra-red emission (Chroma; 485±10-nm
excitation; 505-nm beam splitter; 535±20-nm band-pass
emission); III, 365-nm excitation; 395-nm beam splitter;
460±25-nm emission (Chroma); IV, 365-nm excitation;
395-nm beam splitter; 535±20-nm band-pass emission.
Images were acquired through a 10× objective with a
12-bit MicroMax cooled CCD camera (Princeton
Instruments, Trenton, NJ ) operated by IPLab software
(Signal Analytics, Vienna, VA) on a Macintosh 7100
PowerPC. Images were processed for printing via Adobe
Photoshop software and printed on a Kodak XLS8600
dye sublimation printer.
2. Materials and methods
2.1. Plasmid construction
Standard molecular cloning procedures (Sambrook
et al., 1989) were employed, and details of the clone
construction are available upon request. All GFP-vectors are based on the expression cassette of pRTL2
(Restrepo et al., 1990). This vector contains a dual 35S
promoter from cauliflower mosaic virus (CaMV ), the
translational leader sequence from tobacco etch virus
( TEV ), and the 35S polyadenylation signal from CaMV.
Except for pAVA68, which contains a wild-type gfp
cDNA (Prasher et al., 1992), all gfp cDNAs are based
on mGFP4 (Haseloff et al., 1997) and therefore express
correctly spliced transcripts in Arabidopsis. Unless stated
otherwise in Table 1, all vectors contain the TEV translational enhancer and support the cloning of aminoterminal fusions to GFP via a unique NcoI-site, and the
cloning of carboxy-terminal fusions via BglII. An XbaIsite is located beyond the gfp stop codon to facilitate
directional cloning. Vectors containing the NcoI cloning
site also carry a S1G substitution and a deletion of E6
of GFP, mutations that do not interfere with GFPfluorescence. Fusion protein genes were constructed
based on published full-length cDNA sequences using
oligonucleotides to introduce suitable restriction enzyme
recognition sites. PCR amplification of cDNAs was
2.3. Quantification of nuclear versus cytoplasmic
fluorescence
GFP-images were acquired with a cooled CCD
camera through the FITC-filter cube (Cube II ), adjusting the exposure time to prevent saturation of the CCD.
Using IPLab software, the fluorescence level was quantified separately for the nucleus and for the whole cell by
circumscribing the respective area as a Region of Interest
( ROI ). Background correction was applied by estimating both nuclear and cytoplasmic fluorescence levels in
a neighboring untransformed cell. The fraction of
nuclear versus whole-cell fluorescence was expressed as
a percentage. Typically, between 10 and 20 cells were
imaged per sample. In pilot experiments, we determined
that the arbitrarily chosen plane of focus through the
cell did not significantly affect our results. Specifically,
the variance of the data collected from one GFPexpressing cell at different focal planes is less than the
variance of data collected from different cells at a
common focal plane.
2.4. GFP expression in Arabidopsis
The GFP–COP1 expression cassette was subcloned
to the binary T-DNA vector pBIN19 (Bevan, 1984).
Arabidopsis thaliana (ecotypes Nossen and Columbia)
37
A.G. von Arnim et al. / Gene 221 (1998) 35–43
Table 1
GFP-fusion cloning vectors
Name
Fluorescence
Seriesa
Remarksb
pAVA68
pAVA90
pAVA120
pAVA122
pAVA319
pAVA322
pAVA118
pAVA121
pAVA321
pAVA192
pAVA389
Wild-type
Wild-type
Wild-type
Wild-type
Wild-type
Wild-type
S65T
S65T
S65T
Y66H (BFP)
Y66H (BFP)
I
I
I
I
II
II
I
I
II
I
I
Splicing defect in Arabidopsis C-fusions only
C-fusions only
No translational enhancer
C-fusions only
C-fusions only
C-fusions only
aRefer to Fig. 1 for restriction enzyme sites in Series I and Series II vectors.
bVectors marked ‘C-fusions only’ do not contain the NcoI site displayed in Fig. 1.
was transformed by Agrobacterium-mediated T-DNA
transfer according to the vacuum infiltration procedure
(Bechtold et al., 1993). Kanamycin-resistant transgenic
seedlings were grown on GM medium with 1% sucrose
( Valvekens et al., 1988). Seedlings were mounted
in water on glass slides and observed through 40× or
63× oil-immersion objectives. All eight independent
transgenic lines showed the same subcellular localization
pattern for GFP–COP1. In some experiments, seedlings
were planted in soil after microscopic examination and
grown to seed set without exhibiting any adverse effects
in response to the prior microscopic illumination
treatment.
2.5. Immunofluorescence and GUS assays
The immunofluorescence assay for COP1 using
an affinity-purified polyclonal antiserum against
Arabidopsis COP1 (McNellis et al., 1994a) or a mouse
monoclonal antibody against Arabidopsis COP1
(McNellis et al., 1994b) in conjunction with FITClabelled secondary antibodies was carried out essentially
as described (Matsui et al., 1995). In-situ GUS staining
was carried out as described previously (von Arnim and
Deng, 1994).
3. Results and discussion
3.1. Construction of GFP fusion vectors
We have constructed 11 plasmid cloning vectors for
the expression of fluorescent protein fusions in plants
(Fig. 1 and Table 1). The basic vector (pAVA120) is
composed of a dual 35S promoter from CaMV, the
translational enhancer sequence of TEV, the GFP
coding sequence, and the 35S transcriptional terminator
from CaMV. In addition, all vectors carry the ampicillin
resistance gene and the high copy number ColE1 origin
of replication. Cloning sites are detailed in Fig. 1C.
The vectors differ in the restriction enzyme digestion
sites available for cloning of protein fusions. While one
subset of vectors allows the construction of both aminoand carboxy-terminal fusions via unique NcoI and BglII
sites, respectively (pAVA120, 121, 122, 319, 321, 389),
the other subset supports carboxy-terminal fusions only
(pAVA68, 90, 118, 322; Table 1).
The vectors fall into two subsets with respect to
cloning sites flanking the expression cassette (Fig. 1).
Series I vectors carry identical sites on the left and right
of the expression cassette, useful if subsequent subcloning is to remain unbiased with regard to the orientation
of the expression cassette. In contrast, Series II vectors
carry different restriction sites on either side of the
cassette, facilitating directional subcloning.
Furthermore, pAVA122 has a deletion of the translational enhancer sequence, which resulted in an approximately threefold reduction in fluorescence intensity in
the onion epidermal cell transient expression assay when
compared to the most closely related plasmid, pAVA120
(data not shown). This feature may be helpful if overexpression of the protein is toxic to the cell or otherwise
undesirable.
Vector pAVA68 contains an unmodified GFP cDNA,
which lacks the splice site and codon usage modifications
required for expression in Arabidopsis (Haseloff et al.,
1997). However, GFP expression from pAVA68 is indistinguishable from that of the most closely related vector,
pAVA90, in onion epidermal cells and may potentially
be superior over pAVA90 in other plant species.
3.2. Fluorescence properties
The GFP-fusion vectors differ primarily in the spectral
properties of the encoded fluorescent protein. Six of the
38
A.G. von Arnim et al. / Gene 221 (1998) 35–43
Fig. 1. Structure of cloning vectors for expression of GFP fusion proteins. (A) Series I vectors are based on the plasmid pRTL2 (Carrington et al.,
1990). (B) Series II vectors are based on pBluescript SK+ (Stratagene). (C ) Nucleotide sequences at the cloning sites around the start and stopcodons of GFP. Amino acids are numbered according to the sequence in Prasher et al. (1992) and are given in single-letter code.
vectors contain GFP with wild-type fluorescence, three
contain the GFP–S65T mutant, and two contain the
GFP–Y66H mutant. Expression of the respective fluorescent proteins in a transient onion epidermal cell assay
confirmed the expected fluorescence properties (Heim
et al., 1994, 1995). Whereas wild-type GFP fluoresced
bright green under both ultraviolet ( UV ) and blue light
(not shown), the GFP–S65T mutant fluoresced bright
green under blue light but only faintly under UV
(Fig. 2). GFP–Y66H (BFP) fluoresced blue under
UV, but did not fluoresce under blue light ( Fig. 2).
GFP–S65T and BFP show a limited spectral overlap
(Heim et al., 1994, 1995). Therefore, these two reporter
proteins may be suitable for the concomitant tagging of
two different proteins in the same cell. The feasibility of
double labelling in the widely popular onion epidermal cell transient expression assay ( Varagona et al.,
1992) was tested by co-expressing GFP–S65T and
BFP together, as well as individually. As expected,
GFP–S65T and BFP showed a limited spectral overlap
using the filter combinations described here ( Fig. 2A,
left and central column; Fig. 2B). Therefore, double
labelling should be feasible, at least if BFP and
GFP–S65T do not show one of the following three types
of optical interaction. First, it is conceivable that fluorescence resonance energy transfer (FRET ) from BFP
to GFP–S65T might result in increased green fluorescence emission by GFP–S65T in the presence of BFP,
if BFP and GFP–S65T were physically associated (Mitra
et al., 1996; Miyawaki et al., 1997). Second, GFP–S65T,
which absorbs blue light, might quench blue fluorescence
emission of BFP by reabsorbing blue light. Third, the
presence of BFP, which significantly absorbs blue light,
might inhibit excitation of GFP–S65T by ‘shading’
GFP–S65T. We tested these possibilities by coexpressing
BFP and GFP–S65T in onion epidermal cells at a molar
ratio of 2.5:1. This ratio was chosen as a trade-off
between the ratio that would maximize interaction
between GFP–S65T and BFP (1:1) and the ratio that
would result in equal fluorescence intensity (6:1) based
on the higher quantum yield and absorption coefficient
of GFP–S65T when compared to BFP. We then examined green fluorescence emission under UV excitation
as a sensitive measure of any optical interaction. As
shown in Fig. 2 (bottom row), the combined fluorescence after co-expression of GFP–S65T and BFP was
indistinguishable from the value expected for an additive
interaction of the two proteins (Fig. 2B, arrow labelled
‘minus’), indicating that no significant FRET, quenching, or shading occurs between the two proteins. This is
not surprising, because FRET relies on the immediate
proximity of the two chromophores, whereas quenching
and shading should become measurable only under very
high concentrations of GFP. Therefore, it may be feasible to employ the fusion vectors in conjunction with the
expression assay described here to perform doublelabelling experiments and to examine direct protein–protein interactions in vivo by monitoring FRET.
A.G. von Arnim et al. / Gene 221 (1998) 35–43
39
Fig. 2. (A) GFP–S65T and BFP fluorescence are additive upon co-expression in onion epidermal cells. Representative cells expressing GFP–S65T
alone (top row), BFP alone (middle row) or both GFP–S65T and BFP together (bottom row), were imaged through filter combinations designed
to visualize BFP ( left column, cube III, UVB), GFP–S65T (central column, cube II, BG) and their interaction (right column, cube IV,
UVG). (B) Quantification of fluorescence from (A). Note that GFP–S65T fluoresces more brightly than BFP. In the bottom graph, fluorescence
values expected in the absence of any interaction (−) or in the presence of FRET or fluorescence quenching (+) are indicated by arrowheads.
3.3. A GFP-based nuclear targeting assay
Because GFP is detectable in live cells without the
complication of a histochemical assay, this reporter has
potential advantages over the GUS reporter enzyme.
However, GFP enters the nucleus of diverse plant cells,
even in the absence of an added nuclear localization
signal ( Fig. 2; Grebenok et al., 1997). To address this
potential handicap of GFP, we have employed two
strategies. First, we have established a simple, yet quantitative, assay for the measurement of nuclear and total
cellular GFP fluorescence levels in live onion epidermal
cells (see Section 2). As shown in Fig. 3A and Table 2,
approximately 11% of cellular GFP was localized to the
nucleus, whereas the remainder was cytoplasmic. The
fraction of nuclear GFP was not affected by the expression level of the protein (not shown). We then tested
whether GFP might localize to the nucleus because of
a cryptic nuclear localization signal. Alternatively, GFP
might enter the nucleus by diffusion due to its small size
(28 kDa). If GFP contained an NLS, then a GFPdimer, consisting of two GFP domains arranged in
tandem within one polypeptide, should result in identical
or increased nuclear localization. In contrast, if GFP
did not contain an NLS, dimerization should reduce the
level of nuclear GFP, because the increased size would
inhibit diffusion through the nuclear pore complexes. A
head-to-tail dimer of GFP (55 kDa) showed a reduced
nuclear localization (Fig. 3B; Table 2), and a trimer of
GFP (83 kDa) remained primarily cytoplasmic ( Fig. 3D;
Table 2). A fusion between GUS and GFP (Fig. 3C;
Table 2), previously proposed to represent bona fide
cytoplasmic localization (Grebenok et al., 1997) resembled the GFP-dimer and the GFP-trimer ( Table 2). As
expected, when stained for GUS activity, the GFP–GUS
fusion appeared cytoplasmic (not shown). These data
indicate that GFP does not contain an NLS of its own.
Rather, unfused GFP most likely underpasses the size
exclusion limit for bidirectional diffusion through
nuclear pore complexes, estimated at 40–60 kDa
(Görlich and Mattaj, 1996). Our interpretation is consistent with a previous report that lysed protoplasts
expressing GFP rapidly lose all nuclear fluorescence
(Grebenok et al., 1997).
We then compared our nuclear targeting assay with a
tobacco protoplast assay described previously
(Grebenok et al., 1997) by expressing a fusion between
GFP and the nuclear NIa protein from tobacco etch
40
A.G. von Arnim et al. / Gene 221 (1998) 35–43
Fig. 3. Nuclear localization of GFP fusions. (A) GFP; (B) GFP dimer; (C ) GFP–GUS; (D) GFP-trimer; (E ) GFP–NIa.
virus ( Restrepo et al., 1990). Whereas exclusively
nuclear accumulation of GFP–NIa had been reported
using confocal microscopy (Grebenok et al., 1997), we
observed that GFP–NIa was strongly enriched in the
nucleus, but that a detectable fraction remained cytoplasmic. ( Fig. 4; Table 2). This minor discrepancy may
be due to the different cell types employed or to the
increased sensitivity of our assay, which, other than a
typical confocal imaging protocol, operated at a camera
gain-setting of 1, more closely representing quantitative
imaging. Because the GFP-based assay is carried out in
live cells, affords improved spatial resolution, and is
easily quantifiable, it is generally superior to the GUSbased assay. However, for fusion proteins that are
smaller than the size exclusion limit for nuclear diffusion,
results of the GFP-based assay must be interpreted
cautiously.
Table 2
Percentage of nuclear fluorescence determined for GFP-fusions in the
transient onion epidermal cell assay
Protein
Nuclear fluorescence (%)
Mean±SD
GFP
GFP-dimer
GFP–GUS
GFP-trimer
GFP–NIa
11.0%±1.2%
7.9%±2.2%
7.3%±2.7%
5.5%±1.5%
58.3%±7.0%
a
b
b
b
c
SD, standard deviation.
The letters a, b, and c separate means that are significantly different
at the 95% confidence level.
3.4. Transient expression of GFP-fusion proteins
To establish whether the newly constructed GFPfusion vectors were suitable for studying the subcellular
localization of diverse plant proteins, we created fusions
between GFP and each of a series of proteins involved
in the light control of seedling development, including
Arabidopsis COP9, FUS6, and DET1 (Castle and
Meinke, 1994; Pepper et al., 1994; von Arnim and Deng,
1996; Wei et al., 1994). Data on the subcellular localization of these proteins had previously been obtained by
independent methods. COP9 and FUS6 are part of a
nuclear protein complex of ~550 kDa, but both lack
canonical NLSs (Castle and Meinke, 1994; Wei et al.,
1994; Chamovitz et al., 1996; Staub et al., 1996). DET1
had previously exhibited nuclear uptake as a GUSfusion protein in Arabidopsis protoplasts, but the NLSs
in DET1 remain to be defined (Pepper et al., 1994).
COP9 and DET1 were cloned as carboxy-terminal
fusions to GFP, and FUS6 was cloned as an aminoterminal fusion (Fig. 4). While GFP–COP9 and
FUS6–GFP showed nuclear accumulation to approximately the extent of unfused GFP ( Fig. 4A and B), the
nuclear accumulation of GFP–DET1 was poor
( Fig. 4C ). The GFP–COP9 fusion has a predicted
molecular mass of 50 kDa. Because a GFP-dimer
(55 kDa) showed a reduction in nuclear localization
compared to unfused GFP, the nuclear localization of
GFP–COP9 cannot be explained easily by simple diffusion, but may be more consistent with a form of active
or facilitated transport. It is possible that COP9 contains
A.G. von Arnim et al. / Gene 221 (1998) 35–43
41
Fig. 4. Subcellular localization of Arabidopsis COP9, FUS6, DET1, and COP1 proteins as GFP fusions in onion epidermal cells. (A) GFP–COP9;
(B) FUS6–GFP; (C ) GFP–DET1; (D) GFP–COP1. (D) is enlarged in order to demonstrate the subnuclear speckles formed by GFP–COP1. The
bar corresponds to 10 mm. Arrows point to the nucleus.
its own non-canonical NLS, that COP9 enters the
nucleus by co-transport with another protein, or that
slow diffusion and nuclear retention cooperate in the
nuclear accumulation of GFP–COP9. The FUS6–GFP
fusion has a predicted molecular mass of 82 kDa, and
its diffusion into the nucleus is predicted to be prevented
by its size. The incomplete nuclear localization of
FUS6–GFP may be mediated by piggy-back transport
with a cellular partner protein or by a weak noncanonical NLS. Given that FUS6 and COP9 are part
of a nuclear-localized ~550-kDa protein complex in
Arabidopsis and cauliflower (Chamovitz et al., 1996;
Staub et al., 1996), the efficient nuclear translocation of
GFP–COP9 and FUS6–GFP may be dependent on
additional subunits of the COP9 complex that are ratelimiting in the heterologous onion cell assay.
In our assay, the GFP–DET1 protein demonstrated
a limited nuclear uptake (Fig. 4C ). GFP–DET1 has a
predicted molecular mass of 90 kDa. DET1 may enter
the nucleus by co-transport with a partner protein, and
co-transport may be more efficient in the native species
Arabidopsis (Pepper et al., 1994) than in the heterologous onion system.
The COP1 protein cooperates with DET1, FUS6, and
COP9 in repressing photomorphogenic development in
Arabidopsis. COP1 had previously been shown to be
weakly nucleophilic as a GUS-fusion protein in both
onion epidermal cells and in transgenic Arabidopsis (von
Arnim and Deng, 1994,von Arnim et al., 1997).
Localization of a GFP–COP1 fusion protein in onion
epidermal cells confirmed these results. Moreover,
GFP–COP1 accumulated in discrete subnuclear particles
( Fig. 4D). The particles were of a defined size and shape
and were therefore clearly distinguishable from the
irregularly shaped, much larger, and less numerous
cytoplasmic inclusion bodies that formed at a high
expression level of GFP–COP1 in the cytoplasm (not
shown). The distinct subnuclear localization of the
GFP–COP1 protein suggested that GFP fusions displayed improved resolution at the subcellular level compared to GUS fusions.
3.5. GFP–COP1 fusion protein in transgenic
Arabidopsis
After establishing the suitability of the GFP-fusion
vector system in transient expression assays, we examined whether GFP-fusion proteins may also be visualized
in transgenic Arabidopsis. We selected the GFP–COP1
fusion protein as an example to test whether the subnuclear particles seen in the transient expression assay
were reproducible in transgenic Arabidopsis. As
expected, GFP–COP1 was detected in transgenic seedlings ( Fig. 5A). Moreover, whereas the cytoplasmic fraction of the GFP–COP1 fusion protein accumulated in
a single cytoplasmic inclusion body per cell (not shown),
exactly as observed for the GUS–COP1 fusion protein
(von Arnim and Deng, 1994), the nuclear GFP–COP1
protein was restricted to subnuclear speckles ( Fig. 5A
and B), as previously observed in onion epidermal cells
( Fig. 4D). Typically, one Arabidopsis root epidermal cell
nucleus contained approximately 25 evenly distributed
speckles. Independently, we have confirmed the confinement of COP1 to subnuclear particles in an immuno-
42
A.G. von Arnim et al. / Gene 221 (1998) 35–43
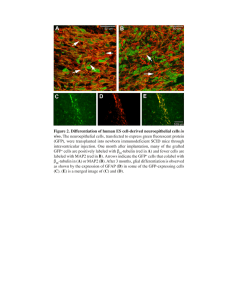
Fig. 5. Subnuclear localization of COP1 in Arabidopsis. (A) Root epidermal cell nucleus of a 35S:gfp–cop1 transgenic seedling. Note that not all
of approximately 25 nuclear speckles are in focus. (B) 4,6-diamidino-2-phenylindole (DAPI )-staining of the nucleus shown in (A). (C )
Immunofluorescence staining of an Arabidopsis mesophyll cell. Note speckled distribution of COP1 within the nucleus. (D) GUS-staining of a
35S:gus–cop1 transgenic Arabidopsis hypocotyl cell. Note nuclear speckles.
fluorescence assay on non-transgenic, wild-type,
Arabidopsis protoplasts using an anti-COP1 specific
polyclonal antiserum (Fig. 5C ) as well as an anti-COP1
monoclonal antibody (not shown). Interestingly, in
transgenic Arabidopsis hypocotyl cells, nuclear speckles
were also formed by the fusion protein between COP1
and GUS (Fig. 5D). Taken together, these data suggest
that the localization of COP1 to subnuclear speckles is
functionally significant. To test this hypothesis further
and to address whether COP1 retains full activity after
fusion to GFP, we crossed the gfp–cop1 transgene into
a cop1-null mutant background, the lethal allele cop1–5
(Deng et al., 1992). We were able to derive a family
that was homozygous for the cop1–5 mutation and that
segregated for the gfp–cop1 transgene. In this family,
all phenotypically mutant, cop1-like, seedlings were
GFP-negative, whereas all wild-type seedlings were
GFP-positive ( Table 3). Moreover, a family segregating
for both the cop1–5 mutation and the gfp–cop1
transgene exhibited a reduction in the number of
cop1-like seedlings ( Table 3). These data indicate that
the gfp–cop1 fusion rescued the cop1 mutation by genetic
complementation. Therefore, the fusion of GFP to
COP1 did not compromise the activity of the COP1
protein.
3.6. Conclusions
(1) The newly constructed GFP-fusion vectors are suitable for visualizing fluorescently labeled proteins at
high levels of subcellular resolution in transient
expression assays and in transgenic plants.
(2) Amino-terminal and carboxy-terminal fusions to
GFP may be constructed easily.
(3) Spectrally distinguishable GFP derivatives are available for double labelling in situ.
(4) A GFP–COP1 fusion protein exhibited biological
activities similar or identical to the native protein,
as shown by genetic complementation of a lethal
cop1 allele in transgenic Arabidopsis.
(5) Individual vectors are available from the authors
upon request and will be distributed through the
Arabidopsis Biological Resource Center (ABRC ) at
Ohio State University (http://aims.cps.msu.edu/aims/).
Table 3
Seedling phenotypes within Arabidopsis populations segregating for the lethal cop1–5 allele (1) or the gfp–cop1 transgene (3) or both (2)
Number
1
2
3
Genotypea
Phenotype
Ratio of wild-type:mutant
cop1
gfp–cop1
Wild-type
gfp+
cop1
gfp+
+/−
+/−
−/−
−/−
+/−
+/−
300
203
165
nd
nd
25/25
93
19
59
nd
nd
0/19
nd, not done.
aGenotype of the parent plant that was selfed to derive the segregating population.
−, cop1 mutant allele; +, COP1 wild-type allele.
3.2:1
11.0:1
2.8:1
A.G. von Arnim et al. / Gene 221 (1998) 35–43
Acknowledgement
We thank Dr J.C. Carrington for the gift of plasmids
pRTL2-GUS and pRTL2-GUS-NIa, Dr J. Chory for a
DET1 cDNA, Dr M. Chalfie for a GFP cDNA, Dr
J. Haseloff for the mGFP4 cDNA, Drs R. Heim and R.
Tsien for the S65T and Y66H mutant GFP cDNAs and
L.-H. Ang for construction of plasmid pAVA192. This
work was supported by a National Institutes of Health
grant (GM47850) to X-W.D. and by a US Department
of Energy grant (DE-FG02-96ER20223) to A.G.v.A.
References
Bechtold, N., Ellis, J., Pelletier, G., 1993. In planta Agrobacterium
mediated gene transfer by infiltration of adult Arabidopsis thaliana
plants. C.R. Acad. Sci. Paris 316, 1194–1199.
Bevan, N., 1984. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 12, 8711–8721.
Carrington, J.C., Freed, D.D., Oh, C.S., 1990. Expression of potyviral
polyproteins in transgenic plants reveals three proteolytic activities
required for complete processing. EMBO J. 9, 1347–1353.
Castle, L., Meinke, D., 1994. A FUSCA gene of Arabidopsis encodes
a novel protein essential for plant development. Plant Cell 6, 25–41.
Chamovitz, D.A., Wei, N., Osterlund, M.T., von Arnim, A.G., Staub,
J.M., Matsui, M., Deng, X.-W., 1996. The COP9 complex, a novel
multisubunit nuclear regulator involved in light control of a plant
developmental switch. Cell 86, 115–122.
Davis, S.J., Vierstra, R.D., 1998. Soluble, highly fluorescent variants
of green fluorescent protein (GFP) for use in higher plants. Plant
Mol. Biol. 36, 521–528.
Deng, X.-W., Matsui, M., Wei, N., Wagner, D., Chu, A.M., Feldmann,
K.A., Quail, P.H., 1992. COP1, an Arabidopsis regulatory gene,
encodes a novel protein with both a Zn-binding motif and a Gb
homologous domain. Cell 71, 791–801.
Görlich, D., Mattaj, I.W., 1996. Nucleocytoplasmic transport. Science
271, 1513–1518.
Grebenok, R.J., Pierson, E., Lambert, G.M., Gong, F.-H., Afonso,
C.L., Haldeman-Cahill, R., Carrington, J.C., Galbraith, D.W., 1997.
Green-fluorescent protein fusions for efficient characterization of
nuclear targeting. Plant J. 11, 573–586.
Haseloff, J., Siemering, K.R., Prasher, D.C., Hodge, S., 1997. Removal
of a cryptic intron and subcellular localization of green fluorescent
protein are required to make transgenic Arabidopsis plants fluoresce
brightly. Proc. Natl. Acad. Sci. USA 94, 2122–2127.
Heim, R., Prasher, D.C., Tsien, R.Y., 1994. Wavelength mutations
and posttranslational autoxidation of green fluorescent protein.
Proc. Natl. Acad. Sci. USA 91, 12501–12504.
Heim, R., Cubitt, A.B., Tsien, R.Y., 1995. Improved green fluorescence. Nature 373, 663–664.
Heinlein, M., Epel, B., Padgett, H.S., Beachy, R.N., 1995. Interaction
of tobamovirus movement proteins with the plant cytoskeleton. Science 270, 1983–1985.
Köhler, R.H., Cao, J., Zipfel, W.R., Webb, W.W., Hanson, M.R.,
1997a. Exchange of protein molecules through connections between
higher plant plastids. Science 276, 2039–2042.
43
Köhler, R.H., Zipfel, W.R., Webb, W.W., Hanson, M.R., 1997b. The
green fluorescent protein as a marker to visualize plant mitochondria
in vivo. Plant J. 11, 613–621.
McNellis, T., von Arnim, A.G., Araki, T., Komeda, Y., Miséra, S.,
Deng, X.-W., 1994a. Genetic and molecular analysis of an allelic
series of cop1 mutants suggests functional roles for the multiple
protein domains. Plant Cell 6, 487–500.
McNellis, T., von Arnim, A.G., Deng, X.-W., 1994b. Overexpression
of Arabidopsis COP1 results in partial suppression of light-mediated
development: evidence for a light-inactivable repressor of photomorphogenesis. Plant Cell 6, 1391–1400.
Matsui, M., Stoop, C.D., von Arnim, A.G., Wei, N., Deng, X.-W.,
1995. Arabidopsis COP1 protein specifically interacts with a novel
cytoskeleton associated protein, CIP1. Proc. Natl. Acad. Sci. USA
92, 4239–4243.
Mitra, R.D., Silva, C.M., Youvan, D.C., 1996. Fluorescence resonance
energy transfer between blue-emitting and red-shifted excitation
derivatives of the green fluorescent protein. Gene 173, 13–17.
Miyawaki, A., Liopis, J., Heim, R., McCaffery, J.M., Adams, J.A.,
Ikura, M., Tsien, R.Y., 1997. Fluorescent indiators for Ca2+ based
on green fluorescent proteins and calmodulin. Nature 388, 882–887.
Olson, K.R., McIntosh, J.R., Olmsted, J.B., 1995. Analysis of MAP4
function in living cells using green fluorescent protein (GFP) chimeras. J. Cell Biol. 130, 639–650.
Pepper, A., Delaney, T., Washburn, T., Poole, D., Chory, J., 1994.
DET1, a negative regulator of light-mediated development and gene
expression in Arabidopsis, encodes a novel nuclear-localized protein.
Cell 78, 109–116.
Prasher, D.C., Eckenrode, V.K., Ward, W.W., Prendergast, F.G., Cormier, M.J., 1992. Primary structure of the Aequorea victoria greenfluorescent protein. Gene 111, 229–233.
Restrepo, M.A., Freed, D.D., Carrington, J.C., 1990. Nuclear transport of plant potyviral proteins. Plant Cell 2, 987–998.
Sambrook, J., Fritsch, E.F., Maniatis, T., 1989. Molecular Cloning:
A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory
Press, Cold Spring Harbor, NY.
Staub, J.M., Wei, N., Deng, X.-W., 1996. Evidence for FUS6 as a
component of the nuclear-localized COP9 complex in Arabidopsis.
Plant Cell 8, 2047–2056.
Valvekens, D., Van Montagu, M., van Lijsebettens, M., 1988. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc. Natl. Acad.
Sci. USA 85, 5536–5540.
Varagona, M.J., Schmidt, R.J., Raikhel, N.V., 1992. Nuclear localization signal(s) required for nuclear targeting of the maize regulatory
protein Opaque-2. Plant Cell 4, 1213–1227.
von Arnim, A.G., Deng, X.-W., 1994. Light inactivation of Arabidopsis
photomorphogenic repressor COP1 involves a cell type specific modulation of its nucleocytoplasmic partitioning. Cell 79, 1035–1045.
von Arnim, A.G., Deng, X.-W., 1996. Light control of seedling development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 215–243.
von Arnim, A.G., Osterlund, M.T., Kwok, S.F., Deng, X.-W., 1997.
Genetic and developmental control of nuclear accumulation of
COP1, a repressor of photomorphogenesis in Arabidopsis. Plant Physiol. 114, 779–788.
Wang, S., Hazelrigg, T., 1994. Implications for bcd mRNA localization
from spatial distribution of exu protein in Drosophila embryogenesis.
Nature 369, 400–403.
Wei, N., Chamovitz, D.C., Deng, X.-W., 1994. Arabidopsis COP9 is a
component of a novel signaling complex mediating light control of
development. Cell 78, 117–124.