2.2.1 Errors and their causes
advertisement
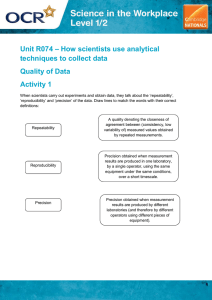
2.2.1 Errors and their causes 2.2 Measurement Error Error 2.2.1 Errors and their causes 2.2.2 The expression of Errors 1 2.2.1 Errors and their causes ①method: imperfection of methods, e.g e.g,, incorrect indicators indicator in titrametry or incomplete precipitation in gravimetry error: measured value subtracted from true value ①systematic error ②random error 1. Classification ③gross error 2. systematic error errorss are biases in measurement which lead the situation where the mean of many separate measurements differs significantly from the actual value of the measured attribute. Sources of them may be imperfect calibration of instruments, imperfect methods of observation, or changes in the environment which interfere with the measurement process. 2 2.2.1 Errors and their causes Incorrect calibration of volumetric flask or pipette precipitation ②instrument instrument:: Incorrect calibration E.g., corrosion of weight or balance 3 2.2.1 Errors and their causes Characterization of systematic errors ①exhibit same direction and magnitude ②relatively constant in repeated measurements measurements;; ③ “measurable measurable”” and reducible (determinate) 3.random error random error is caused by random reasons, such as variations in temperature, pressure or humidity of Lab. And it is difficult to identify the specific reason for it. Therefore random error may change direction and magnitude randomly (indeterminate) (indeterminate),, but it follows the normal distribution. 5 pipette ③reagent reagent:: deionized or distilled water is contaminated ④ personal reason 4 2.2.1 Errors and their causes Statistical characteristics ① equal probability for positive and negative errors ② Probability is inversely proportional to its magnitude 4. gross error: 5. How to avoid the errors (1)systematic error: blank test conduct same measurement procedure without sample check test conduct same measurement procedure with a standard or a sample of “known known”” content 6 1 2.2.1 Errors and their causes (2) random error: increase the measurement time (3) gross error:: carefulness and good habit (professional) 7 2.2.2 Error expression absolute error: Ea = x − xT relative error: Er = Ea xT note: error can be positive or negative 2.2.2 Error expression 1. Accuracy and errors: Accuracy is a measure of how close a measure of central tendency is to the true, or expected value, xT. Accuracy is usually expressed as either an absolute error E a or a percent relative error, Er . true value value:: theoretical value or value agreed by authority, different labs 8 E1: Two samples A and B are weighed to be 1.5268g and 0.1526g respectively by using an electronic balance. If the true weights of A and B are 1.5267g and 0.1525g, please work out the Ea and Er . EaA = 1.5268 - 1.5267 = +0.0001g EaB = 0.1526 - 0.1525 = +0.0001g balance ErA = +0.0001/1.5267 = +0.006% ErB = +0.0001/0.1525 = +0.06% What you can conclude from the calculations? 9 E2: It is known that the reading error of a burette could be ±0.01mL. To keep the relative error of a burette within 0.1%, then at least how much of titrant volume should be used? ∵Ea = 0.01 To obtain a volume, two times of reading are needed, burette Therefore, Ea = 0.02mL. burette And ∵Er ≤0.1%. Er = Ea xT ∴V = 0.02/0.1% = 20mL Conclusion? 11 10 E3 E3:: If titrant consumption is controlled at around 25mL, and reading error of an electronic balance is ± 0.0001g. To standardized 0.2mol 0.2mol·· L-1 NaOH both (A) potassium hydrogen phthalate (MA = 204) and (B) oxalate acid (MB = 126) can be used as the primary standard. Please compare the relative errors caused by using different standards. ①nNaOH = nA mA = 0.2×25×10-3×204 = 1g ∴Er A = 0.0002/1 = 0.02% ∵H 2C2O4 + 2OH- = C2O 42- + H2O ②∵ nNaOH = 2nB mB = 0.2×25×10-3×126/2 = 0.3g ∴Er B = 0.0002/0.3 = 0.07% Conclusion? 12 2 2.2.2 Error expression 2.2.2 Error expression 2. Precision and deviation Precision is a measure of the spread of data about a central value and may be expressed as the range, the deviation, the standard deviation, or the variance. Deviation can also be expressed in an absolute or relative way Precision is commonly divided into two categories: repeatability and reproducibility. Repeatability is the precision obtained when all measurements are made by the same analyst during a single period of laboratory work, using the same solutions and equipment. Reproducibility is the precision obtained under any other set of conditions,including that between analysts, or between laboratory sessions for a single analyst. The relationship between accuracy and precision: Note Note that that there there are are no no direction direction notations notations for for deviation deviation Systematic errors decide the error of an analysis, while random errors decide the precision. If systematic errors are not excluded, than the accuracy can not be assured even with a good precision 13 14 E4. Shooting results of 4 students (A, B, C and D) A B C D Conclusion Conclusion:: precision is the premise of accuracy, but good precision can not guarantee a satisfactory accuracy 15 3