Physical Properties
advertisement

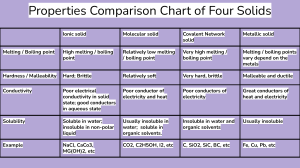
Physical Properties …can be observed without changing composition Property = a characteristic or a quality Composition = the ingredients or make-up of a substance, often represented by a chemical formula Examples: Water is composed of Hydrogen and Oxygen Color Shape Magnetism Emeralds are composed of Al, Be, Si and O Boiling Point Melting Point Diamonds are composed of Carbon only Density Buoyancy Physical Property Chemical Property • can be observed without changing composition • Only can be observed when composition changes. • depends only on the substance itself • “depends on its reaction with other matter” Methanol / Water Physical Property Chemical Property • can be described using the word “is” • can be described using the word “can” or “will” • (or will not, can not) Methanol / Water Physical Properties …can be observed without changing composition Shape and Form • Crystalline: solid with a repeating pattern. Examples: • Massive: shapeless, no pattern or order to shape. Color Shape Magnetism • Granular: made of grains or tiny crystals Boiling Point Melting Point Density Buoyancy 1 Color and Light • Transparent: clear, all light passes through (“see-through”) • Translucent: not clear, some light passes through (“light-through”) • Opaque: light cannot pass through; solid color. • Reflective: light bounces off; shiny • Dull: Non-reflective. Changes in Shape • Rigid: does not change shape • Brittle: rigid and fragile; breaks easily • Malleable: can change shape without breaking • Ductile: can be drawn out or stretched • Elastic: always returns to original shape (“shape memory”) Viscosity: Conductivity: the ability to conduct (carry) heat or electricity a fluid’s resistance to flow (how slow it flows) Conductors Molasses is very viscous. carry heat and electricity well. Miscibility: the ability of two fluids to mix evenly. Insulators prevent or slow the flow of heat/electricity. Juice and water are miscible. Oil and water are immiscible. Miscible vs. Immiscible These terms describe the ability of a liquid to Soluble vs. Insoluble These terms describe the ability of a solid to form a solution in a given solvent. form a solution in a given solvent. Alcohol is miscible in water. Oil is immiscible in water. Salt is highly soluble in water. Chalk is insoluble in water. CuCl2 is soluble in water. CuCl2 is insoluble in toluene. 2 Solubility the ability to dissolve in water to dissolve in water : to form a homogeneous mixture with water Soluble : dissolves in water Insoluble : cannot dissolve in water Boiling Point The temperature at which evaporation or condensation occur. Melting Point The temperature at which melting or freezing occur. 3