480 Real-Time PCR System in Human Genetics
advertisement

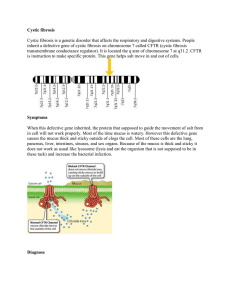
® LightCycler® 480 Real-Time PCR System in Human Genetics Featured project: High-Resolution Melting for CFTR Mutation Scanning Stefanie Beck-Wödl and Peter Bauer Molecular Pathology Research and Development Laboratory, Department of Pathology, University of Tübingen, Germany Read in this article: Introduction Materials and Methods Results and Discussion Conclusions References Introduction Cystic fibrosis (CF) is the most common autosomal recessive monogenic disease (1:2500, with a carrier frequency of 1 in 25). More than 1,000 mutations are described in the CFTR (cystic fibrosis transmembrane conductance regulator) gene. In Germany, 86% of all CFTR alleles are defined by 19 different mutations, with F508del being the most prevalent (73%). These alleles are located in nine different PCR fragments. Complete gene analysis by scanning and/or sequencing is seldom performed because of the cost, time, and labor involved. High-resolution melting analysis (HRM) is a rapid, closed-tube alternative. To assess the feasibility of using HRM in CFTR mutation screening for research purposes, we set up PCR protocols for CFTR exons 4, 7, 10, 11, 14b, 19, 20, and 21, enabling the analysis of the most prevalent CFTR mutations in the German population. In parallel, unlabeled probes were tested to directly discern known CFTR mutations in these exons. Back to Top Materials and Methods Several CFTR-mutation-positive samples were selected for this study, including samples for F508del, I507del, R117H, R347P, G542X, G551D, R553X, 1717-1G>A, 2789+5G>A, S1255X, W1272X, and N1303K. CFTR genotypes had been previously confirmed for all samples, either by direct sequencing or RFLP-analysis. Unlabeled probes were designed complementary to the wildtype sequences. PCR fragments usually had amplicon lengths of less than 400 bp. Amplification was carried out for 45 cycles followed by a melting curve from +50 to +85°C. Ten picomoles of each primer and 3 pmol of each unlabeled probe were used for detection. Back to Top Results and Discussion So far, we have been able to establish an HRM analysis panel with 18 CFTR mutations amplified in one PCR assay. This panel covers approximately 85% of the German population. All mutations in this panel were determined to be heterozygous, as indicated by aberrant melting curves. F508del and compound heterozygote alleles in exon 11 (G551D/R553X) where clearly discernible by analysis of the melting curves of the unlabeled probes. Figure 1: Genetic variation in the human CFTR gene analyzed by high-resolution melting. A 198 bp fragment of the human CFTR gene was amplified using the LightCycler® 480 High Resolution Melting Master and subjected to amplicon melting at high resolution. Difference plot analysis revealed three different groups of heterozygotes (blue, red, and green) in addition to the homozygous samples (black). Figure 2: Genotype differentiation using high-resolution melting analysis in the presence of unlabeled probes. Samples not differentiated by amplicon melting alone (green in Figure 1) could be separated based on the Tm of an unlabeled probe covering both mutated sites (see insert). Back to Top Conclusions High-resolution melting analysis with unlabeled probes enables accurate genotyping and mutation screening in the CFTR gene. The sensitivity obtained in this experiment was almost 100%, and specificity was guaranteed by mutation-specific melting peaks. Back to Top References ● ● ● ● ● [1] Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066-1073. [2] Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations: correlation with incidence data and application to screening. Hum Mutat 2002;19:575-606. [3] Dork T, Mekus F, Schmidt K, Bosshammer J, Fislage R, Heuer T, et al. Detection of more than 50 different CFTR mutations in a large group of German cystic fibrosis patients. Hum Genet 1994;94:533-542. [4] Chou LS, Lyon E, Wittwer CT. A comparison of high-resolution melting analysis with denaturing high-performance liquid chromatography for mutation scanning: cystic fibrosis transmembrane conductance regulator gene as a model. Am J Clin Pathol 2005;124:330-338. [5] Wittwer CT, Marshall BC, Reed GH, Cherry JL. Rapid cycle allele-specific amplification: studies with the cystic fibrosis delta F508 locus. Clin Chem 1993;39:804-809. Back to Top