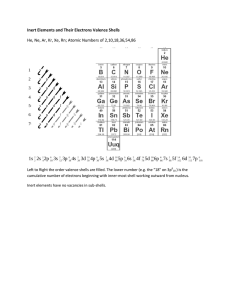
Diagrams showing the composition of an atom: Models include:
advertisement

Diagrams showing the composition of an atom: ◦ number of protons and neutrons in the nucleus ◦ arrangement of electrons in the outer shells Outermost shell called the valence Electrons in the valence level are valence electrons Models include: ◦ Bohr-Rutherford Model ◦ Energy Level Diagrams Basic Rules for drawing a Bohr-Rutherford diagram (first 20 elements only): ◦ Draw a circular nucleus identify the number of protons (P) and neutrons (N) inside it ◦ Draw circular shells around the nucleus for the orbiting electrons. For the first 20 elements: The The The The first shell can hold 2 electrons. second shell can hold 8 electrons. third shell can hold 8 electrons. fourth shell will hold any remaining electrons. ◦ These rules become more complex for other elements in the periodic table (more electrons). Similar to the Bohr Model ◦ instead of drawing circular shells around the nucleus, lines are drawn above the nucleus This is an energy level diagram for Neon: Ions are atoms that have an electrical charge: ◦ Cation – positive charge ◦ Anion – negative charge The periodic table will usually indicate the most common ion charge(s) for each element based on its tendency to gain (-) or lose (+) electrons. BUT WHY DO ATOMS GAIN OR LOSE ELECTRONS? To have the same number of electrons as the nearest noble gas. ◦ The outer energy level wants to be full! This is done by losing its own valence electrons or by gaining another atom’s valence electrons. The first group of elements on the left side of the table (alkali metals – group 1), each have a charge of 1+ The halogens (group 17) on the right of the table have an ion charge of 1- There are some other general patterns in the ion charges for each group and each period. The ion charge is usually written with a plus or minus sign as a superscript (raised like an exponent) next to the element. ◦ Ca2+ is a calcium ion with a charge of 2+ meaning it has lost 2 electrons. electrons ◦ F 1- is a fluorine ion with a charge of 1- meaning it has gained 1 electron. electron Part 1: Draw BohrBohr-Rutherford diagrams for elements 110 on the Periodic Table. Draw Energy Level diagrams for elements 11–20 on the Periodic Table. Part 2: Read pages 34–38 (starting with “Formation of Ions”) Complete “Check and Reflect” on page 39: #1 – 3, 5 – 8, 10 – 12 Answer key will be posted on Online Homework