Lecture 15: The Nernst Equation
advertisement

Lecture 15: The Nernst Equation
• Reading: Zumdahl 11.4
• Outline:
– Why would concentration matter in
electrochemistry?
– The Nernst equation (contains Concentration
effects on Battery Voltage)
– Applications
– An entropically-driven battery
• Problems (Ch 11 Zumdahl 5th Ed.)
– 49, 50, 52 (Show you can do this), 53 (how much does
entropy contribute), 55, 57d.
1
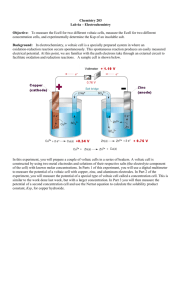
Concentration and Ecell
• Consider the following redox reaction:
Zn(s) + 2H+ (aq)
Zn2+(aq) + H2(g) Ecello = +0.76V
nFEcell = −ΔGrxn > 0
(spontaneous)
• What if [H+] = 2 M?
Expect driving force for product formation to increase.
LeChatelier Prinicple
Therefore ΔGrxn decreases, and Ecell increases
How does Ecell depend on concentration?
2
Ecell depends on Concentration
The Nernst Equation
ΔGrxn = ΔGrxn + RT ln Q
0
Recall, in general:
Putting this with our recent result:
nFEcell = −ΔGrxn = −ΔGrxn − RT ln Q
0
Ecell =E
0
cell
Ecell =E
0
cell
RT
−
ln Q
nF
0.059
log10 Q V
−
n
0
ΔGrxn = ΔGrxn
+ 5.7 log10 Q kJ
3
Ecell: The Nernst Equation
• With the Nernst Eq., we can determine the effect
of concentration on cell potentials.
Ecell =E
0
cell
60
− log10 Q mV
n
• Example. Calculate the cell potential for the
following:
2+
2+
Fe( s ) + Cu (aq ) → Fe (aq) + Cu ( s )
When [Cu2+] = 1 M and [Fe2+] = 1 M
When [Cu2+] = 0.3 M and [Fe2+] = 0.1 M
Do you expect the potential to be greater/less than the
Standard potential?
4
Ecell example
Fe( s ) + Cu 2+ (aq ) → Fe 2+ (aq) + Cu ( s )
First, need to identify the 1/2 cells (table, 11.1).
Get the voltage under standard conditions
Cu 2+ (aq) + 2e − → Cu ( s )
EEcell0 = +0.34V
Fe 2+ (aq ) + 2e − → Fe( s )
Ecell0 = −0.44V
Fe( s ) → Fe 2+ (aq ) + 2e −
Ecell0 = +0.44V
_______________________________________________
Fe( s ) + Cu 2+ (aq ) → Fe 2+ (aq) + Cu ( s )
Ecell0 = +0.78V
Turn the iron half cell around and add. Note n=2
5
Ecell example
Fe( s ) + Cu 2+ (aq ) → Fe 2+ (aq ) + Cu ( s )
Ecell0 = +0.78V
Now, calculate Q and then Ecell.
Notice, the concentrations are in separate containers.
⎡⎣ Fe 2+ ⎤⎦ a 0 ⎧⎪ ⎡⎣ Fe 2+ ⎤⎦ ⎫⎪ ⎧⎪ a 0 ⎫⎪
(0.1)
Cu
Cu
Q=
⋅
=⎨
=
= 0.33
⎬ ⋅⎨
2+
2+ ⎬
(0.3)
⎡⎣Cu ⎤⎦ aFe0 ⎪ aFe0 ⎪ ⎪ ⎡⎣Cu ⎤⎦ ⎪
⎩
⎭ An ⎩
⎭Cat
n=2
0.06
o
ECell =ECell −
log10 Q V
n
0.06
ECell = 0.78 −
log10 0.33 = 0.78 + 0.014 = 0.80 V
2
6
Ecell another example
• If [Cu2+] = 0.3 M, what [Fe2+] is needed so that
Ecell = 0.76 V?
Fe( s) + Cu 2+ (aq ) → Fe 2+ (aq ) + Cu ( s )
0
Ecell
= +0.78V
0.059
log10 Q
Ecell = E −
n
0.059
0.76 = 0.78 −
log10 Q
2
2 ⋅ 0.02 2
log10 Q =
=
0.059 3
⎡⎣ Fe 2+ ⎤⎦ ⎡⎣ Fe 2+ ⎤⎦
Q = 4.7 =
=
2+
0.3
⎡⎣Cu ⎤⎦
0
cell
⎡⎣ Fe 2+ ⎤⎦ = 4.7 ⋅ 0.3 = 1.4 M
7
Concentration Cells
• Consider the cell
presented on the left.
• The 1/2 cell reactions
are the same, it is just
the concentrations that
differ.
• Will there be electron
flow? Why?
8
Concentration Cells: Concentration Effects Only
Ag+ + e-
Ag
E°1/2 = 0.80 V
• E°1/2 is measured when all
species are in standard state, so
this means both sides have 1 M
concentrations of Ag+.
Therefore , E°cell = 0.
9
Silver Concentration Cell
Driving force for the reaction: Get the concentration up on the
left, so produce more ions there. Will stop when concentrations
in both beakers are equal (Q=1 but not Std State)
Anode: Ag
Cathode: Ag+ + e-
Ag+ + e- E°1/2 = -0.80 V
Ag
E01/2 =+ 0.80 V
⎡⎣ Ag + ⎤⎦
(0.1)
Anode
Q=
=
= 0.1
+
(1.0)
⎡⎣ Ag ⎤⎦
Cathode
n =1
0.059
log10 Q
n
0.059
= 0.0 −
log10 0.1 = 0. + 0.059 = 0.06V
1
0
Ecell = ECell
−
Ecell
10
Concentration Cells (2nd example)
Another Example:
The number of electrons per rxn is different
What is Ecell?
11
Iron Concentration Cells
Fe2+ + 2e- Fe
2 e- transferred…n = 2
e-
⎡⎣ Fe 2+ ⎤⎦
(0.01)
Anode
Q=
=
= 0.1
2+
(0.1)
⎡⎣ Fe ⎤⎦
Cathode
n=2
0.059
log10 Q
n
0.059
= 0.0 −
log10 0.1 = 0. + 0.03 = 0.03V
2
0
−
Ecell = ECell
Ecell
anode
cathode
Ecell = 30mV Same Result for Cu/Cu2+ battery
12
Measurement of pH
• pH meters use electrochemical reactions.
• Ion selective probes: respond to the presence of a
specific ion. pH probes are sensitive to H+.
• Specific reactions:
Hg2Cl2(s) + 2eH2(g)
Hg2Cl2(s) + H2(g)
2Hg(l) + 2Cl-(aq) E°1/2 = 0.27 V
2H+(aq) + 2e-
E°1/2 = 0.0 V
2Hg(l) + 2H+(aq) + 2Cl-(aq)
E°cell = 0.27 V
13
Measurement of pH
2Hg(l) + 2H+(aq) + 2Cl-(aq)
Hg2Cl2(s) + H2(g)
• What if we let [H+] vary?
2
2
⎡⎣ H ⎤⎦ ⎡⎣Cl ⎤⎦
+ 2
− 2
Q=
= ⎡⎣ H ⎤⎦ ⎡⎣Cl ⎤⎦
PH 2
+
−
n=2
ECell = E
0
Cell
0
ECell = ECell
0
ECell = ECell
0.059
−
log10 Q
n
0.059
+ 2
− 2
−
log10 ⎡⎣ H ⎤⎦ ⎡⎣Cl ⎤⎦
2
− 0.059 log10 ⎡⎣ H + ⎤⎦ − 0.059 log10 ⎡⎣Cl − ⎤⎦
{
Saturate the Chloride ion so that it is constant.
}
14
Application of pH Measurement
0
Ecell = { Ecell
+ Eoffset } − (0.0591) log10 [ H + ]
Ecell = Eref + 59.1⋅ pH
mV
• Ecell is directly proportional to pH or log [H+]
electrode
15
Summary
electricl
wrev
= ΔG = ΔGrxn ΔX
0
ΔGrxn = ΔGrxn
+ RT ln Q
0
ΔGrxn = ΔGrxn
+ 2.48ln Q kJ
ΔGrxn = − nF ⋅ECell
ECell =E
0
Cell
0.0591
−
⋅ log Q V
n
None of these ideas is separate. They are all connected,
and are all derived directly from thermodynamics.
16
Nernst Equation and half reactions (Z11.52)
Show that the Nernst Equation can be applied to half
reactions as well:
0.06
⋅ log Q V
n
=E 1 −E 2
Q = Q1Q2−1
0
ECell =ECell
−
ECell
E 1 =E1 0 −
0.06
⋅ log Q1
n
E 2 =E2 0 −
0.06
⋅ log Q2
n
However each half reaction has to be multiplied by some factor so that the
number of electrons is the same for both half reactions.
n = n1m1 = n2 m2
0.06
0.06
0.06
m1
0
0
m1 ⋅ log Q1 =E1 −
⋅ log ( Q1 ) =E1 −
⋅ log ( Q1m1 )
E =E1 −
n1 ⋅ m1
n1 ⋅ m1
n
1
0
So this shows that yes one can do each half reaction separately and get the same
result as doing both half reactions together as a single reaction.
17
Nernst Equation half reactions (Z11.52a)
Apply the Nernst Equation to the Half reaction Cu/Cu2+
So you need to write and balance the half reaction in the
same direction as the half cell EMF is specified, which is
written as a reduction potential in table 11.1
E o = 0.34V
Cu 2+ ( aq ) + 2e − → Cu ( s )
n = 2 ⎡⎣Cu 2+ ( aq ) ⎤⎦ = 0.1M
Q=
1
= 10
⎡⎣Cu ( aq ) ⎤⎦
0.06
0.06
E 1 =E1 0 −
⋅ log Q1 = 0.34 −
⋅ log10 = 0.34 − .07 = 0.27V
n1
2
2+
18
Sample Problems (Z11.49, 52)
• Analyze the galvanic cell for the reaction:
Au 3+ + 3e − → Au
Tl + + e − → Tl
E o = 1.5V
E o = −0.34V
As you can see it really doesn’t matter what the metals are: The overall
cell is just the sum of the half cell potentials
For later we need to know that n=3 and have the balance reaction for the
cell:
Au 3+ + 3Tl → Au + 3Tl +
E o = 1.84V
cell
Get Gibbs energy and K:
o
o
ΔGrxn
= − nF ⋅ECell
= − RT ln K
So a 2V battery is VERY
o
ΔGrxn
= −550kJ
Spontaneous.
o
ECell
1.84
=3
= 220
ln K = n
0.025
0.025
19
Sample (Z11.49, 52)
• How much change in EMF if product and reactant
concentrations are low? There is a trade off.
Au 3+ + 3Tl → Au + 3Tl +
Ecello = 1.84V
⎡⎣ Au 3+ ⎦⎤ = 1⋅10−2
⎡⎣Tl + ⎦⎤ = 1⋅10−4 M
ECell −E
0.0591
=−
⋅ log Q
n
ECell −E
0.6
=
⋅ log10 = 0.46
3
0
Cell
0
Cell
3
⎡⎣Tl ⎤⎦
10−12
−10
Q=
10
=
=
⎡⎣ Au 3+ ⎤⎦ 10−2
+
ECell = 1.8 + 0.46 = 2.3V
The voltage will stay up until the Gold ion concentration goes way down.
That’s the way batteries are: When they start to go out, they go quickly.
20
Lead Acid Car Battery (Z11.53)
• For the car battery, calculate the standard EMF at -20C.
Pb ( s ) + PbO2 ( s ) + 4 H + → 2 Pb 2+ ( aq ) + 2 H 2O
Pb 2+ ( aq ) + SO4 2− ( aq ) → PbSO4 ( aq )
K sp = 1.3 ⋅10−8
Pb ( s ) Pb 2+ PbO2 ( s ) Shorthand/no salt bridge
Ecello = 2.04V
The EMF is the total for both reactions; does the
second reaction increase the EMF or decrease it?
o
o
o
o
ΔGRxn
= − nF ⋅ECell
= ΔH Rxn
− T ΔS Rxn
The reaction is exothermic and the entropy is positive. So both parts contribute
to the battery functioning. Because the entropy is positive the EMF of the cell
will be smaller at lower temperatures. It is not surprising that a battery works
worse at low temperatures.
An extra question: What percentage of the battery is driven
entropically under standard conditions?
o
T ΔS Rxn
0.298 ⋅ 263
78
=
=
= 0.2 = 20%
o
−ΔGRxn 316 + 0.298 ⋅ 263 395
21
The Concentration Battery (Z11.55)
NiA ( s ) Ni 2+ A ( aq ) Ni 2+ B ( aq ) NiB ( s )
•
•
•
•
Two cells, each contains Ni metal and Ni 2+ ions.
Why would there be any potential at all?
The concentration in the different Beakers is different.
Eg (c) 1 M Ni 2+ in beaker A and 0.1M Ni 2+ in beaker B.
–
–
–
–
–
What is the standard EMF ?
What is the EMF?
Which way do the electrons go?
What is the concentration of Ni 2+ in A/B at equilibrium?
What is EMF at equilibrium?
0.0591
0
ECell =ECell
−
⋅ log Q = −.03log10 10 = −0.03V = −30mV
n
Not spontaneous in
2+
⎡
⎤⎦
Ni
direction written. E 0 = 0 Q = ⎣
A
= 10
n=2
Cell
2+
⎡⎣ Ni ⎤⎦
B
It is a totally Entropic
22
battery!!!!!