Polar Covalent Bonds and Hydrogen Bonds
advertisement
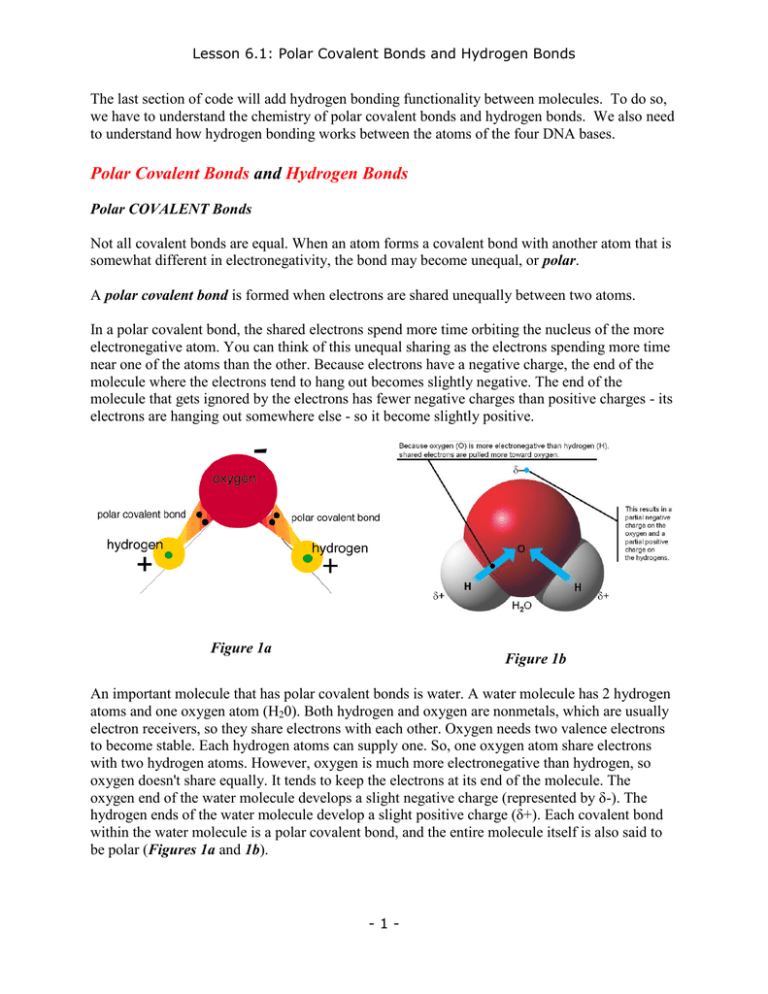
Lesson 6.1: Polar Covalent Bonds and Hydrogen Bonds The last section of code will add hydrogen bonding functionality between molecules. To do so, we have to understand the chemistry of polar covalent bonds and hydrogen bonds. We also need to understand how hydrogen bonding works between the atoms of the four DNA bases. Polar Covalent Bonds and Hydrogen Bonds Polar COVALENT Bonds Not all covalent bonds are equal. When an atom forms a covalent bond with another atom that is somewhat different in electronegativity, the bond may become unequal, or polar. A polar covalent bond is formed when electrons are shared unequally between two atoms. In a polar covalent bond, the shared electrons spend more time orbiting the nucleus of the more electronegative atom. You can think of this unequal sharing as the electrons spending more time near one of the atoms than the other. Because electrons have a negative charge, the end of the molecule where the electrons tend to hang out becomes slightly negative. The end of the molecule that gets ignored by the electrons has fewer negative charges than positive charges - its electrons are hanging out somewhere else - so it become slightly positive. Figure 1a Figure 1b An important molecule that has polar covalent bonds is water. A water molecule has 2 hydrogen atoms and one oxygen atom (H20). Both hydrogen and oxygen are nonmetals, which are usually electron receivers, so they share electrons with each other. Oxygen needs two valence electrons to become stable. Each hydrogen atoms can supply one. So, one oxygen atom share electrons with two hydrogen atoms. However, oxygen is much more electronegative than hydrogen, so oxygen doesn't share equally. It tends to keep the electrons at its end of the molecule. The oxygen end of the water molecule develops a slight negative charge (represented by δ-). The hydrogen ends of the water molecule develop a slight positive charge (δ+). Each covalent bond within the water molecule is a polar covalent bond, and the entire molecule itself is also said to be polar (Figures 1a and 1b). -1- Lesson 6.1: Polar Covalent Bonds and Hydrogen Bonds HYDROGEN Bonds After polar covalent bonds are formed, they set up conditions for the formation of another type of bond: the hydrogen bond. Hydrogen bonds are weak electrical attractions that form between the ends of polar molecules. As always, opposites attract, so the negatively charged end of one molecule would be attracted to the positively charged end of another. The term hydrogen bond doesn't refer to a covalent bond with a hydrogen atom. The bond got it's name because the hydrogen atoms on polar molecules are often seen sticking to atoms on other polar molecules. For example, in the water molecules shown in Figures 2a and 2b, the hydrogen bonds occur between the water molecules, NOT within them. Hydrogen bonds are only about 1/10 as strong as covalent bonds. The length of a hydrogen bond averages about 1.97 Angstroms, about twice as long as the 0.97 Angstrom length of a normal covalent bond. Figure 2a Figure 2b Hydrogen bonds are the molecular equivalent of Velcro. If you look closely at a piece of Velcro, you'll see that one side has tiny little hooks, and on the other side are soft fuzzy material for the hooks to grab. Each little hook on its own forms a very weak attachment to the fuzzy material - it easily is pulled apart. However, a strip of hundreds of these bonds collectively forms a very powerful grip. Another analogous structure would be a zipper. Hydrogen bonds work the same way. Each individual hydrogen bond is very weak. However, many hydrogen bonds together perform important functions inside cells. -2- Lesson 6.1: Polar Covalent Bonds and Hydrogen Bonds 1. Hydrogen bonds hold the two half strands of DNA molecules together so that they form a twisting, ladder-like double helix. 2. Hydrogen bonds are also important in holding proteins together in their proper shapes (called conformations), without which the proteins would be unable to perform their specific functions. 3. Finally, without hydrogen bonds, water would not have its amazing properties. Hydrogen bonds between the positively and negatively charged ends of the water molecules cause them to cling together. 4. (a) Water's high boiling point is due to hydrogen bonding - it takes a lot of heat/energy to break the hydrogen bonds holding water molecules together so that they vaporize. (b) Surface tension is also due to hydrogen bonding. Surface tension accounts for water being able to form droplets. Surface tension also allows small water bugs to skim along the surface of lakes and streams. -3- Lesson 6.2: Hydrogen Bonds in DNA Imagining the HYDROGEN BONDS between DNA Base Pairs After a double helix structure was deemed the most likely structure for DNA, it was still not immediately obvious to investigators how the 4 DNA bases were arranged. The Austrian-American biochemist Erwin Chargaff had established that in all natural DNA, the number of Guanine molecules equals the number of Cytosine molecules and the number of Adenine molecules equals the number of Thymine molecules. That is, there is a one-to-one correspondence between each matching Purine and Pyrimidine. Watson and Crick hypothesized that hydrogen bonds holding together individual C-G and A-T pairs within the double helix would be consistent with Chargaff's data. The problem then was to determine the physical arrangement of these Purine-Pyrimidine pairs, that is, how did they actually fit together? There was also a further requirement: the C-G and A-T molecules being paired on the inside of the double helix needed to take up the same amount of space. If the base pairs were different sizes, the double helix would bulge in some areas and narrow in others. The x-ray diffraction data showed, however, that the helix was of uniform width. To solve this problem, we need to look at the available electro-Positive Hydrogens and electroNegative Atoms on the 4 DNA bases. Remember that an ELECTRO-POSITIVE Hydrogen is one that is bonded to either a Nitrogen or Oxygen atom. An ELECTRO-NEGATIVE atom is a Nitrogen or Oxygen atom that is receptive to an electropositive Hydrogen. Below are images of the four bases showing the available electro-positive Hydrogens and the electro-negative atoms. Adenine Thymine Guanine Electro-POSITIVE HYDROGENS Figure 6.2A -4- Cytosine Lesson 6.2: Hydrogen Bonds in DNA Adenine Thymine Guanine Cytosine Electro-NEGATIVE ATOMS NOTE that the Pyrimidine N-1 and Purine N-9 atoms are not available for hydrogen bonding because they are already covalently bonded to the double helix backbone through deoxyribose. Figure 6.2B Hydrogen Bonds formed between A-T and G-C Immediately above you can see normal base pairing between A-T and G-C. This is what DNA looks like when everything is working correctly. But what happens when things occasionally DON'T work as they should? Take a look at the two base pair MISMATCHES below. -5- Lesson 6.2: Hydrogen Bonds in DNA In the first mismatch, Adenine pairs with Thymine through the N-7 Nitrogen on the Imidazole ring, rather than via the usual N-1 Nitrogen on its Pyrimidine ring (the Adenine shown is a mirror image). In the second mismatch, Guanine is paired incorrectly with Thymine. Such mismatches are NOT anomalies: although infrequent, they are part of the normal functioning of nature and they have both beneficial and deleterious (damaging) effects. The deleterious effects are, of course, mutations that can cause disease and/or death. The beneficial effects are that such molecular base-pair mismatches are the mechanism by which organisms evolve, conferring advantageous traits that enable them to compete and survive. What determines whether a mutation is beneficial or deleterious is the specific location in an organism's genome (genetic code). We can use the 2-D Molecular Modeling program we have been writing to help us understand the Watson-Crick structure of DNA proposed long ago in an article in Nature April 25, 1953 and later confirmed by subsequent experiments and measurements. This is an important DESCRIPTIVE use of the program. What makes a program useful, though, is its PREDICTIVE power, its ability to reveal things that we don't already know. One important use of this very simple program vis-a-vis (regarding) DNA is that it can help us explore all possible base pair mismatches, and thereby enhance our understanding of the different types of point mutations. -6-