Cardiovascular Research (2009) 83, 123–130
doi:10.1093/cvr/cvp120
Aspirin acetylates nitric oxide synthase type 3
in platelets thereby increasing its activity
Peter O’Kane1†, Liping Xie2†, Zhen Liu2, Lindsay Queen3, Graham Jackson1,
Yong Ji2*, and Albert Ferro3*
1
Department of Cardiology, Guy’s and St Thomas’ NHS Foundation Trust, London, UK; 2Key Laboratory of Human Functional
Genomics, Atherosclerosis Research Centre, Nanjing Medical University, Nanjing 210029, P.R. China; and 3Department of
Clinical Pharmacology, Cardiovascular Division, King’s College London, Franklin-Wilkins Building, 150 Stamford Street,
London SE1 9NH, UK
Received 8 August 2008; revised 30 March 2009; accepted 6 April 2009; online publish-ahead-of-print 17 April 2009
Time for primary review: 17 days
KEYWORDS
Aims Acute administration of aspirin increases nitric oxide (NO) synthesis by platelets, an effect not
shared by other non-steroidal anti-inflammatory drugs. The aim of the present study was to determine
the mechanism by which aspirin acutely increases the activity of NO synthase type 3 (NOS-3), the predominant NOS isoform expressed by platelets, and specifically whether this occurs through an increase
in its acetylation.
Methods and results Platelets isolated from the blood of healthy human subjects were exposed in vitro
to vehicle or aspirin at different concentrations (5 mmol/L–4 mmol/L). Changes in intraplatelet Ca2+
concentration were determined from fura-2 fluorescence. Following immunoprecipitation of NOS-3
from platelet lysates, its activity was determined from L-[3H]arginine to L-[3H]citrulline conversion,
and its serine phosphorylation quantified by western blotting. Acetylation of NOS-3 in platelets was
assessed by the incorporation of radioactivity into the immunoprecipitated enzyme from [acetyl-14C]aspirin. Following transfection of HeLa cells with NOS-3, NO biosynthesis in response to aspirin was determined from cyclic GMP measurement, and sites of NOS-3 acetylation were ascertained by liquid
chromatography–tandem mass spectrometry. At all concentrations tested, aspirin increased the activity
of NOS-3 from platelets. This was not associated with any measurable change in intraplatelet Ca2+ concentration. Serine phosphorylation of NOS-3 in platelets was decreased, and this was especially marked
for serine-1177 phosphorylation, whereas acetylation of NOS-3 was increased, by aspirin incubation.
HeLa cells transfected with NOS-3 exhibited an increase in NO biosynthesis following aspirin exposure,
and this was associated with acetylation of the enzyme on both serine-765 and serine-771.
Conclusion Aspirin acetylates NOS-3 acutely in platelets, and this causes an increase in its activity as
well as a decrease in its phosphorylation. It is also possible that aspirin indirectly affects NOS-3 activity
by acetylating other substrates within the platelet, but this remains to be determined.
1. Introduction
Aspirin is widely used for cardiovascular prophylaxis in
patients either with established atherosclerotic disease or
at high risk of developing such disease. Its effectiveness in
preventing arterial thrombotic disease has been established
by numerous large clinical trials.1–5 Other non-steroidal
anti-inflammatory drugs may not confer the same degree
of cardioprotection and indeed may even increase cardiovascular events; the cyclooxygenase type 2 inhibitors
have particularly been implicated in causing adverse
* Corresponding authors. Tel: þ86 25 8686 2886; fax: þ86 25 8650 8960.
E-mail address: yongji@njmu.edu.cn (Y.J.); Tel: þ44 20 7848 4283;
fax: þ44 20 7848 3743.
E-mail address: albert.ferro@kcl.ac.uk (A.F.)
† These two authors have contributed equally.
cardiovascular outcomes, although this may also be true of
the non-selective cyclooxygenase inhibitors other than
aspirin.6,7
Aspirin acts predominantly by acetylating cyclooxygenase
type 1, the predominant isoform of cyclooxygenase present
in platelets, on serine residue 529, thereby irreversibly inhibiting platelet synthesis of prostaglandin H2 and thus subsequent formation of thromboxane A2. Although it has a
small inhibitory effect on cyclooxygenase type 2, it is
150–200-fold selective for the type 1 isoform; and since
the type 2 isoform is expressed in the vascular endothelium,
this gives rise to preferential suppression of platelet thromboxane A2 formation with relative sparing of endothelial
prostacyclin synthesis in response to aspirin therapy.
However, this may not be the only action by which aspirin
is cardioprotective. Indeed, studies have shown that
Published on behalf of the European Society of Cardiology. All rights reserved. & The Author 2009.
For permissions please email: journals.permissions@oxfordjournals.org.
Downloaded from by guest on September 30, 2016
Aspirin;
Nitric oxide;
Nitric oxide synthase;
Acetylation;
Phosphorylation
124
2. Methods
2.1 Subjects
Subjects were healthy, asymptomatic non-smokers, with no history
of serious disease, with normal plasma biochemistry (electrolytes,
fasting glucose, lipid, renal, and liver profiles), and on no regular
medication; in particular, they had taken no aspirin or other antiplatelet medication for at least 2 weeks before study. The investigation conforms with the principles outlined in the Declaration of
Helsinki (Cardiovascular Research 1997;35:2–4). All subjects gave
informed consent. The study was approved by the St Thomas’ Hospital Research Ethics Committee. Subjects (n = 6 men; 18–30 years
old) were recruited sequentially in response to advertisement.
2.2 Preparation of platelets
Subjects attended in the morning, having fasted overnight and
refrained from alcohol and caffeine since the previous evening.
Using a 19G Butterflyw needle, 70–80 mL venous blood was taken
from a large antecubital vein, collected into tri-sodium citrate
(0.38% final concentration), and centrifuged (200g, 10 min, room
temperature) to obtain platelet-rich plasma (PRP). Gel-filtered
platelets were obtained by eluting PRP through a Sepharose gel
column with balanced salt solution (BSS) buffer, of the following
composition (mmol/L): NaCl 125, KCl 5.4, NaHCO3 16.2, HEPES 15,
NaH2PO4 1, MgSO4 0.8, CaCl2 1.8, glucose 5.5 (pH 7.4). Platelet
count in the eluate was obtained using a Coulter counter, and
samples were normalized to a final concentration of 108 platelets/
mL for all experiments, using BSS.
2.3 Assessment of changes in intraplatelet Ca2+
concentration in response to aspirin
Gel-filtered platelets in BSS were incubated with fura-2-AM 3 mmol/
L for 1 h at 378C, following which the mixture was acidified to pH 6.5
by the addition of citric acid (final concentration 6 mmol/L). Following centrifugation (650g, 15 min, room temperature), the platelet
pellet was resuspended (at a final concentration of 108 platelets/
mL) in the buffer of the following composition (in mmol/L): NaCl
140, KCl 5, MgCl2 1, glucose 5, NaH2PO4.H2O 0.42, NaHCO3 12,
HEPES 10, pH 7.35, with freshly added apyrase (1 U/mL) and indomethacin (3 mmol/L). Following subsequent addition of aspirin
(10 or 400 mmol/L) and/or 1 U/mL thrombin (as a positive
control), changes in cytoplasmic Ca2+ concentration were examined
as a function of time, over 30 min, from the ratio of emission at
510 nm following excitation at 340 nm and 380 nm, in an LS50 luminescence spectrometer.10
2.4 Immunoprecipitation of NOS-3 from platelets
following treatment with aspirin or vehicle
Gel-filtered platelets (1 mL aliquots, in BSS) were exposed to aspirin
at different concentrations (5 mmol/L–4 mmol/L), or to corresponding vehicle, at 378C for 30 min. At the end of the incubation,
samples were placed on ice and platelets were pelleted (650g,
15 min, 48C) and lysed by sonication in 0.5 mL of lysis buffer, of
the following composition: Tris–HCl 25 mmol/L, NaCl 150 mmol/L,
phenylmethanesulfonyl fluoride 1 mmol/L, aprotinin 1 mg/mL, leupeptin 10 mg/mL, EDTA 1 mmol/L, NaF 50 mmol/L, sodium orthovanadate 1 mmol/L, Triton-X 1%, pH 7.6. This was left on ice for
30 min and subsequently diluted with an equal volume of Trisbuffered saline (TBS, composition: Tris–HCl 25 mmol/L, NaCl
150 mmol/L, pH 7.6) containing bovine serum albumin 5 mg/mL,
CaCl2 2 mmol/L, and sodium azide 0.02%. Debris was pelleted at
15 000g for 15 min, and 0.5 mL of the supernatant was added to
protein A-Sepharose beads precoated with rabbit anti-NOS-3 antibody (Santa Cruz Biotechnology Inc., Heidelberg, Germany), for
2 h at 48C. Following extensive washing, the resultant bead suspension was used for NOS activity measurement or for western blotting.
2.5 Western blotting of immunoprecipitated
NOS-3 from platelets
Immunoprecipitates were boiled in SDS–PAGE sample buffer (glycerol 16%, SDS 3.2%, dithiothreitol 64 mmol/L, Tris–HCl 0.1 mol/L,
pH 6.8) for 5 min and separated by SDS–PAGE on a 10% gel, followed
by electroblotting for 1 h onto a nitrocellulose membrane. Membranes were blocked by overnight incubation at 48C in TBS containing 5% non-fat dry milk, followed by 2 h incubation with rabbit
anti-NOS-3 antibody, dilution 1:1000 in blocking buffer at room
temperature. Following extensive washing in TBS with 0.1%
Tween-20 (TBS-Tween), membranes were incubated for 30 min
with goat anti-rabbit horseradish peroxidase-conjugated IgG (Dako
Ltd, High Wycombe, UK), dilution 1:1000 in TBS-Tween containing
5% non-fat dry milk, at room temperature. They were then extensively washed in TBS-Tween and developed using enhanced chemiluminescence (ECL) substrate (Amersham Life Science Ltd, Little
Chalfont, Buckinghamshire, UK). Bands thus revealed were analysed
by scanning densitometry (Pharmacia ImageMaster, version 2.0
software).
Membranes were then submerged in stripping buffer (composition: 62.5 mmol/L Tris–HCl, pH 6.7, 2% SDS, 100 mmol/L 2mercaptoethanol), at 508C for 30 min, with gentle agitation. Following washing two times (10 min each) in TBS-Tween, membranes
were blocked overnight once again in 5% non-fat dry milk in TBS,
at 48C, followed by 2 h incubation with mouse anti-phosphoserine
or mouse anti-phosphoserine-1177-NOS-3 antibody (both from
Calbiochem-Novabiochem Ltd, Nottingham, UK), dilution 1:1000 in
blocking buffer at room temperature. Membranes were then
washed extensively in TBS-Tween, followed by 30 min incubation
with goat anti-mouse horseradish peroxidase-conjugated IgG (Dako
Ltd, High Wycombe, UK), dilution 1:1000 in TBS-Tween containing
5% non-fat dry milk, at room temperature. They were then
washed, developed, and scanned as before.
In preliminary experiments, we found that, following stripping of
the blots, with subsequent re-blocking and re-probing with the secondary antibody alone (in the absence of any primary antibody), no
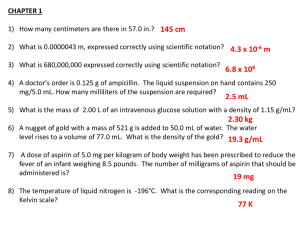
Downloaded from by guest on September 30, 2016
aspirin can cause serine acetylation of a variety of other proteins,8,9 although it is unclear whether any of these actions
is of therapeutic importance.
We have previously demonstrated that aspirin has important effects on nitric oxide (NO) production by human platelets. In common with other cyclooxygenase inhibitors, it
impairs the ability of NO synthase (NOS) to undergo activation by albuterol (an agonist which increases platelet
NOS activity in a Ca2+-independent manner10); on the
other hand, unlike other cyclooxygenase inhibitors, it
causes an increase in basal platelet NOS activity upon
acute exposure.11 Similarly, Madajka et al.12 have reported
that whereas acute aspirin treatment has no effect on NO
production by cultured endothelial cells, it increases platelet NO biosynthesis considerably, and other workers have
confirmed that aspirin stimulates platelet NO generation.13,14 However, the mechanism by which it does this is
presently unknown.
The aim of the present study was to determine the
mechanism by which the exposure of platelets to aspirin
causes the activation of NOS-3. Specifically, we hypothesized that aspirin may activate NOS-3 through acetylation,
perhaps on serine-1177, since the modification of this
residue by phosphorylation is well established to cause
Ca2+-independent activation,15 and acetylation may have a
similar functional effect to phosphorylation.
P. O’Kane et al.
Aspirin and platelet nitric oxide synthase
residual bands were seen when ECL was performed, thus confirming
the effectiveness of the stripping procedure.
2.6 Activity of immunoprecipitated NOS-3
from platelets
NOS-3 immunoprecipitated and immobilized on protein A-Sepharose
beads was resuspended in 100 mL NOS assay buffer of the following
composition: Tris–HCl 50 mmol/L, EDTA 0.1 mmol/L, EGTA
0.1 mmol/L, leupeptin 2 mmol/L, pepstatin 1 mmol/L, aprotinin
1 mmol/L, phenylmethanesulfonyl fluoride 1 mmol/L, NaF
10 mmol/L, sodium orthovanadate 1 mmol/L, NADPH 1 mmol/L,
tetrahydrobiopterin 3 mmol/L, calmodulin 100 nmol/L, CaCl2
2.5 mmol/L, L-arginine 10 mmol/L, pH 7.5. L-[3H]arginine 0.2 mCi
was added with or without the NOS inhibitor NG-nitro-L-arginine
methyl ester (L-NAME, 100 mmol/L), and, following incubation for
30 min at 378C, the reaction was quenched by the addition of
1 mL ice-cold stop buffer (HEPES 20 mmol/L, EDTA 2 mmol/L,
EGTA 2 mmol/L, pH 5.5). An excess of Dowex resin (Na+ form) was
added, and this was mixed thoroughly and allowed to settle for
10 min. Two hundred microlitres of the resultant supernatant, containing the L-citrulline fraction, was counted using a Wallac Beta
liquid scintillation counter. Results were converted from counts
per minute (cpm) to fmol L-citrulline by the following formula:
fmol L-citrulline ¼
cpm
14000;
cpms
vehicle, for 30 min. All incubations were at 378C in the presence of
3-isobutyl-1-methylxanthine (500 mmol/L) and were performed in
the absence or presence of L-NAME (100 mmol/L). The reaction
was ended by adding 1 mL ice-cold perchloric acid 0.3 mol/L.
Cells were lysed by sonication for 30 min and by rapid freezing
(2208C) and thawing, and cell debris was pelleted by centrifugation
(20 min, 2000g, 48C). The supernatants containing cyclic GMP
(cGMP) were collected and stored at 2208C until ready for assay
using a proprietary cGMP radioimmunoassay kit (Amersham Biosciences, Buckinghamshire, UK). The cGMP attributable to NO
activity (NO-attributable cGMP) was taken as the difference in
measured cGMP when co-incubated with and without L-NAME and
was corrected for cell count.
In separate experiments, cells transfected with NOS-3 were incubated with aspirin (400 mmol/L), or with corresponding vehicle, for
30 min at 378C, following which cells were lysed and NOS-3 immunoprecipitated as outlined in Section 2.4. Immunoprecipitates were
run on a 7.5% SDS–PAGE and stained with Coomassie Brilliant Blue.
A band was identified at 135 kDa, the known molecular mass of
NOS-3, and this was excised and subjected to tryptic digestion followed by liquid chromatography–mass spectrometry (MS) analysis
(performed by the proteomics facility at the Key Laboratory of
Reproductive Medicine, Nanjing Medical University, P.R. China).
Having confirmed the identity of the protein band as NOS-3,
further characterization of acetylation sites was then performed
using tandem MS (MS/MS).
2.9 Statistical analysis
All data are expressed as mean + standard deviation and were analysed using Student’s paired t-test or repeated measures ANOVA
with Dunnett’s post-test, as appropriate (GraphPad Prism version 5
software). A value of P , 0.05 (two-tailed) was considered statistically significant.
2.7 Assessment of acetylation of NOS-3 in platelets
Gel-filtered platelets (400 mL) were incubated with either [acetyl-14
C]aspirin (ARC UK Ltd, Cardiff, UK) 0.25 mmol/L or corresponding
vehicle (30 min, 378C). The reaction was terminated by placing
the reaction tubes in ice for 5 min, and platelets were pelleted
(2000g, 48C, 20 min). The pellets were sonicated and resuspended
in lysis buffer (composition as mentioned before) on ice for 30 min.
NOS-3 was immunoprecipitated from platelet lysates as described
earlier. In parallel experiments, normal rabbit serum was substituted for the NOS-3 antibody as a negative control. Beads were pelleted (2000g, 48C, 5 min), the supernatant was removed, and the
beads washed three times with TBS. The beads were resuspended
in TBS, and the radioactivity in 100 mL of this suspension was
measured using a Wallac Beta liquid scintillation counter.
2.8 Transfection of wild-type NOS-3 and assessment
of the effect of aspirin on NO production and NOS-3
acetylation in HeLa cells
HeLa cells (Genetimes Technology Inc., P.R. China), cultured in Dulbecco’s Modified Eagle Medium (GIBCO) supplemented with penicillin (100 U/mL), streptomycin (100 mg/mL), and 10% foetal bovine
serum, were cultured in six-well plates to 80% confluence (2 105 cells/6 cm plate), at which point they were transfected with
plasmid (pcDNA3.1, Invitrogen) containing the gene for wild-type
NOS-3; control transfections were also performed with empty
pcDNA3.1 vector. Transfections were performed using LipofectAMINE (Life Technologies Inc.) according to the manufacturer’s
protocol. The transfection efficiency was determined by the use
of a pcDNA3.1 plasmid encoding green fluorescent protein (GFP)
compared with empty vector control. The NOS-3 construct was a
gift from Professor Stephanie Dimmeler (University of Frankfurt,
Germany).
Transfected and non-transfected cells were incubated with
aspirin (10 mmol/L, 400 mmol/L, or 4 mmol/L), or to corresponding
3. Results
3.1 Aspirin does not affect intraplatelet Ca2+ levels
Since aspirin increases platelet NOS activity over a short
time course (within 30 min), and NOS-3 (the predominant
NOS isoform expressed by platelets) is a Ca2+-sensitive
enzyme, we examined the effect of different concentrations
of aspirin on intraplatelet Ca2+ concentration by fura-2 fluorescence. No change was seen in intraplatelet Ca2+ concentration, over a 30 min incubation period with aspirin (10 or
400 mmol/L), whereas thrombin (1 U/mL, used as a positive
control) elicited a reproducible large increase in intracellular Ca2+ concentration (Figure 1A). Co-incubation of aspirin
(10 or 400 mmol/L) with thrombin (1 U/mL) did not affect
the Ca2+ responses to thrombin (Figure 1B).
3.2 Aspirin increases NOS-3 activity in platelets
To confirm that aspirin increases NOS-3 activity in platelets
after short-term incubation, platelets were exposed to
aspirin (5 mmol/L, 10 mmol/L, 400 mmol/L, or 4 mmol/L)
for 30 min, following which NOS-3 was immunoprecipitated
from platelet lysates and its activity measured in vitro
from the rate of L-[3H]arginine to L-[3H]citrulline conversion.
At each concentration tested, aspirin elicited an increase in
the activity of NOS-3 from baseline; the degree of activation
was of the order of 20% in the presence of 5 mmol/L aspirin,
and of the order of 45% in the presence of 10 mmol/L aspirin.
At higher aspirin concentrations, the effect was not different to that at 10 mmol/L, suggesting that aspirin exerts its
maximal effect on NOS-3 activity at 10 mmol/L (Figure 2).
Downloaded from by guest on September 30, 2016
where cpm is the cpm of the sample, and cpms is cpm in the standard (all standards contained 1 mCi L-[3H]-arginine, corresponding
to 14 000 fmol). NOS activity was taken as the difference in
measured L-citrulline in the absence and presence of L-NAME, and
corrected for the amount of protein.
125
126
P. O’Kane et al.
Figure 1 Aspirin does not affect intraplatelet Ca2+ concentration. Changes in intraplatelet Ca2+ levels were determined over 30 min by fura 2 fluorescence and
expressed as the ratio of emission at 510 nm after excitation at 340 and 380 nm (R340/380). (A) Tracings show no effect of aspirin at either 10 or 400 mmol/L,
whereas thrombin 1 U/mL used as a positive control elicits an increase in intracellular Ca2+ concentration. (B) Co-incubation with aspirin at either 10 or
400 mmol/L does not affect the change in intracellular Ca2+ concentration in response to thrombin 1 U/mL. Arrow indicates the addition of aspirin and/or thrombin. Figures are each representative traces of n = 6 experiments.
Vehicle
[Acetyl-14C]aspirin
Figure 2 Aspirin increases platelet NOS-3 activity. Platelets were incubated
for 30 min with different concentrations of aspirin, as indicated. Following
immunoprecipitation of NOS-3 from lysed platelets, L-[3H]arginine to
3
L-[ H]citrulline conversion was determined in the immunoprecipitate, and
NOS activity expressed as the difference in measured L-[3H]citrulline in the
absence and presence of L-NAME. Results are shown for n = 6 experiments
and are expressed as fmol L-citrulline/mg protein. *P , 0.05 and ***P ,
0.001, compared with vehicle.
3.3 Aspirin causes acetylation of NOS-3 in platelets
To ascertain whether the observed increase in NOS-3 activity
caused by aspirin might be explained by acetylation of the
enzyme, platelets were treated with [acetyl-14C]aspirin or
vehicle for 30 min, following which NOS-3 was immunoprecipitated from platelet lysates, and radioactivity in the immunoprecipitate was counted. Treatment with [acetyl-14C]aspirin
caused a large increase in 14C counts in the NOS-3 immunoprecipitates. This increase was not seen if normal rabbit serum
was substituted for the NOS-3 antibody for immunoprecipitation (Table 1).
3.4 Aspirin treatment decreases phosphorylation
of NOS-3 on serine-1177 in platelets
Since aspirin can acetylate serine residues on a variety of
proteins, we hypothesized that it may activate NOS-3 in
Normal rabbit serum
Anti-NOS-3 antibody
100
176.9 + 45.9
75.7 +16.4
876.7 +258.9*
Following the incubation of platelets with either [acetyl-14C]aspirin or
vehicle, platelets were lysed, and the lysates were incubated with
protein A-Sepharose beads coated with either anti-NOS-3 antibody or
normal rabbit serum. 14C counts in the bead suspensions were measured,
and results were all expressed as the percentage of counts measured for
the combination (vehicle: normal rabbit serum); statistical analysis
(repeated measures one-way ANOVA) was performed on non-normalized
data. Results are shown for n = 6 replicates.
*P , 0.05 compared with the combination (vehicle: normal rabbit
serum).
platelets by acetylating a serine residue that normally can
undergo phosphorylation. We therefore determined the
degree of serine phosphorylation of NOS-3 following incubation of platelets for 30 min with aspirin (10 mmol/L,
400 mmol/L, or 4 mmol/L) or vehicle. We found that there
was a small but significant decrease in serine phosphorylation of NOS-3 following aspirin treatment, as detected by
western blotting of NOS-3 immunoprecipitates using a
mouse anti-phosphoserine antibody; no corresponding
change was seen in total NOS-3 expression (Figure 3).
Several serine residues on NOS-3 can undergo phosphorylation and thus lead to an increase in its activity,15 but of
these, serine-1177 is the best established. We therefore proceeded to specifically measure phosphoserine-1177-NOS-3
(by western blotting using a specific mouse antiphosphoserine-1177-NOS-3 antibody) in NOS-3 immunoprecipitates from platelets following 30 min incubation with
aspirin (10 mmol/L, 400 mmol/L, or 4 mmol/L) or vehicle.
Aspirin treatment, at all concentrations tested, led to a substantial decrease in measured serine-1177 phosphorylation of
NOS-3 (Figure 4).
Downloaded from by guest on September 30, 2016
Table 1 NOS-3 acetylation by aspirin
Aspirin and platelet nitric oxide synthase
127
Figure 3 Aspirin decreases serine phosphorylation of platelet NOS-3. (A) Western blot depicting the presence of a 135 kDa band (the known molecular mass of
NOS-3) in NOS-3 immunoprecipitates prepared from platelet lysates (following the incubation of platelets as indicated, for 30 min) probed with antiphosphoserine or anti-NOS-3 antibody. (B) Accumulated results (n = 6) of NOS-3 band density, as determined from densitometry of blots. (C ) Accumulated
results (n = 6) of phosphoserine/total NOS-3 densitometric ratio. *P , 0.05, compared with vehicle.
3.5 Aspirin causes acetylation of serine residues
765 and 771 on NOS-3, thereby activating it
To determine precisely which residues on NOS-3 are acetylated, we undertook experiments to isolate NOS-3 from cells
4. Discussion
Aspirin exerts its antithrombotic effect predominantly through
the irreversible inhibition of platelet cyclooxygenase-1, by
acetylating the serine-529 residue of this enzyme. It is
known to acetylate serine residues on a variety of other
proteins,8,9 but it is not clear whether such effects have
therapeutic consequences. The data presented here suggest
Downloaded from by guest on September 30, 2016
Figure 4 Aspirin decreases serine-1177 phosphorylation of platelet NOS-3.
(A) Western blot depicting the presence of a 135 kDa band (the known molecular mass of NOS-3) in NOS-3 immunoprecipitates prepared from platelet
lysates (following the incubation of platelets as indicated, for 30 min)
probed with anti-phosphoserine-1177-NOS-3 or anti-NOS-3 antibody. (B) Accumulated results (n = 6) of phosphoserine-1177/total NOS-3 ratio, as determined from densitometry of blots. ***P , 0.001, compared with vehicle.
following treatment with aspirin or vehicle, followed by
tryptic digestion and subsequent liquid chromatography–
MS/MS. However, the amount of NOS-3 which could be isolated from platelet preparations was insufficient for this
analysis. We therefore performed these experiments in
HeLa cells transfected with either NOS-3 or empty vector.
Transfection efficiency of HeLa cells was 70%, as determined using GFP (Figure 5A). The expression of NOS-3 in
HeLa cells transfected with NOS-3 was confirmed by
western blotting (Figure 5B). In non-transfected cells and
cells transfected with empty vector, NO-attributable cGMP
was undetectable. Moreover, in cells transfected with
NOS-3, NO-attributable cGMP was not detectable in the
absence of aspirin co-incubation, presumably reflecting a
lack of enzymatic activity in the absence of an NO agonist.
In contrast, aspirin elicited a concentration-dependent
increase in NO-attributable cGMP in HeLa cells transfected
with NOS-3 (Figure 5C).
Liquid chromatography–MS/MS analysis of tryptic digests
of NOS-3 following the exposure of HeLa cells transfected
with the NOS-3 construct to aspirin revealed that aspirin elicited acetylation of NOS-3 at serine residues 765 and 771
(Figure 6). No evidence was found of acetylation at
serine-1177. Moreover, no acetylation of NOS-3 was seen in
NOS-3 isolated from cells exposed to vehicle, and no
NOS-3 could be detected in non-transfected cells or cells
transfected with empty vector.
128
that aspirin acetylates NOS-3 in platelets, and that this
appears to increase the activity of this enzyme. This would
give rise to an increase in platelet NO biosynthesis, and,
since NO inhibits platelet activation, this would be expected
to contribute further to the anti-platelet effect of aspirin.
Although NOS-3 was originally described in endothelial
cells, it is now known to be expressed in a variety of other
cell types, including platelets. The regulation of NOS-3 in
endothelial cells and platelets is very similar, and we have
recently reviewed this.16 Classically, NOS-3 can undergo
activation in response to an increase in intracellular Ca2+
concentration. More recently, it has become clear that
NOS-3 phosphorylation on a variety of residues can have
important modulatory effects on its activity, independent
of any changes in Ca2+ concentration.17–21 Phosphorylation
of NOS-3 has been shown to occur on serine residues 114,
615, 633, and 1177, as well as on threonine-495. Phosphorylation of serine-633 and 1177 increases NOS-3 activity,
whereas phosphorylation of threonine-495 inhibits; the
effects of phosphorylation of serine-114 and serine-615
remain controversial. We hypothesized that acetylation of
one or more of these serine residues by aspirin might functionally mimic phosphorylation, leading to Ca2+-independent
NOS-3 activation. We therefore measured the phosphorylation state of NOS-3 isolated from platelets exposed to
aspirin or vehicle for 30 min. We found that NOS-3
phosphorylation was decreased by aspirin treatment. The
degree of the suppression of phosphorylation observed
was small. Since serine-1177 is the residue whose phosphorylation appears to activate NOS-3 to the greatest
extent,15 we therefore went on to specifically examine
whether the suppression of phosphorylation by aspirin
might be particularly marked on this residue.
We found that platelet exposure to aspirin for 30 min elicited a marked suppression of NOS-3 phosphorylation on
serine-1177. This suggests strongly that aspirin treatment
gives rise to a marked suppression of phosphorylation of
NOS-3 at serine-1177. On the other hand, aspirin treatment
caused an increase in NOS-3 activity, despite this decrease in
serine-1177 phosphorylation. In support of these data, we
also found that aspirin elicited a marked concentrationdependent increase in NO biosynthesis in HeLa cells transfected with NOS-3. It is noteworthy that much higher concentrations were necessary to cause the activation of
NOS-3 in transfected HeLa cells compared with platelets.
Although the reason for this is not clear, it is likely to
reflect differences in cell penetration of the drug; alternatively, it is possible that aspirin is degraded more rapidly
by HeLa cells than by platelets. Nevertheless, our experiments confirm that aspirin treatment causes an increase
in NOS-3 activity, both in platelets and in HeLa cells transfected with NOS-3, despite the demonstrated suppression
of serine-1177 phosphorylation of NOS-3 in platelets by
aspirin. We therefore hypothesized that this may be
caused by acetylation of NOS-3 by aspirin, either at
serine-1177 itself (thereby explaining the decrease in phosphorylation at this site) or elsewhere in the molecule. In
view of this, we examined directly the effects of aspirin
on NOS-3 acetylation in HeLa cells, using liquid
chromatography-MS/MS. Our results demonstrate that
aspirin elicits acetylation on serine residues 765 and 771,
with no evidence of acetylation at other sites (including
serine-1177). These data suggest that acetylation at either
or both of these residues may give rise to a conformational
change in the NOS-3 protein such that serine-1177
becomes less liable to undergo phosphorylation and that,
irrespective of its phosphorylation status at serine-1177,
such acetylation gives rise to an increase in NOS-3 activity.
We considered the possibility that acetylation of NOS-3,
rather than reducing the ability of NOS-3 to undergo phosphorylation, gives rise to dephosphorylation of serine-1177
and/or of threonine-495 on the enzyme by facilitating the
action of phosphatases. However, the presence of sodium
fluoride (at a concentration of 10 mmol/L) in the buffer
should have ensured complete inhibition of protein phosphoseryl and phosphothreonyl phosphatases; so this possibility is
unlikely.
In this study, we have not examined possible effects of
aspirin on NOS-3 activity and NO production by the vascular
endothelium. Endothelial cells synthesize larger quantities
of NO than do platelets, and endothelium-derived NO may
contribute substantially to the inhibition of platelet activation.22–24 However, it is clear that platelet-derived NO
has important effects on platelet activation and recruitment,25 and impaired platelet NO biosynthesis may contribute to thrombosis in the context of acute coronary
syndrome, myocardial infarction, and diabetes.26,27 In
future work, effects of aspirin on endothelial NOS-3 should
be examined; although previously published work suggests
that any such effects are likely to be small or even
non-existent.12
Downloaded from by guest on September 30, 2016
Figure 5 Aspirin activates NO biosynthesis in HeLa cells transfected with
NOS-3. (A) Fluorescence micrograph showing successful transfection (70%
efficiency) of HeLa cells with pcDNA3.1 plasmid containing the gene for
GFP. (B) Western blot demonstrating the expression (arrow) of NOS-3 in
HeLa cells transfected with pcDNA3.1 containing the gene for NOS-3, but
not in cells transfected with empty pcDNA3.1 vector. (C ) Graph showing
that 30 min incubation with aspirin causes a concentration-dependent
increase in NO biosynthesis, as measured by NO-attributable cGMP, in HeLa
cells transfected with NOS-3, but not in those transfected with empty
vector. Results are shown for n = 6 experiments. *P , 0.05, **P , 0.01,
***P , ,0.001, compared with vehicle.
P. O’Kane et al.
Aspirin and platelet nitric oxide synthase
129
Downloaded from by guest on September 30, 2016
Figure 6 Aspirin acetylates NOS-3 at serine residues 765 and 771. (A) HeLa cells transfected with pcDNA3.1 containing the gene for NOS-3 were exposed to
aspirin or vehicle, and, following SDS–PAGE of cell lysates and staining with Coomassie Brilliant Blue, the band running at 135 kDa (not present in cells transfected
with empty pcDNA3.1 vector) was excised, and tryptic digests were analysed by peptide mass fingerprinting. The mass spectrum is shown for cells both treated
and not treated with aspirin, and positive identification as NOS-3 was confirmed with a MASCOT search. (B) Most of the constituent peptides showed no evidence
of acetylation. However, the modified (+Aspirin) peptide SVENLQSSK (amino acids 765–773 of NOS-3) showed a precursor ion at m/z 1075.558, whereas the unmodified (2Aspirin) peptide SVENLQSSK displayed a precursor ion at m/z 991.505. The mass of the precursor ion of the modified peptide therefore showed a
+84.053 Da shift (equivalent to the molecular mass of two acetyl groups) when compared with that of the unmodified peptide. (C ) The modified peptide
SVENLQSSK (amino acids 765–773 of NOS-3) was further analysed by MS/MS; sequence-specific ions are labelled as y and b ions on the spectra. The peptide
was found to be acetylated at serine residues 765 and 771.
In the present study, we chose to incubate with aspirin at
different concentrations: 5–10 mmol/L, representative of
plasma levels found during aspirin treatment at the low doses
used in cardiovascular disease prophylaxis28; 400 mmol/L,
which is representative of plasma levels obtained during
aspirin treatment at anti-inflammatory doses11,29; and
130
4 mmol/L, which is within the range of plasma concentration
found in patients with aspirin toxicity. Our findings are therefore
applicable to plasma concentrations of aspirin which are of clinical relevance. In addition, it should be noted that, following oral
administration, aspirin is believed mainly to act on platelets as
they pass through the portal circulation, where the concentrations achieved may be much higher than those achieved in
the systemic circulation—and especially so if higher (antiinflammatory) doses of aspirin are being administered.
In conclusion, aspirin acutely increases platelet NOS-3
activity, and this is associated with acetylation of the
enzyme. The present study suggests that such acetylation
occurs on serine-765 and serine-771, although it is also possible that aspirin additionally affects NOS-3 indirectly by
acetylating other substrates. This effect may contribute
importantly to the anti-platelet effect of aspirin.
Conflict of interest: none declared.
Funding
This project was supported by the Coronary Research Fund
UK, by the National Natural Science Foundation of China
(grant no. 30770891), and by the QingLan Project.
1. Steering Committee of the Physicians’ Health Study Research Group.
Final report on the aspirin component of the ongoing Physicians’ Health
study. N Engl J Med 1989;321:129–135.
2. Antiplatelet Trialists’ Collaboration. Collaborative overview of randomized trials of antiplatelet therapy. I: Prevention of death, myocardial
infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81–106.
3. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of
randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86.
4. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel
in Unstable Angina to Prevent Recurrent Events Trial Investigators.
Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST segment elevation. N Engl J Med 2001;345:
494–502.
5. ISIS-2 (Second International Study of Infarct Survival) Collaborative
Group. Randomised trial of intravenous streptokinase, oral aspirin,
both, or neither among 17,187 cases of suspected acute myocardial
infarction ISIS-2. Lancet 1988;13:349–360.
6. Zarraga IG, Schwarz ER. Coxibs and heart disease: what we have learned
and what else we need to know. J Am Coll Cardiol 2007;49:1–14.
7. Krotz F, Hellwig N, Schiele TM, Klauss V, Sohn HY. Prothrombotic potential
of NSAID in ischemic heart disease. Mini Rev Med Chem 2006;6:
1351–1355.
8. Hawkins D, Pinckard RN, Farr RS. Acetylation of human serum albumin by
acetylsalicylic acid. Science 1968;160:780–781.
9. Pinckard RN, Hawkins D, Farr RS. In vitro acetylation of plasma proteins,
enzymes and DNA by aspirin. Nature 1968;219:68–69.
10. Queen LR, Xu B, Horinouchi K, Fisher I, Ferro A. b2-Adrenoceptors activate nitric oxide synthase in human platelets. Circ Res 2000;87:39–44.
11. O’Kane PD, Queen LR, Ji Y, Reebye V, Stratton P, Jackson G et al. Aspirin
modifies nitric oxide synthase activity in platelets: effects of acute versus
chronic aspirin treatment. Cardiovasc Res 2003;59:152–159.
12. Madajka M, Korda M, White J, Malinski T. Effect of aspirin on constitutive
nitric oxide synthase and the biovailability of NO. Thromb Res 2003;110:
317–321.
13. Karmohapatra SK, Chakraborty K, Kahn NN, Sinha AK. The role of nitric
oxide in aspirin induced thrombolysis in vitro and the purification of
aspirin activated nitric oxide synthase from human blood platelets. Am
J Hematol 2007;82:986–995.
14. Chakraborty K, Khan GA, Banerjee P, Ray U, Sinha AK. Inhibition of human
blood platelet aggregation and the stimulation of nitric oxide synthesis
by aspirin. Platelets 2003;14:421–427.
15. Fleming I, Busse R. Molecular mechanisms involved in the regulation of
the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp
Physiol 2003;284:R1–R12.
16. Gkaliagkousi E, Ritter J, Ferro A. Platelet-derived nitric oxide signaling
and regulation. Circ Res 2007;101:654–662.
17. Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I,
Witters LA et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 1999;43:285–289.
18. Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K et al. Regulation of endothelium-derived nitric oxide production by the protein
kinase Akt. Nature 1999;399:597–601.
19. Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H et al. Compensatory phosphorylation and protein–protein interactions revealed by loss of
function and gain of function mutants of multiple serine phosphorylation
sites in endothelial nitric-oxide synthase. J Biol Chem 2003;278:
14841–14849.
20. Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA et al.
Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem
2002;277:42344–42351.
21. Matsubara M, Hayashi N, Jing T, Titani K. Regulation of endothelial nitric
oxide synthase by protein kinase C. J Biochem (Tokyo) 2003;133:
773–781.
22. Alheid U, Frolich JC, Forstermann U. Endothelium-derived relaxing factor
from cultured human endothelial cells inhibits aggregation of human
platelets. Thromb Res 1987;47:561–571.
23. de Graaf JC, Banga JD, Moncada S, Palmer RMJ, de Groot PG, Sixma JJ.
Nitric oxide functions as an inhibitor of platelet adhesion under flow conditions. Circulation 1992;85:2284–2290.
24. Broekman MJ, Eiroa AM, Marcus AJ. Inhibition of human platelet reactivity by endothelium-derived relaxing factor from human umbilical vein
endothelial cells in suspension: blockade of aggregation and secretion
by an aspirin-insensitive mechanism. Blood 1991;78:1033–1040.
25. Freedman JE, Loscalzo J, Barnard MR, Alpert C, Keaney JF, Michelson AD.
Nitric oxide released from activated platelets inhibits platelet recruitment. J Clin Invest 1997;100:350–356.
26. Freedman JE, Ting B, Hankin B, Loscalzo J, Keaney JFJ, Vita JA. Impaired
platelet production of nitric oxide in patients with unstable angina.
Circulation 1998;98:1481–1486.
27. Queen LR, Ji Y, Goubareva I, Ferro A. Nitric oxide generation mediated by
b-adrenoceptors is impaired in platelets from patients with type 2 diabetes mellitus. Diabetologia 2003;46:1474–1482.
28. Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic
acetylation of platelet cyclooxygenase. N Engl J Med 1984;311:
1206–1211.
29. Nia B, Vergnaud JM. Comparative pharmacokinetics of Aspegic 1000 mg
i.v. versus 1000 mg i.m. thrice daily. Eur J Drug Metab Pharmacokinet
1996;21:333–338.
Downloaded from by guest on September 30, 2016
References
P. O’Kane et al.