QOI 0809 C=C
advertisement
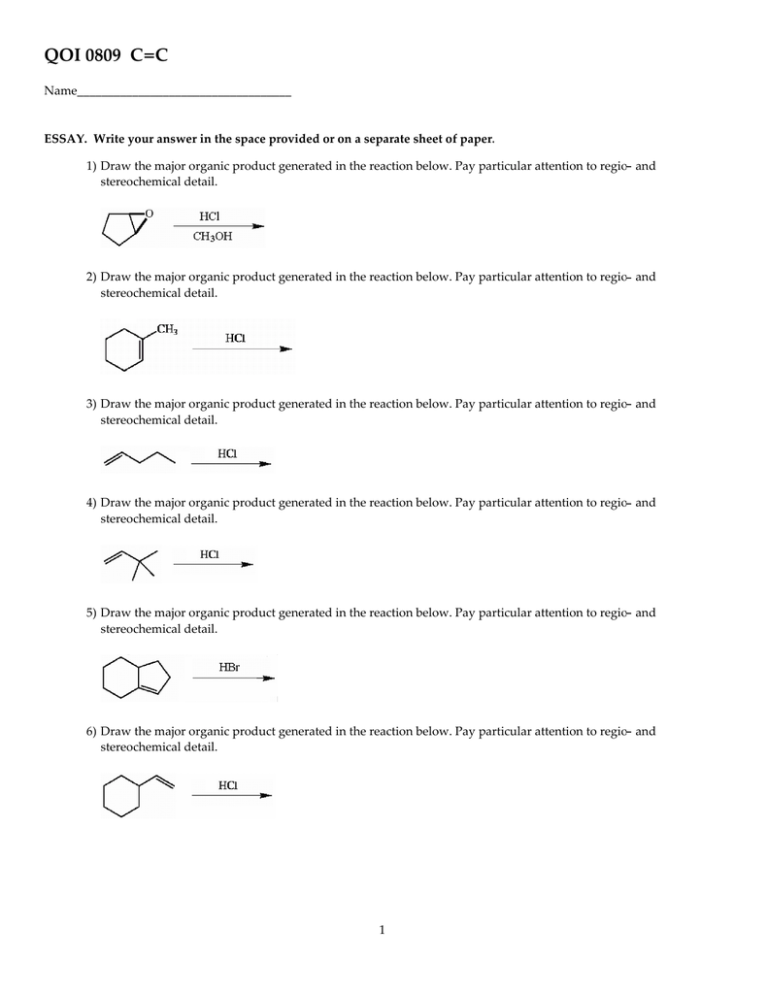
QOI 0809 C=C Name___________________________________ ESSAY. Write your answer in the space provided or on a separate sheet of paper. 1) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 2) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 3) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 4) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 5) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 6) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 1 7) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 8) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 9) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 10) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 11) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 12) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 13) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 2 14) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 15) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 16) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 17) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. (Z)-3-hexene 1. BH3 ·THF → 2. H2 O2 , -OH 18) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 19) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 20) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 3 21) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 22) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 23) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 24) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 25) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 26) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 27) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 4 28) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 29) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 30) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 31) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 32) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 33) Humulene is a monocyclic terpene constituent of carnations. When 0.25 mol of humulene is hydrogenated in the presence of platinum catalyst, 0.75 mol of H2 reacts with the humulene to produce a monocyclic hydrocarbon of formula C15H30. What is the molecular formula of humulene? MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 34) Treatment of cyclopentene with peroxybenzoic acid __________. A) gives the same product as treatment of cyclopentene with OsO4 B) results in oxidative cleavage of the ring to produce an acyclic compound C) yields an equimolar mixture of enantiomeric epoxides D) yields a meso epoxide E) none of the above 5 34) ESSAY. Write your answer in the space provided or on a separate sheet of paper. 35) Complete the following reaction and provide a detailed, step-by-step mechanism for the process. 36) Complete the following reaction and provide a detailed, step-by-step mechanism for the process. 37) Complete the following reaction and provide a detailed, step-by-step mechanism for the process. 38) Provide a detailed, step-by-step mechanism for the reaction shown below. 39) Provide a detailed, step-by-step mechanism for the reaction shown below. 40) When isobutylene [CH2C(CH3 )2 ] is treated with BF3, polyisobutylene is formed. Provide a step-by-step mechanism for this polymerization reaction. 41) Provide the reagents necessary to complete the following transformation. 42) Provide the reagents necessary to complete the following transformation. 6 43) Provide the reagents necessary to complete the following transformation. 44) Provide the reagents necessary to complete the following transformation. 45) Provide the reagents necessary to convert 3-methyl-2-butanol to 2-methyl-2-butanol. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 46) Both (E)- and (Z)-hex-3-ene can be subjected to a hydroboration-oxidation sequence. How are the products from these two reactions related to each other? A) The products of the two isomers are not structurally related. B) The products of the two isomers are related as constitutional isomers. C) The (E)- and (Z)-isomers generate the same products in exactly the same amounts. D) The products of the two isomers are related as diastereomers. E) The (E)- and (Z)-isomers generate the same products but in differing amounts. 46) 47) Both (E)- and (Z)-hex-3-ene can be treated with D2 in the presence of a platinum catalyst. How are the products from these two reactions related to each other? A) The (E)- and (Z)-isomers generate the same products but in differing amounts. B) The (E)- and (Z)-isomers generate the same products in exactly the same amounts. C) The products of the two isomers are related as enantiomers. D) The products of the two isomers are related as constitutional isomers. E) The products of the two isomers are related as diastereomers. 47) SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 48) Give the structure of the alkene which would yield the following products upon ozonolysis-reduction. 48) CH3 CH2CH2 CH2 CHO + CH2 O 49) Give the structure of the alkene which would yield the following products upon ozonolysis-reduction. CH3 COCH3 + CH3 CH2CHO 7 49) MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 50) Addition of Br2 to (E)-hex-3-ene produces __________. A) a mixture of enantiomeric dibromides which is optically inactive B) a meso dibromide C) (E)-3,4-dibromo-3-hexene D) (Z)-3,4-dibromo-3-hexene E) a mixture of enantiomeric dibromides which is optically active 50) ESSAY. Write your answer in the space provided or on a separate sheet of paper. 51) The following reaction is known to proceed by a free radical chain mechanism. Suggest a reasonable, step-by-step mechanism for this reaction. CH3 CHCH2 + CHCl3 ROOR CH3 CH2CH2 CCl3 52) Consider how the ICl bond is polarized and predict the product which results when this mixed halogen adds to 1-methylcyclohexene. 53) β-Ocimene is a natural product with a pleasant odor. Based on the information below, deduce the structure of β-ocimene. 54) Based on the relative stabilities of the intermediates involved, explain the basis for Markovinkov's rule in the addition of hydrogen halides to alkenes. 55) Explain the regioselectivity observed in the radical addition of HBr to 2-methylpropene. SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 56) The mechanism for the acid-catalyzed hydration of alkenes is simply the reverse of the mechanism by which alcohols are dehydrated using concentrated acid. This is an illustration of the principle of __________. 56) MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 57) Which of the following is the best reaction sequence to use if one wants to accomplish a Markovnikov addition of water to an alkene with minimal skeletal rearrangement? A) water + dilute acid B) oxymercuration-demercuration C) hydroboration-oxidation D) water + concentrated acid E) none of the above 8 57) ESSAY. Write your answer in the space provided or on a separate sheet of paper. 58) Draw the Lewis structure of dibromocarbene. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 59) Which of the following additions to alkenes occur(s) specifically in an anti fashion? A) addition of H2 B) addition of H2 O in dilute acid C) addition of Br2 D) hydroboration-oxidation E) both A and B 59) 60) Which of the following additions to alkenes occur(s) specifically in an syn fashion? A) addition of H2 B) dihydroxylation using OsO4 , H2 O2 C) hydroboration D) addition of HCl E) A, B, and C 60) 61) HBr can be added to an alkene in the presence of peroxides (ROOR). What function does the peroxide serve in this reaction? A) electrophile B) acid catalyst C) radical chain initiator D) nucleophile E) solvent 61) SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 62) Name the major product which results when HBr is added to 3-ethyl-3-hexene. 62) MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 63) Which of the following intermediates is thought to occur in the mechanism by which alkenes are hydrated in the presence of acid? A) free radical B) carbene C) carbocation D) alkyne E) carbanion SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 64) Name the major alcohol product which results when 3,3-dimethylbut-1-ene is treated with dilute acid. 9 64) 63) MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 65) What synthetic goal is achieved by subjecting an alkene to an oxymercuration-demercuration sequence? A) anti-Markovnikov addition of H2 O wherein skeletal rearrangement is prevented B) Markovnikov addition of H2O wherein skeletal rearrangement is promoted C) anti-Markovnikov addition of H2 O wherein skeletal rearrangement is promoted D) syn-hydroxylation E) Markovnikov addition of H2O wherein skeletal rearrangement is prevented 65) 66) When an alkene is subjected to treatment with Hg(OAc)2 in alcohol followed by reaction with NaBH4 , what new class of compound is formed? A) syn diol B) alkane C) epoxide D) ether E) alkyne 66) 67) Which of the following compounds is (are) appropriate to promote the cationic polymerization of isobutylene? A) BF3 B) NaOH C) H2SO4 D) ROOR E) both H2 SO4 and BF3 67) ESSAY. Write your answer in the space provided or on a separate sheet of paper. 68) Why can methyl acrylate (H2 CCHCO2 CH3 ) be polymerized through anionic polymerization? 69) Name the compound PhCO3 H and give its most common use as a reagent. 70) Provide the structure of the product which results when the alkene below is treated with O3 followed by (CH3 )2S. SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 71) Provide the structure of the major organic product of the reaction below. 10 71) 72) Provide the structure of the major organic product of the reaction below. 72) 73) Provide the structure of the major organic product of the reaction below. 73) 74) Provide the structure of the major organic product of the reaction below. 74) 75) Provide the structure of the major organic product of the reaction below. 75) 76) Provide the structure of the major organic product of the reaction below. 76) 77) Provide the structure of the major organic product of the reaction below. 77) 11 78) Provide the structure of the major organic product of the reaction below. 78) 79) Provide the structure of the major organic product of the reaction below. 79) 80) Provide the structure of the major organic product of the reaction below. 80) 81) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 81) 82) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 82) 83) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 83) 12 84) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 84) 85) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 85) 86) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 86) 87) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 87) 88) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 88) 89) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 89) 13 90) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 90) MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 91) Which of the following alkenes will yield a meso dihalide when reacted with Br2 /CCl4 at room 91) temperature? A) B) C) D) E) both Band D 92) Acid catalyzed hydration (H2 SO4 /water/△) of an unknown compound (A)with a chemical formula C6 H12, yielded a racemic mixture of product C6 H13OH. Which, if any, of the following compounds is/are possible structures for the initial compound (A)? A) compounds 2 and 3 B) compound 2 only C) compound 1 only D) compounds 1 and 3 E) none of the above 14 92) 93) A reaction of an unknown alkene with MCPBA in dichloromethane followed by work-up with H2O/H+ yielded, as the major product, a racemic mixture of (2S, 3S) and (2R, 93) 3R)-3-methylpentan-2,3-diol. What is the specific structure of the alkene used in the reaction? A) (E)-3-methylpent- 2-ene B) 2-methylpent-2-ene C) (Z)-3-methylpent-2-ene D) 2,3-dimethylbut-2-ene E) none of the above SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 94) When 3,6-dimethylcyclohexene is reacted with dry gaseous HBr, one of the products is 1-bromo-1,4-dimethylcyclohexane. Provide a detailed step-by-step mechanism to explain the formation of this product 94) MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 95) Treatment of 2-methylpropene with which of the following reaction conditions results in an anti-Markovnikov addition product? A) dry gaseous HBr with peroxides present B) BH3-THF, followed by alkaline H2 O2 C) aqueous Hg(OAc)2 , followed by alkaline NaBH4 D) dilute H2 SO4 and heat E) both A and B 15 95) 96) An unknown compound with empirical formula C3 H5 was treated with Br2 /CCl4. The bromine solution went from orangish/red to clear immediately at room temperature. Upon treatment with O3 followed by work-up with dimethylsulfide the following products were identified. From the information provided what is/are the most likely structure(s) for this unknown compound. A) B) C) D) E) both A and D 16 96) Answer Key Testname: UNTITLED1 1) ID: oc6w 8-1 Diff: 3 2) ID: oc6w 8-2 Diff: 1 3) ID: oc6w 8-3 Diff: 1 4) ID: oc6w 8-4 Diff: 3 5) ID: oc6w 8-5 Diff: 2 6) ID: oc6w 8-6 Diff: 3 7) ID: oc6w 8-7 Diff: 2 17 Answer Key Testname: UNTITLED1 8) ID: oc6w 8-8 Diff: 2 9) ID: oc6w 8-9 Diff: 2 10) ID: oc6w 8-10 Diff: 1 11) ID: oc6w 8-11 Diff: 3 12) ID: oc6w 8-12 Diff: 2 13) ID: oc6w 8-13 Diff: 2 14) ID: oc6w 8-14 Diff: 2 18 Answer Key Testname: UNTITLED1 15) ID: oc6w 8-15 Diff: 3 16) ID: oc6w 8-16 Diff: 3 17) ID: oc6w 8-17 Diff: 3 18) ID: oc6w 8-18 Diff: 2 19) ID: oc6w 8-19 Diff: 1 20) ID: oc6w 8-20 Diff: 3 21) ID: oc6w 8-21 Diff: 3 22) ID: oc6w 8-22 Diff: 1 19 Answer Key Testname: UNTITLED1 23) ID: oc6w 8-23 Diff: 3 24) ID: oc6w 8-24 Diff: 3 25) ID: oc6w 8-25 Diff: 3 26) ID: oc6w 8-26 Diff: 3 27) ID: oc6w 8-27 Diff: 2 28) ID: oc6w 8-28 Diff: 2 29) ID: oc6w 8-29 Diff: 3 20 Answer Key Testname: UNTITLED1 30) ID: oc6w 8-30 Diff: 3 31) ID: oc6w 8-31 Diff: 2 32) ID: oc6w 8-32 Diff: 2 33) C15H24 ID: oc6w 8-33 Diff: 2 34) D ID: oc6w 8-34 Diff: 3 35) ID: oc6w 8-35 Diff: 1 36) ID: oc6w 8-36 Diff: 2 37) ID: oc6w 8-37 Diff: 2 21 Answer Key Testname: UNTITLED1 38) ID: oc6w 8-38 Diff: 2 39) ID: oc6w 8-39 Diff: 3 40) ID: oc6w 8-40 Diff: 2 41) 1. Br2 , hν 2. H2 O, Δ or 1. Br2 , hν 2. NaOCH3 , CH3 OH 3. H3 O+ or Hg(OAc)2 , H2 O; NaBH4 ID: oc6w 8-41 Diff: 3 42) 1. NaOCH3 , CH3 OH 2. OsO4, H2O2 or cold, dilute KMnO4 , - OH ID: oc6w 8-42 Diff: 2 43) 1. NaOCH3 , CH3 OH 2. MCPBA or CH3 CO3H 3. H3 O+ or -OH ID: oc6w 8-43 Diff: 3 22 Answer Key Testname: UNTITLED1 44) 1. Br2 , hν 2. NaOCH3 , CH3 OH 3. CH2 I2 , Zn(Cu) ID: oc6w 8-44 Diff: 3 45) 1. conc. H2 SO4 2. H3 O+ or Hg(OAc)2, H2O or 1. PBr3 2. NaOCH3 , CH3 OH 3. NaBH4 ID: oc6w 8-45 Diff: 2 46) C ID: oc6w 8-46 Diff: 2 47) E ID: oc6w 8-47 Diff: 2 48) CH3 CH2CH2 CH2 CHCH2 ID: oc6w 8-48 Diff: 1 49) (CH3 )2CCHCH2 CH3 ID: oc6w 8-49 Diff: 1 50) B ID: oc6w 8-50 Diff: 2 51) ROOR → 2 RO· RO· + HCCl3 → ROH + ·CCl3 Cl3 C· + CH2 CHCH3 → Cl3 CCH2 CH CH3 · Cl3 CCH2 CH CH3 + HCCl3 → Cl3 CCH2 CH2 CH3 + ·CCl3 · ID: oc6w 8-51 Diff: 3 52) ID: oc6w 8-52 Diff: 3 23 Answer Key Testname: UNTITLED1 53) ID: oc6w 8-53 Diff: 2 54) The rate-determining step in this reaction is the production of a carbocation intermediate. Since this step is endothermic, Hammond's postulate allows one to gauge the relative stabilites of the transition states by comparing the relative stabilities of the carbocation intermediates. The reaction pathway which produces the more substituted carbocation will thus occur more rapidly. ID: oc6w 8-54 Diff: 2 55) The reaction proceeds via the addition of Br· to the alkene. Two competing pathways are possible, but the transition state leading to the more substituted alkyl radical is lower in energy. This process ultimately makes the addition anti-Markovnikov in nature. ID: oc6w 8-55 Diff: 3 56) microscopic reversibility ID: oc6w 8-56 Diff: 1 57) B ID: oc6w 8-57 Diff: 1 58) ID: oc6w 8-58 Diff: 3 59) C ID: oc6w 8-59 Diff: 1 60) E ID: oc6w 8-60 Diff: 1 61) C ID: oc6w 8-61 Diff: 2 62) 3-bromo-3-ethylhexane ID: oc6w 8-62 Diff: 2 63) C ID: oc6w 8-63 Diff: 2 64) 2,3-dimethyl-2-butanol ID: oc6w 8-64 Diff: 2 65) E ID: oc6w 8-65 Diff: 2 24 Answer Key Testname: UNTITLED1 66) D ID: oc6w 8-66 Diff: 2 67) E ID: oc6w 8-67 Diff: 2 68) The intermediate carbanion and the transition state leading to it are stabilized by the electron-withdrawing capacity of the carbonyl group. ID: oc6w 8-68 Diff: 2 69) peroxybenzoic acid; an oxidizing agent which converts alkenes to epoxides ID: oc6w 8-69 Diff: 1 70) ID: oc6w 8-70 Diff: 3 71) ID: oc6w 8-71 Diff: 1 72) ID: oc6w 8-72 Diff: 2 25 Answer Key Testname: UNTITLED1 73) ID: oc6w 8-73 Diff: 3 74) ID: oc6w 8-74 Diff: 3 75) ID: oc6w 8-75 Diff: 2 76) ID: oc6w 8-76 Diff: 2 77) ID: oc6w 8-77 Diff: 2 26 Answer Key Testname: UNTITLED1 78) ID: oc6w 8-78 Diff: 3 79) ID: oc6w 8-79 Diff: 2 80) ID: oc6w 8-80 Diff: 3 81) ID: oc6w 8-81 Diff: 3 82) ID: oc6w 8-82 Diff: 3 27 Answer Key Testname: UNTITLED1 83) ID: oc6w 8-83 Diff: 3 84) ID: oc6w 8-84 Diff: 3 85) ID: oc6w 8-85 Diff: 3 86) ID: oc6w 8-86 Diff: 3 87) ID: oc6w 8-87 Diff: 3 28 Answer Key Testname: UNTITLED1 88) ID: oc6w 8-88 Diff: 3 89) ID: oc6w 8-89 Diff: 3 90) ID: oc6w 8-90 Diff: 3 91) B ID: oc6w 8-91 Diff: 2 92) E ID: oc6w 8-92 Diff: 2 93) C ID: oc6w 8-93 Diff: 3 94) ID: oc6w 8-94 Diff: 3 29 Answer Key Testname: UNTITLED1 95) E ID: oc6w 8-95 Diff: 1 96) A ID: oc6w 8-96 Diff: 2 30