Chapter 2: Fundamentals of Chemistry The main subatomic particles
advertisement

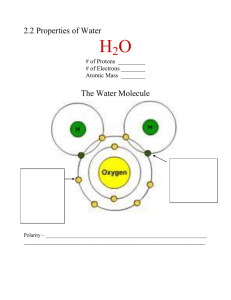
You need to know this for the Chemistry Quiz Atoms Chapter 2: Fundamentals of Chemistry Building blocks of matter Dr. Amy Rogers Made up of subatomic particles Invisible but not indivisible: Bio 139 Microbiology Some figures courtesy of Biology: A Guide to the Natural World by Krogh The main subatomic particles Charge Location • Proton Positive Nucleus Mass • Neutron Neutral Nucleus Mass • Electron Negative Orbital “shell” Space/Volume Can only be broken down by extraordinary means (for example, particle accelerators) Atomic Model If drawn to scale, electrons would be 1/3 mile away! Atomic Number = # of protons This defines an element Isotopes Same element, different number of Neutrons – Hydrogen: one proton (+ one electron) – Gold: 79 protons (+79 electrons) Atomic Weight: sum of protons & neutrons Mole (also called gram molecular weight): the weight of a substance in grams equal to total atomic weight of all the atoms in a molecule of the substance e.g. Glucose (C6H12O6) molecular weight = 180 1 Mole glucose = 180 grams Some isotopes are unstable and undergo radioactive decay 1 Interactions between atoms: Chemical Bonding Stable energy states • Energy always seeks its lowest state • Matter is transformed from pure elements into everything else by joining atoms together • Bonding converts the atoms to a lower, more stable energy state • This is called Chemical Bonding • Stable energy state = Filled outer electron shell • Electrons are responsible for atoms bonding together Outer Shells • Electrons move within defined “energy levels” outside the nucleus • The volume of each energy level, or shell, fits a specific number of electrons • First shell: Second shell: Third shell: 2 electrons 8 electrons 8 electrons Stable atoms: Largely inert Outer electron shells are full Unstable atoms: Highly reactive Outer electron shells are unfilled (fewer than 2 or 8 valence electrons) Ionic Bonding: Give and Take Electrons are given from one atom to another, leading to a net transfer of charge. This charge separation creates ions, which are attracted to one another electrostatically. Cation: (2 or 8 valence electrons) positively charged ion; lost electron(s) ex. Na+ Anion: negatively charged ion; gained electron(s) ex. Cl- 2 Covalent Bonding: Polar Covalent Bonding: Share and share alike Sharing of the electrons is unequal, leading to slight charge separation (polarity). Electrons are NOT transferred, but shared. Oxygen has greater electronegativity Carbon more electronegative but molecule is symmetric Hydrogen Bonds: A special kind of weak electrostatic attraction that results from polar covalent bonds involving hydrogen • Hydrogen bonds are WEAK but MANY of them together are a significant force • Critically important for biology • Hydrogen bonds form from attraction between partial + and - charges of polar molecules Metabolism: Forming & Breaking chemical bonds Catabolism: X—Y breaking of bonds (digestion) X + Y + energy exergonic reaction • Hydrogen bonds are very important for: – Protein folding – DNA base pairing – Receptor/ligand interactions – Properties of water Metabolism: Forming & Breaking chemical bonds Anabolism: synthesis (bond creation) X + Y + energy X—Y endergonic reaction 3 Some Biologically Important Properties of Water • Solvent: for Polar & charged solutes (hydrophilic) • Specific Heat: high • Reactant in biochemical reactions Water as a Solvent: Water is polar & forms hydrogen bonds Solubility: The solution to many problems Solute Solvent Solution Hydrophobic molecules (with itself and with solutes) •Do not dissolve in water •Crucial for maintaining biological “compartments” •Nonpolar molecules Hydrophilic: “water loving” interacts with or dissolves in water •Hydrocarbons, lipids Polar or Ionic Solutes Specific Heat: The amount of energy required to raise the temperature of a substance by 1o C Heat buffering by water maintains (relatively) stable temperatures on planet earth and in animals’ bodies Specific Heat of Water: Why so high? • Heat is the kinetic energy of moving molecules • It takes a lot of energy to get water molecules moving because: Hydrogen Bonds must be broken 4 Water as a reactant • Most biochemical reactions take place in water • Water is often not only a solvent, but also a reactant X—H + HO—Y Æ X—Y + H2O Dehydration synthesis X—Y + H2O Æ X—H + HO—Y Hydrolysis (often endergonic) (ex. anabolic formation of organic polymers such as complex carbohydrates, some lipids, and proteins) (often exergonic) (ex. catabolism of complex carbohydrates, lipids, proteins to simple sugars, fats, amino acids) Acids & Bases: All things in moderation • Opposite of acidic is alkaline (basic) • Alkaline/Basic = low [H+] or high [OH-] • Low [H+] = high pH - • OH (hydroxide ion) is a strong base: It accepts H+ to yield water • pH is a measure of concentration of hydrogen ions (H+, or protons) in a solution • More hydrogen ions = more acidic • High acidity = low pH pH • On the pH scale, 7 is neutral OH- + H+ H2O Carbon: The primary ingredient of life – 0 is highly acidic – 14 is highly basic/alkaline • Logarithmic scale: one change in pH unit = 10x change in [H+] e.g., pH 5 = 100 times more acidic than pH 7 •Organisms use buffers to maintain appropriate pH (usually 6-8) •pH extremes can denature proteins & disrupt membranes 5 Carbon Carbon Structures: Hydrocarbons • Atomic number = 6 • 4 valence electrons; wants 8 • Most stable with 4 covalent bonds per carbon Propane C3 H8 • Stability, Variety, Complexity of organic molecules Hydrocarbons are reduced: saturated with hydrogen, no oxygen atoms present Lots of energy stored in these bonds; can be released by oxidation Functional Groups drastically alter chemical properties Functional Groups Change Everything Ethane (hydrocarbon gas) Ethanol (liquid alcohol) Nonpolar Polar Won’t dissolve in water Water soluble Complex organic molecules: Carbohydrates •Main energy source for most living things •Monosaccharides (simple sugars): •carbon ring with several alcohol groups (+ one aldehyde or ketone) •Some are isomers (same molecular formula but different structures) •e.g., glucose & fructose (C6H12O6) Most common monosaccharide: Glucose Groups of atoms that confer a special property on a carbon-based molecule Other examples: ribose & deoxyribose 6 Disaccharides e.g., lactose, maltose, sucrose (table sugar) two monosaccharides connected by the removal of water and the formation of a glycosidic bond Polysaccharides (complex carbohydrates) • Starch • Glycogen • Cellulose energy storage in plants energy storage in animals structural role (cell walls) These 3 examples are all very long (thousands of subunits) polymers of glucose Example of dehydration synthesis! Polymers large molecules made up of many similar or identical chemical subunits Polymer Monomer Subunits Polysaccharides & Complex carbohydrates Monosaccharides or simple sugars Proteins Amino acids Nucleic acids Nucleotides Lipids Fatty acids: Long hydrocarbon chains with carboxyl group at end • Diverse category including fats, phospholipids, steroids • Relatively more hydrogen & less oxygen than carbohydrates; therefore more energy-rich • Hydrophobic • Many important functions including – Energy storage – Cell membranes 7 Saturated fatty acid: •maximum hydrogen content; all single bonds between carbons. Fats 3 carbon glycerol “head” + 1 to 3 fatty acid “tails” •Usually solid at room temp. Unsaturated fatty acid: •one or more double bonds; not “saturated” with hydrogen; “kink” in chain. •Often liquid at room temp, esp. if polyunsaturated (more than one double bond) + If: 3 fatty acids: triacylglycerol (or triglyceride) 2 fatty acids: diacylglycerol 1 fatty acid: monoacylglycerol Formation of triacylglycerols Phospholipids Diacylglycerols (glycerol head + 2 fatty acids tails) + a charged phosphate group R = long hydrocarbon chain (do NOT need to be all the same) Dehydration synthesis forming three ester bonds Phospholipids In an aqueous environment, phospholipids will spontaneously form structures to “hide” their hydrophobic parts Hydrophobic end (nonpolar fatty acid tails) Hydrophilic end (charged phosphate group) VERY IMPORTANT property Principle component of all cell membranes 8 Lipids: Steroids e.g. cholesterol, steroid hormones, vitamin D Basic structure of all steroids: Four carbon rings Proteins are polymers of Amino Acids Specific steroids have various side chains, bond arrangements. Here: cholesterol Proteins begin as polypeptide chains Peptide bonds form between Amino acids differ in the structure of their side chain (R group) Examples at left (do NOT memorize) carboxyl group of one amino acid and amino group of another amino acid Note similarity of basic structure despite variety of R groups This tremendous chemical diversity is linked like letters of the alphabet into protein “words” Dehydration synthesis again! • Polypeptides fold into complex shapes Protein folding: Telephone cord analogy • Final protein has a very specific 3-D conformation critical to its function • Most folding is mediated by hydrogen bonds • At high temperature or extreme pH, these bonds are broken and the protein unfolds or denatures 9 Nucleotides: DNA & RNA: polymers of nucleotides Subunits of nucleic acids Energy carriers 3 components: Phosphate(s) Sugar (ribose or deoxyribose) Base (ATGCU) ATP: adenosine triphosphate High energy bonds between 2 outer phosphate groups DNA RNA Double stranded Single stranded Deoxyribose Ribose Bases: Bases: Adenine Guanine Cytosine Thymine Adenine Guanine Cystosine Uracil 10