Chapter Outline Law of Conservation of Mass Law of Conservation
advertisement
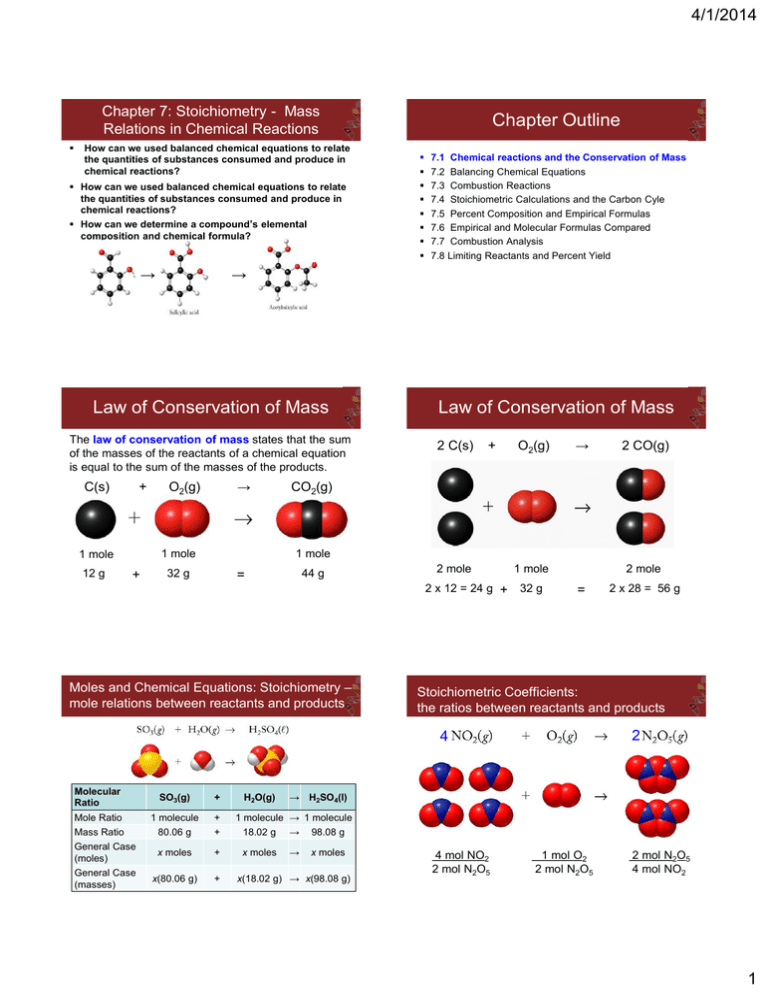
4/1/2014 Chapter 7: Stoichiometry - Mass Relations in Chemical Reactions How can we used balanced chemical equations to relate the quantities of substances consumed and produce in chemical reactions? How can we used balanced chemical equations to relate the quantities of substances consumed and produce in chemical reactions? How can we determine a compound’s elemental composition and chemical formula? → The law of conservation of mass states that the sum of the masses of the reactants of a chemical equation is equal to the sum of the masses of the products. + 12 g O2(g) → 1 mole 1 mole + 7.1 Chemical reactions and the Conservation of Mass 7.2 Balancing Chemical Equations 7.3 Combustion Reactions 7.4 Stoichiometric Calculations and the Carbon Cyle 7.5 Percent Composition and Empirical Formulas 7.6 Empirical and Molecular Formulas Compared 7.7 Combustion Analysis 7.8 Limiting Reactants and Percent Yield → Law of Conservation of Mass C(s) Chapter Outline Law of Conservation of Mass 2 C(s) + O2(g) → 2 CO(g) CO2(g) 1 mole 32 g 44 g = 2 mole 2 x 12 = 24 g + Moles and Chemical Equations: Stoichiometry – mole relations between reactants and products 1 mole 32 g 2 mole = Stoichiometric Coefficients: the ratios between reactants and products 2 4 Molecular Ratio SO3(g) + Mole Ratio 1 molecule + Mass Ratio 80.06 g + 18.02 g → 98.08 g General Case (moles) x moles + x moles → x moles General Case (masses) x(80.06 g) + H2O(g) 2 x 28 = 56 g → H2SO4(l) 1 molecule → 1 molecule x(18.02 g) → x(98.08 g) 4 mol NO2 2 mol N2O5 1 mol O2 2 mol N2O5 2 mol N2O5 4 mol NO2 1 4/1/2014 Balancing Chemical Equations Chapter Outline SO2(g) + 7.1 Chemical reactions and the Conservation of Mass 7.2 Balancing Chemical Equations 7.3 Combustion Reactions 7.4 Stoichiometric Calculations and the Carbon Cyle 7.5 Percent Composition and Empirical Formulas 7.6 Empirical and Molecular Formulas Compared 7.7 Combustion Analysis 7.8 Limiting Reactants and Percent Yield H2O(l) → H2O(l) → + SO2(g) + H2O(l) → 2 HNO3(aq) + NO(aq) 2 HNO3(aq) + NO(aq) Step 3: Atoms = 3 N + 2 H + 7 O → 3 N + 2 H + 7 O Combustion Reactions The reaction of an organic compound with oxygen to produce CO2 + H2O CH4(g) + O2(g) → CO2(g) + H2O(g) Step 1: Atoms = 1 C + 4 H + 2 O → 1 C + 2 H + 3 O CH4(g) + O2(g) → CO2(g) + 2 H2O(g) Step 1: Atoms = 1 C + 4 H + 2 O → 1 C + 4 H + 4 O CH4(g) + 2 O2(g) → CO2(g) O2(g) → 2 SO3(g) Step 2: Atoms = 1 S + 4 O → 2 S + 6 O 2 SO2(g) + O2(g) → 2 SO3(g) Step 3: Atoms = 2 S + 6 O → 2 S + 6 O NO(aq) Step 2: Atoms = 1 N + 2 H + 3 O → 3 N + 2 H + 7 O 3 NO2(g) + SO3(g) Chapter Outline HNO3(aq) Step 1: Atoms = 1 N + 2 H + 3 O → 2 N + 1 H + 4 O NO2(g) + → Step 1: Atoms = 1 S + 4 O → 1 S + 3 O A more challenging example: NO2(g) + O2(g) + 2 H2O(g) Step 1: Atoms = 1 C + 4 H + 4 O → 1 C + 4 H + 4 O 7.1 Chemical reactions and the Conservation of Mass 7.2 Balancing Chemical Equations 7.3 Combustion Reactions 7.4 Stoichiometric Calculations and the Carbon Cyle 7.5 Percent Composition and Empirical Formulas 7.6 Empirical and Molecular Formulas Compared 7.7 Combustion Analysis 7.8 Limiting Reactants and Percent Yield Procedure for Balancing Combustion Reactions C2H6 (ethane) reacts with oxygen to form CO2 and H2O 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. C 2 H 6 + O2 CO2 + H2O 2. Change the numbers in front of the formulas (coefficients) to make the number of atoms of each element the same on both sides of the equation. Do not change the subscripts. 2 C2H6 NOT C4H12 2 4/1/2014 Procedure for Balancing Combustion Reactions Procedure for Balancing Combustion Reactions 3. Start by balancing those elements that appear in only one reactant and one product. C2H6 + O2 CO2 + H2O start with C or H but not O 1 carbon on right 2 carbons on left C2H6 + O2 multiply CO2 by 2 2 CO2 + H2O 6 hydrogens on left 2 hydrogens on right C2H6 + O2 multiply H2O by 3 4. Balance those elements that appear in two or more reactants or products. C2H6 + O2 2 oxygen on left 2CO2 + 3H2O multiply O2 by 7 2 4 oxygen + 3 oxygen = 7 oxygen (3x1) on right (2x2) C2H6 + 7 O2 2 2CO2 + 3H2O 2C2H6 + 7O2 4CO2 + 6H2O remove fraction multiply both sides by 2 2 CO2 + 3 H2O Procedure for Balancing Combustion Reactions 5. Check to make sure that you have the same number of each type of atom on both sides of the equation. 2C2H6 + 7O2 The reaction of CH4 with O2 actually follows a series of steps due to incomplete combustion: 4CO2 + 6H2O Step 1: CO is the product when O2 is deficient: Reactants 4C 12 H 14 O Products 4C 12 H 14 O Step 2: the H2 is combusted to form H2O Step 3: the CO reacts further to form CO2 3 4/1/2014 The individual steps can be added together to get the full reaction - 4