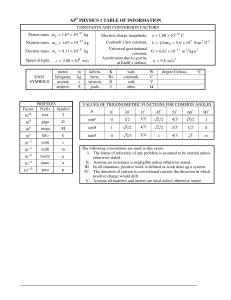
Y θ φ θ π 0 θ φ, θ π θ
advertisement

Chem. 140B S2008 HW set 7 Quiz this week: SHO (7.1) Rotation in 2-D (7.2) I will skip ch. 8 for now, and then come back to it after ch.9 & 10 Quantum Chemistry and Spectroscopy CH.7: P7.25, 7.30 Ch. 9: Q9.2, 3, 9 P9.2, 5, 6 1/ 2 5 P7.25) At what values of θ does Y (θ ,φ ) = 16π lines, planes, or other surfaces? 0 2 b g c ( 3cos θ − 1) have nodes? Are the nodes points, 2 h Y20 θ , φ has nodes when 3 cos 2 θ − 1 has nodes. This occurs for θ = 0.955 and π − 0.955 radians or 54.7 and 125.3 degrees. These surfaces are cones. P7.30) Draw a picture (to scale) showing all angular momentum cones consistent with l = 5. Calculate the half angles for each of the cones. ml = +5 ml = +4 ml = +3 ml = +2 ml = +1 ml = 0 ml = -1 ml = -2 ml = -3 ml = -4 ml = -5 The half angles of the cones measured from the positive and negative z axis are 5 = 0.420 radians θ ml =5 = cos−1 5 ( 5 + 1) 1 4 = 0.752 radians θ ml =4 = cos 5 ( 5 + 1) 3 = 0.991radians θ ml =3 = cos−1 5 ( 5 + 1) 2 −1 = 1.20 radians θ ml =2 = cos 5 ( 5 + 1) 1 = 1.39 radians θ ml =1 = cos −1 5 ( 5 + 1) 0 = 1.57 radians θ ml =0 = cos −1 5 ( 5 + 1) as well as π minus these values for the negative values of ml. −1 Q9.2) Use an analogy with the particle in the box to explain why the energy levels for the H atom are not equally spaced. Given the functional form of the Coulomb potential, one can think of a “box” that is very narrow for low values of energy and very wide near the dissociation limit of zero energy. Therefore, by analogy with the particle in the box, we expect the low lying levels to be widely spaced, and the energy levels near the dissociation limit to be closely spaced. Q9.3) What transition gives rise to the highest frequency spectral line in the Lyman series? The transition n → 1 to n → ∞, effectively the dissociation limit, will have the frequency. 1/ 2 highest 3/ 2 1 r r2 1 2 Q9.9) Is the total energy wave function ψ 310 ( r, θ , φ ) = 6 − 2 e − r / 3a0 cos θ an 81 π a0 a0 a0 eigenfunction of any other operators? If so, which ones? What are the eigenvalues? Yes, it is an eigenfunction of the operator lˆ 2 with the eigenvalue 2= 2 , and an of the operator lˆ with the eigenvalue zero. eigenfunction z P9.2) The total energy eigenvalues for the hydrogen atom are given by En = − e2 , 8π ε 0 a0 n 2 n = 1, 2, 3, 4,..., and the three quantum numbers associated with the total energy 2 eigenfunctions are related by n = 1, 2, 3, 4,.....; l = 0, 1, 2, 3, ..., n − 1; and ml = 0, ± 1, ± 2, ± 3, ... ± l. Using the nomenclature, list all eigenfunctions that have the following total energy eigenvalues e2 a) E = − 32π ε 0 a 0 b) E = − c) E = − e2 72π ε 0 a 0 e2 128π ε 0 a 0 d) What is the degeneracy of each of these energy levels? e2 corresponds to n = 2. The total energy eigenfunctions are ψ 200 , ψ 210 , ψ 211 , ψ 21−1. 32π ε 0 a 0 The level has a degeneracy of 4. a) E = − b) E = − e2 corresponds to n = 3. The total energy eigenfunctions are 72π ε 0 a 0 ψ 300 , ψ 310 , ψ 311 , ψ 31−1 , ψ 320 , ψ 321 , ψ 32−1 , ψ 322 , ψ 32− 2 . The level has a degeneracy of 9. c) E = − e2 corresponds to n = 4. The total energy eigenfunctions are 128π ε 0 a 0 ψ 400 , ψ 410 , ψ 411 , ψ 41−1 , ψ 420 , ψ 421 , ψ 42−1 , ψ 422 , ψ 42− 2 , ψ 43− 2 , ψ 43−1 , ψ 430 , ψ 431 , ψ 432 , ψ 433 , ψ 43−3 . The level has a degeneracy of 16. P9.5) Locate the radial and angular nodes in the H orbitals ψ 3 p ( r ,θ , φ ) and ψ 3 p ( r ,θ , φ ) . x 3/ 2 z r r2 1 1 r r − r / 3a0 ψ 3 px ( r , θ , φ ) = sin θ cos φ has radial nodes where 6 − 2 has 6 − 2 e a0 a0 81 2π a0 a0 a0 zeros. This is at r = 0, which does not count as a node, and r = 6a0 . The angular nodes correspond to 2 the angles at which the function is zero, or θ = 0D and φ = 90D , corresponding to the y-z plane. 1/ 2 3/ 2 1 r r 2 − r / 3a0 ψ 3 pz cos θ also has a radial node at r = 6a0 . The angular 6 − 2 e a0 a0 a0 node is for θ = 90D , corresponding to the x-y plane. 1 2 ( r, θ , φ ) = 81 π P9.6) How many radial and angular nodes are there in the following H orbitals set? a) ψ 2 px ( r , θ , φ ) b) ψ 2s ( r ) c) ψ 3d xz ( r, θ , φ ) d) ψ 3d 2 x − y2 ( r, θ , φ ) The functions have n–l–1 radial nodes and l angular nodes. Therefore a) ψ 2 px ( r , θ , φ ) has no radial nodes and one angular node. 3 b) ψ 2s ( r ) has one radial node and no angular nodes. c) ψ 3d xz ( r, θ , φ ) has no radial nodes and 2 angular nodes. d) ψ 3d 2 x − y2 ( r, θ , φ ) has no radial nodes and 2 angular nodes. 4