Fabrication of Mn/Mn oxide core-shell electrodes with three
advertisement

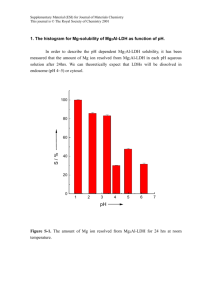
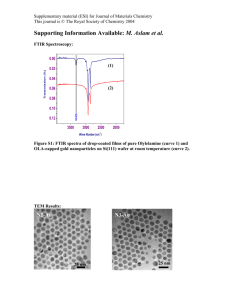
Electronic Supplementary Material (ESI) for Energy & Environmental Science This journal is © The Royal Society of Chemistry 2013 Fabrication of Mn/Mn oxide core-shell electrodes with three-dimensionally ordered macroporous structures for highcapacitance supercapacitors Ming-Jay Denga*, Pei-Jung Hob, Cheng-Zhao Song b, Shin-An Chen a,c, Jyh-Fu Leea, Jin-Ming Chen a*, Kueih-Tzu Lu a* a National Synchrotron Radiation Research Center (NSRRC), Hsinchu, Taiwan b Department of Applied Science, National Hsinchu University of Education, Hsinchu, Taiwan c Department of Engineering and System Science, National Tsing Hua University, Hsinchu, Taiwan E-mail: deng.mj@nsrrc.org.tw or martinez730523@yahoo.com.tw ; jmchen@nsrrc.org.tw; ktlu@nsrrc.org.tw Electronic Supplementary Material (ESI) for Energy & Environmental Science This journal is © The Royal Society of Chemistry 2013 Figure S1 SEM micrographs of the cross-sectional PS template on an Au/ITO electrode. (a) (b) (c) Figure S2 (a) higher magnification TEM image of 3DOM Mn/Mn oxide–CV Electronic Supplementary Material (ESI) for Energy & Environmental Science This journal is © The Royal Society of Chemistry 2013 electrode; (b) energy-dispersive spectrometry (EDS) pattern of Area A; (c) EDS pattern of Area B Figure S3 The SEM micrographs of the prepared MnO2 electrodes after removing the PS spheres. Figure S4 XRD pattern of the 3DOM Mn and the 3DOM Mn/Mn oxide–CV films on Au/ITO substrate, respectively. (● indicate the peaks originated from ITO substrate.) Electronic Supplementary Material (ESI) for Energy & Environmental Science This journal is © The Royal Society of Chemistry 2013 Figure S5 Mn K-edge XANES spectra of the 3DOM Mn and the 3DOM Mn/Mn oxide–CV samples, respectively. Figure S6 SEM image of 3DOM Mn/Mn oxide–CV electrode after 2000 cycles. Electronic Supplementary Material (ESI) for Energy & Environmental Science This journal is © The Royal Society of Chemistry 2013 Figure S7 Nyquist plots of three electrodes for supercapacitors. Figure S8 Schematic illustration of the electrochemical cell used in-situ XANES studies. Electronic Supplementary Material (ESI) for Energy & Environmental Science This journal is © The Royal Society of Chemistry 2013 The specific capacitance (Csp), energy density (E), and power density (P) were calculated from the chronopotentiometric curves according to Eqs (1~3):1-3 Csp = I Δt /w ∆V (1) E = (C(Δ V )2)/2 (2) P = E /Δt (3) where I is the charge/discharge current, Δt is the time for a full charge or discharge, w is the mass of the active electrode material, and Δ V is the voltage change after a full charge or discharge. Reference 1. Q. Li, Z. Wang, G. Li, R. Guo, L. Ding and Y. Tong, Nano Lett. 2012, 12, 3803. 2. Q. Lu, M. W. Lattanzi, Y. Chen, X. Kou, W. Li, X. Fan, K. M. Unruh, J. G. Chen, and J. Q. Xiao, Angew. Chem., Int. Ed., 2011, 50, 6847. 3. C. Liu, Z. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863.