Polar Covalent Bonds: Electronegativity
advertisement
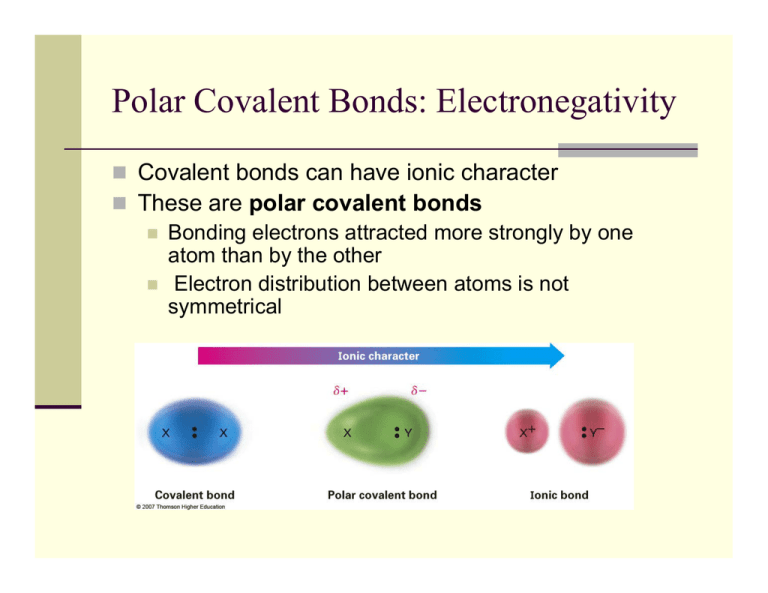
Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character These are polar covalent bonds Bonding electrons attracted more strongly by one atom than by the other Electron distribution between atoms is not symmetrical Bond Polarity and Electronegativity Symmetrical Covalent Bonds C–C C–H Polar Covalent Bonds + C–O (non-polar) (polar) Electronegativity (EN): intrinsic ability of an atom to attract the shared electrons in a covalent bond Inductive Effect: shifting of sigma bonded electrons p to nearby y electronegative g atom in response The Periodic Table and Electronegativity C–H ( (non-polar) l ) C - Br and C - I ((polar) l ) Bond Polarity and Inductive Effect Nonpolar p Covalent Bonds: atoms with similar EN Polar Covalent Bonds: Difference in EN of atoms < 2 Ionic Bonds: Difference in EN > 2 C–H bonds, relatively nonpolar C-O, C-X bonds (more electronegative elements) are polar Bonding electrons shift toward electronegative atom C acquires q p partial p positive charge, g , + Electronegative atom acquires partial negative charge, Inductive effect: shifting of electrons in a bond in response to EN of nearby atoms Electrostatic Potential Maps Electrostatic potential p maps show calculated charge distributions Colors indicate electronrich (red) and electronpoor (blue) (bl ) regions i Arrows indicate direction of bond polarity Polar Covalent Bonds: Net Dipole Moments Molecules as a whole are often polar from vector summation of individual bond polarities and lone lone-pair pair contributions Strongly polar substances soluble in polar solvents like water; nonpolar substances are insoluble in water. Dipole moment () - Net molecular polarity polarity, due to difference in summed charges - magnitude of charge Q at end of molecular dipole times distance r between charges = Q r, in debyes (D), 1 D = 3.336 1030 coulomb meter length of an average covalent bond, the dipole moment would be 1.60 1029 Cm, or 4.80 D. Absence of Net Dipole Moments In symmetrical y molecules,, the dipole p moments of each bond has one in the opposite direction The effects of the local dipoles cancel each other Drawing Lewis Structures Draw molecular skeleton: this will come with practice, remember atom valence Determine number of available valence electrons: add an electron for each negative charge, remove an electron for each positive charge Draw all single covalent bonds and lone pairs: give as many atoms as possible full octets, assigning lone pairs to most electronegative atoms Convert lone pairs to multiple bonds if needed: to satisfy octet rule for as many atoms as possible Assign formal charges to all atoms: the sum of all charges must equal total charge of the molecule Example: Nitromethane: CH3NO2 H Step 1&2 H C O N O H •• H H C •• O •• H C H O N •• •• O •• Step 5…………… N •• H O •• •• H •• Step 3&4 Valence E = 3(1) + 1(4) + 1(5) + 2(6) = 24 e- •• Formal Charges Sometimes it is necessary to have structures with formal charges on individual i di id l atoms t We compare the bonding of the atom in the molecule to the valence electron structure If the atom has one more electron in the molecule, it is shown with a “-” charge If the atom has one less electron, it is shown with a “+” charge Neutral molecules with both a “+” and a “-” are dipolar • Atomic sulfur has 6 valence electrons. Dimethyl suloxide sulfur has only 5. • It has lost an electron and has positive charge. • Oxygen atom in DMSO has gained electron and has (-) charge. Common Formal Charges charges are typically shown (not necessarily the lone pairs) add necessary lone pairs and charges to the following compounds: Resonance Some molecules have structures that cannot be shown with a single i l representation i In these cases we draw structures that contribute to the final structure but which differ in the position of the bond(s) or lone pair(s) Such a structure is delocalized and is represented by resonance forms The resonance forms are connected by a double-headed arrow We say the pi bond is delocalized, and valence bond theory cannot describe where Pi bond and one lone pair are located. This is why we must draw multiple versions And use molecular orbital theory to show delocalization Curved Arrows and Resonance Forms We can imagine g that electrons move in p pairs to convert from one resonance form to another A curved arrow shows that a pair of electrons moves from the atom or bond at the tail of the arrow to the atom or bond at the head of the arrow Drawing Resonance Forms Redraw all atoms and sigma g bonds in same p position Only move pi electrons and lone pair electrons (note that electrons must remain with same atom) using curved arrows to illustrate All resonance forms must have same net charge Never violate the octet rule for 2nd row atoms Resonance forms may be equivalent (degenerate) but do not have to be Not all resonance forms contribute equally to the overall resonance hybrid There are two additional resonance structures for nitromethane can you draw them??? nitromethane, Guidelines to determine major resonance contributor ib 1. Structures with maximum octets are preferred N O N O C major minor O C O major minor 2. Charges should be located on atoms with compatible electronegativity H H C N N major H C H N N minor 3. Structures with minimum charge separation are preferred O H C O O major H H C O minor H Resonance Hybrids A structure with resonance forms does not alternate between the h fforms (i (individual di id l resonance fforms are iimaginary) i ) Instead, it is a hybrid of all resonance forms, so the structure is called a resonance hybrid For F example, l b benzene (C6H6) h has ttwo resonance fforms with ith alternating double and single bonds In the resonance hybrid, the actual structure, all its C-C bonds equivalent, equivalent midway between double and single Different Atoms in Resonance Forms O O - - Are any resonance structures degenerate? What is major resonance contributor? Can you draw any more resonance structures? Formal Charge Problems Provide formal charges for all heteroatoms (non carbon atoms) atoms). Remember, all H's on heteroatoms must be (and are) drawn in structure. H N O H O N H need to determine number of valence electrons to know how many lone pairs to add For each compound below, provide all lone pairs and hydrogens required to be consistent with the given formal charges (again hydrogens are provided on heteroatoms along with any necessary charges). O O H O Resonance/Skeletal Problems O O O O N N N H Resonance Practice Problems 1. Draw the important resonance structures for each of the following molecules. O H 3C O CH2 O N H 3C O Br CH3 OCH 3 N CH3 O CH3 O Put in answer keys to the previous slides also put in slide about hydridization in resonance forms like pyrrole if sp3 in one, sp2 in another must have sp2 hybrid y for p orbital and not move other atoms Brønsted Acids and Bases A Brønsted acid is a substance that donates a hydrogen ion (H+) A Brønsted ø base is a substance that accepts p the H+ Acids are shown in red, bases in blue. Curved arrows go from bases to acids Ka – the Acidity Constant The concentration of water as a solvent does not change significantly when it is protonated The molecular weight of H2O is 18 and one liter weighs 1000 grams, so the concentration is ~ 55.6 M at 25° The acidity y constant,, Ka for HA Ke times 55.6 M ((leaving g [water] out of the expression) Ka ranges from 1015 for the strongest acids to very small values (10-60) for the weakest pKa – the Acid Strength Scale p pKa = -log g Ka The free energy in an equilibrium is related to –log of Keq (G = -RT log Keq) A smaller value of pKa indicates a stronger acid and is proportional to the energy difference between products and reactants The Th pK Ka off water t is i 15.74 15 74 Trends in Acidity: What makes a strong acid? id 1. St 1 Strength th off th the H-A H Ab bond d The stronger this bond is, the less likely it will break to “give-up” an H+. For strong acids, this is a relatively weak bond. Important factor when comparing p g bonds between hydrogen y g and atoms within the same column (not row) of the periodic table Why is HI a weaker bond than the others? pKa = H-II H -10 > H-Br H Br -9 > H-Cl H Cl -7 > H H-F F 3.2 Trends in Acidity: What makes a strong acid? id 2. Stability of Conjugate Base, 2 Base AIf the resulting anion from the dissociation is stabilized (A-), it will be easier to form resulting in a stronger acid. Two major influences operate here: A: Effect of Electronegativities. If the hydrogen is connected to a very electronegative atom the bond is polarized and the eventual breaking of the bond to form A-, a stable anion since A is very electronegative, will become easier and result in a stronger acid (unless you are comparing atoms in the same column of the periodic table as in the above example where you must consider bond strength). pKa = H-F H F 3.2 > H-OH H OH 15.7 > H-NH H NH2 38 > H-CH H CH3 49 Trends in Acidity: What makes a strong acid? id B: Resonance Effects Effects. Delocalization of charge (through resonance showing the negative charge is located on more than one atom) will stabilize the anion, making it easier to form and result in a stronger acid. O H3C C O O H H3C C O O H3C pKa = 5 H3 C O H pKa = 16 H2O versus HNO3 H3 C O C O Trends in Acidity: What makes a strong acid? id 3. Inductive Effects 3 Electronegative groups can inductively “pull” electron density towards themselves. This can result in a net stabilization of the conjugate base, A-. This effect is much weaker than that of resonance noted above. An inductive effect rapidly decreases with distance. CF3O O-H H pKa = 10 > CH2FO FO-H H 12 > CH2FCH2O O-H H 13 Some Common Organic Acids and their pKa’s Functional Group approx pKa Functional Group approx pKa pKa Table: Acidity and Basicity Conjugate Base Acid Strong Base CH4 49 CH3 NH3 36 NH2 H C C H 25 H C C H3C 16 H3 C Weak Acid OH H2 O 15 7 15.7 H3C SH 11 O OH H3 C S Increasing Nucleophilicty NH4 9.2 NH3 H C N 91 9.1 C N H2S 7.0 SH H3 C ** OH 4.8 O 4.7 N N N H-F 3.2 F H3O -1.7 O S OH O -6.5 p-toluene sulfonic acid Strong Acid H3C H N N N H3 C This chart will be VERY useful for sub-elim sub elim chemistry O O H2O O S O O H3 C p-toluene sulfonate or Tosylate H-Cl C -7.0 0 Cl C H-Br -9.0 Br H-I -10.0 I ** Increasing Leaving Group Capabilities Weak Base Predicting pKa Values Identify most acidic hydrogen and rank from most acidic (1) to least acidic (4) (rank by functional groups first, then compare like functional groups) The reactivity patterns of organic compounds often are acid-base combinations Predicting Acid–Base Reactions from pKa Values l For any given reaction involving an acid and a base, the equilibrium will be established such that it favors the weaker acid and weaker base: H-A acid + B base A + B-H conj base conj acid A new equilibrium expression can be written for this reaction which simplifies to the following: g Ka reactant acid (HA) K= = 10 -(pKa HA - pKa HB) Ka product acid (HB) A simple inspection of this new equilibrium expression illustrates that if the reactant acid has a pKa value 2 units lower than the product conjugate acid then the equilibrium constant will be 102, or 100. This means that, as the reaction is written, the concentration of products to reactants will be approximately 100:1. Another way of stating this is that the reaction has gone to 99% completion (100/101). Predicting Acid–Base Reactions from pKa Values l Which side of the following g reaction is favored? What is K for this reaction? Does the reaction proceed from left to right? D Draw iin th the curved d arrows tto show h electron l t flflow. Acids and Bases: The Lewis Definition Lewis acids are electron pair acceptors (have + or + charge, charge or unfilled octet) H+, Li+, AlCl3, CH3O-H Lewis bases are electron pair donors (have lone pairs to donate) H-O-H, CH3-O-H, CH3-NH2 no scale l off strengths t th as in i B Brønsted t d definition d fi iti off pK Ka Illustration of Curved Arrows in Following L i Acid-Base Lewis A id B Reactions R i The combination of a Lewis acid and a Lewis base can be shown with a curved arrow from f base b to t acid id Lewis Acids Lewis Bases Lewis bases can accept protons as well as Lewis acids, therefore the definition encompasses that for Brønsted bases Most oxygen- and nitrogen-containing organic compounds are Lewis bases because they have lone pairs of electrons Some compounds p can act as both acids and bases,, depending p g on the reaction C=C -bond will also act as a weak Lewis Base (chapter 6 & 7) R-X not a Lewis base, but X-1 is Predicting Reactions Identifyy Lewis acids,, Lewis bases,, predict p product, p , add curved arrows O N + O H O AlCl3 + O H Sample Problems 1 D 1. Draw ttwo additional dditi l resonance structures t t with ith curved d arrows and d id identify tif major resonance contributor 2. Predict product if Bronsted-Lowry acid-base reaction occurs. Provide curved arrows. Which side is fa favored? ored? 3. Predict Lewis acid-base product, with curved arrows, for the following reaction.