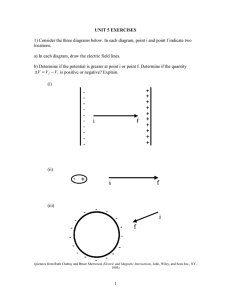
Three-dimensional structure of the shell plate assembly of the
advertisement

Journal of Structural Biology xxx (2012) xxx–xxx Contents lists available at SciVerse ScienceDirect Journal of Structural Biology journal homepage: www.elsevier.com/locate/yjsbi Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences Matthew J. Connors a, Hermann Ehrlich b, Martin Hog b, Clemence Godeffroy b, Sergio Araya c, Ilan Kallai d, Dan Gazit d,e, Mary Boyce f, Christine Ortiz a,⇑ a Department of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA Institute of Bioanalytical Chemistry, Dresden University of Technology, 01069 Dresden, Germany Department of Architecture, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA d Skeletal Biotech Laboratory, The Hebrew University – Hadassah Faculty of Dental Medicine, Ein Kerem, Jerusalem 91120, Israel e Department of Surgery and Cedars-Sinai Regenerative Medicine Institute (CS-RMI), Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA f Department of Mechanical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA b c a r t i c l e i n f o Article history: Received 2 September 2011 Received in revised form 12 December 2011 Accepted 14 December 2011 Available online xxxx Keywords: Chiton Shell microstructure Exoskeleton Natural armor Micro-computed tomography a b s t r a c t This study investigates the three-dimensional structure of the eight plate exoskeletal (shell) assembly of the chiton Tonicella marmorea. X-ray micro-computed tomography and 3D printing elucidate the mechanism of conformational change from a passive (slightly curved, attached to surface) to a defensive (rolled, detached from surface) state of the plate assembly. The passive and defensive conformations exhibited differences in longitudinal curvature index (0.43 vs. 0.70), average plate-to-plate overlap (62% vs. 48%), cross-sectional overlap heterogeneity (60–82.5% vs. 0–90%, fourth plate), and plateto-plate separation distance (100% increase in normalized separation distance between plates 4 and 5), respectively. The plate-to-plate interconnections consist of two rigid plates joined by a compliant, actuating muscle, analogous to a geometrically structured shear lap joint. This work provides an understanding of how T. marmorea achieves the balance between mobility and protection. In the passive state, the morphometry of the plates and plate-to-plate interconnections results in an approximately continuous curvature and constant armor thickness, resulting in limited mobility but maximum protection. In the defensive state, the underlying soft tissues gain protection and the chiton gains mobility through tidal flow, but regions of vulnerability open dorsally, due to the increase in plate-to-plate separation and decrease in plate-to-plate overlap. Lastly, experiments using optical and scanning electron microscopy, mercury porosimetry, and Fourier-transform infrared spectroscopy explore the microstructure and spatial distribution of the six layers within the intermediate plates, the role of multilayering in resisting predatory attacks, and the detection of chitin as a major component of the intra-plate organic matrix and girdle. Ó 2012 Elsevier Inc. All rights reserved. 1. Introduction Chitons (Polyplacophorans) are marine mollusks found worldwide in all seas with more than 940 extant (Schwabe, 2005; Stebbins and Eernisse, 2009) and 430 fossil species (Puchalski et al., 2008) which extend to the late Cambrian (Runnegar et al., 1979). Chitons are one of the earliest diverging groups of living mollusks and are often referred to as ‘‘living fossils’’ since their body plan has not significantly changed for over 300 million years (Sigwart, 2009). They are typically oval in shape, bilaterally ⇑ Corresponding author. Department of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Room 13-4022, Cambridge, MA 02139, USA. Fax: +1 617 253 5620. E-mail address: cortiz@mit.edu (C. Ortiz). symmetric, and generally range from a few mm to 15 cm in length (Eernisse and Reynolds, 1994). Chitons are of great interest from a biomechanical perspective because instead of a single continuous shell, they possess an assembly of eight dorsal aragonite-based plates (valves) (Fig. 1a). The first (head) and eighth (tail) plates are semicircular in outline while the intermediate plates are ‘‘butterfly’’-shaped. These plates provide protection while still allowing for some degree of flexibility during locomotion over uneven, rough surfaces as well as when rolling defensively into a ball-like conformation (Fig. 1b) if dislodged from a surface. In many species, the head plate nearly touches the tail plate in the rolled state (Vermeij, 1993). This defensive conformation covers and protects the ventral side of the chiton from imminent predation, and may allow it to be carried out of harm’s way by a passing wave to a more favorable location to right itself (Arey and Crozier, 1919; 1047-8477/$ - see front matter Ó 2012 Elsevier Inc. All rights reserved. doi:10.1016/j.jsb.2011.12.019 Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 2 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx (a) 4 5 6 3 2 7 1 8 Girdle Posterior Anterior 0.5 cm x • z y (b) Girdle Fig.1. Images of Tonicella marmorea; (a) side view optical microscopy image of a dried shell; (b) side view photograph of a recently thawed chiton in a defensive, curved posture. Eernisse, 2007). The curvature of the plate assembly is most often convex, although less frequent concave curvatures have been observed. Convex flexure is provided by the ‘‘enrolling’’ muscle, which encircles the shell underneath the outer margin of the plates (Haszprunar and Wanninger, 2000). Plates 2–8 possess two anterior projections, the apophyses (sutural laminae), which overlap with the ventral surface of the adjacent anterior plate. Transverse muscles lie in the overlapping region between neighboring plates and connect them together (Wingstrand, 1985). Also extending between the shell plates is a thin cuticular layer (Beedham and Trueman, 1967), which laterally attaches to a thick chitinous cuticle that covers a leathery girdle structure surrounding the plates. Resistance to penetration is provided by the internal, multilayered microstructure of the individual exoskeletal units (Bruet et al., 2008), in this case the chiton plates, which are known to possess one of the most complex multilayered structures of any mollusk (Bøggild, 1930). The multilayered structure of the plates have been studied by a number of authors including Haas (1972, 1976), Laghi and Russo (1978, 1980), Baxter and Jones (1981), Kreusch (1981), Poulicek and Kreusch (1983), Carter and Hall (1990), Currie (1992), and Chen (2010). In addition to the periostracum, an outer thin organic layer that is often eroded away on older shells, chiton shell plates are generally composed of seven aragonite-based layers as follows (described from outer to inner): 1) Tegmentum: a granular or irregular simple prismatic layer which is penetrated by the aesthetes, an intricate network of tissue-filled channels that open on each plates’ dorsal surface as sensory organs (Vendrasco et al., 2008). 2) Dorsal Mesostracum: a thin simple crossed layer which may be divideddorsoventrallyinto two sub-layers by a row of aesthete channels. 3) Articulamentum: a composite prismatic layer that constitutes the bulk of the apophyses and insertion plates. 4) Ventral Mesostracum: a simple crossed-lamellar layer. The dorsal and ventral mesostracum layers are collectively referred to the as the Pallial Myostracum. 5) Anterior Myostracum: a granular or irregular simple prismatic layer which stems from the impressions of the oblique and post-apophysial latero-pedal muscles. 6) Posterior Myostracum: a granular or irregular simple prismatic layer which lies in the impression of the transverse muscle. 7) Hypostracum: a simple crossed-lamellar layer that constitutes the bulk of the central callus. The objectives of this study were to (1) to quantify the threedimensional geometric structure of individual plates, as well as of the entire plate assemblies of chitons in passive (slightly curved, attached to surface) and defensive (rolled, detached from surface) conformations, (2) to determine the spatial distribution of microstructures present in intermediate plates and microstructural orientations relative to macroscopic plate dimensions, (3) to elucidate relationships between the internal multilayering and microstructures of the internal plates (‘‘inherent materiality’’) and the larger length scale geometric design to better understand the balance between threat protection and biomechanical mobility, and (4) to gain insights into the functional and physiological consequences related to survivability of the segmented exoskeleton (structurally, not biologically). To accomplish these goals, the chiton Tonicella marmorea was analyzed by X-ray micro-computed tomography (microCT) to quantify biomechanically-relevant features such as the three-dimensional geometry of the individual plates, the inter-plate registration and overlap, and the curvature and continuity of the entire plate assemblies. Three-dimensional printing was employed to create a scaled-up macroscopic synthetic prototype directly from the microCT data to elucidate the detailed plate morphometry and to better visualize the inter-plate articulation. The intra-plate microstructure was characterized via mercury porosimetry, optical microscopy, scanning electron microscopy (SEM), and X-ray diffraction (XRD). Fourier-transform infrared spectroscopy (FTIR) and amino acid analysis (AAA) were used to identify and compare the composition of organic materials from the girdle, shell plates, and inter-plate region. The obtained results are discussed in the context of other species with articulating natural armor, in particular the balance between the local protection mechanisms of the individual plates and the larger length scale design principles of the plate-to-plate interconnections. The new scientific information obtained holds potential for comparative morphometric analyses of chitons, as well as for the development of improved bio-inspired protective materials, for example with regards to human extremity protection (Arciszewski and Cornell, 2006; Bruet et al., 2008; Ortiz and Boyce, 2008; Song et al., 2010; Yao et al., 2010). 2. Materials and methods 2.1. Samples and terminology Chitons were collected on the coast of Pembroke, Maine and stored frozen until experimentation. When needed, individual plates were separated using forceps and razor blades. Plates were cleaned by sonication (Branson 1510, Danbury, CT) in deionized water twice for approximately 45 s. Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx Species were identified as T. marmorea and distinguished from Tonicella rubra by the height/width aspect ratio of the fourth plate (0.44 vs. 0.29), and appearance of the girdle (leathery to the naked eye vs. densely clothed with club-shaped, calcareous corpuscles), respectively (Kaas and Van Belle, 1985). Macroscopic morphological chiton terminology used was defined and/or standardized by Schwabe (2010) while chiton shell plate layer nomenclature used was derived from Bergenhayn (1930) and Laghi and Russo (1978, 1980). Molluscan microstructure terminology used was defined by Taylor and Layman (1972) and Carter and Clark (1985). 3 2.4. 3D-printing A scaled-up macroscopic prototype of the chiton shell plate assembly was fabricated using a three-dimensional printer (3DP, ZPrinterÒ 310 Plus, ZCorporation, USA) using the stereolithography (STL) files that were created from the micro-computed tomography data. The material used was a plaster powder (ZPÒ 131 powder, ZCorporation, USA). Eighty-nine micrometer thick layers were laid down using a commercially available binder (ZCorporation, USA) at a vertical build speed of 25 mm/h. After printing, the prototype was immersed in a wax bath to ensure a smooth surface. 2.2. Micro-computed tomography (microCT) 2.5. Optical and scanning electron microscopy (SEM) The complete shells (plates 1–8 and girdle) of T. marmorea were scanned with a micro-computed tomography system (lCT40, Scanco Medical AG, Switzerland) operated at 70 kV and 114 lA. Microtomographic slices were recorded every 10 lm and were reconstructed with 10 10 lm voxels in plane. For the chiton presented in Fig. 1a, the 3D information of the plate assembly was partitioned into contributions from each individual plate using the threshold and contour functions of Scanco MicroCT Software. For the chiton presented in Fig. 1b, the 3D information of the plate assembly was segmented using the threshold and region growth functions of image processing software package Mimics (Materialise, Belgium). Mimics was also used to create all three-dimensional images, including those with transparency effects. The threedimensional geometric information of the plate assemblies was converted into three-dimensional polygonal meshes (stereo lithography) using Scanco MicroCT Software for the chiton presented in Fig. 1a or Mimics for the chiton presented in Fig. 1b. Meshes were reduced in size as needed using the smooth, reduce, and remesh tools of 3-matic (Materialise, Belgium). Cross-sectional images were created from the surface meshes using the cut function of the simulation module of Mimics. Open source image analysis software ImageJ (Rasband, 1997–2011) was used to measure the dimensions of cross-sectional images. The spatial distribution of thickness of each individual plate and of the entire plate assembly (Fig. 3a and b, respectively) of the chiton presented in Fig. 1a was calculated using a spherical method in which each point in 3D space is assigned a thickness value corresponding to the diameter of the largest sphere centered on that point which can fit within the boundaries of the plate (Bouxsein et al., 2010). 2.3. Calculations of plate-to-plate overlap and curvature In Fig. 2f, overlap was quantized using aerial projections (in the ‘‘y’’–‘‘z’’ plane) of the plates and plate-to-plate overlapping regions of Fig. 2d and e. For each intermediate plate, total overlap percentage was calculated by summing the projected areas of the two overlapping regions, and expressing the sum as a percentage of the projected area of the entire plate which encompasses them. For the first and eighth plates, total overlap percentage was calculated by expressing the projected area of the single overlapping region (the overlapping region of plates 1 and 2, and plates 7 and 8, respectively) as a percentage of the projected area of the respective plate. In Fig. 5c, overlap was calculated by projecting the overlapping regions of plates 3 and 5 onto the long axis (‘‘l’’) of plate 4, summing the projected lengths, and expressing the sum as a percentage of the cross-sectional length of plate 4. The transverse radii of curvature of individual plates and the longitudinal radii of curvature of the plate assemblies were calculated using open source ‘‘Circle Fit (Pratt Method)’’ MatLab (MathWorks, MA) code (http://www.mathworks.com/matlabcentral/fileexchange/22643) written by Nikolai Chernov based on an algorithm developed by Pratt (1987). For cross-sectional imaging, plates were embedded in a fast cure epoxy (Loctite, USA) and sectioned with a diamond impregnated saw (Buehler Isomet 5000, Lake Bluff, IL) at 875 rpm. Embedded samples were polished stepwise with aluminum oxide pads with roughness varying from 9 to 0.1 lm (South Bay Technologies, CA) and then with 500 nm silica nanoparticles on microcloth (Buehler, IL). Some samples were slightly etched with 0.5 M EDTA for 5 min to improve contrast between microstructurally distinct layers. Additionally, some plates were cryofractured by immersing them in liquid nitrogen for 5 min and then breaking them with a hammer. Optical images were taken with a Nikon ECLIPSE LV100 microscope (Tokyo, Japan). Cross-sectional SEM samples were fixed on a steel support using a conductive tape and then sputter-coated with 5 nm of gold–palladium in a Denton Vacuum Desk II (Moorestown, NJ). A JEOL JSM 6700 (Peabody, MA) scanning electron microscope was used for imaging at an acceleration voltage 10 kV. Demineralized SEM samples were secured in a holder, covered with carbon for 1 min using an Edwards S150B sputter coater (West Sussex, United Kingdom) and imaged with an ESEM XL 30 Philips (Amsterdam, Netherlands) or LEO DSM 982 Gemini (Oberkochen, Germany) scanning electron microscope. 2.6. Thermogravimetric analysis (TGA) Plates were lightly ground with a pestle and mortar. Samples were then vacuum dried at 110 °C overnight to remove residual water. TGA was carried out from 110 to 500 °C at 2.5 °C/min on a TA Instruments TGA Q50 (New Castle, DE). Weight loss due to combustion of organic material was attributed to the temperature range 110–475 °C (Zaremba et al., 1998; Neves and Mano, 2005). 2.7. Mercury porosimetry The volume percent porosity of three plates (2–4) was measured by a mercury porosimeter (Autopore IV 9500, Micromeritics, USA), which operated at pressures between 3.7 kPa and 14 MPa, corresponding to pore diameters of 404 and 0.107 lm, respectively. A standard contact angle of 140°, which was used previously by Gane et al. (2004) to study the porosity of calcium carbonate structures, was assumed in the pore size calculations. 2.8. X-ray diffraction (XRD) The mineral phase of the plates was verified using XRD (Philips PANalytical X’ Pert PRO diffractometer with CuKa radiation, Netherlands), operating at 45 kV and 40 mA between 10° and 70° (2h). 2.9. Chitin isolation and staining The organic matrix of chiton plates was isolated by decalcification using 3 M HCl solution as well as a 7.4 pH Osteosoft™ (Merck) Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 4 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx Dorsal View (a) Anterior (b) Ventral View Slits T1 z • x z Posterior y y x x x L1 (c) z (e) y x z • y T1 (d) Dorsal x 0.5 cm z • y Ventral Posterior Anterior (f) 35 100 Posterior 80 25 60 20 15 40 10 20 5 0 0 1 2 3 4 5 6 7 Percentage Overlap Projected Area (mm2) 30 Anterior Plate Area (Fig. 2d) Plate Area (Fig. 2e) Plate-Plate Overlap Area (Fig. 2d) Plate-Plate Overlap Area (Fig. 2e) % Total Overlap (Fig. 2d) % Total Overlap (Fig. 2e) 8 Plate Number Fig.2. microCT images of the three-dimensional structure of the armor plate assembly of Tonicella marmorea; (a–d) correspond to the chiton of Fig. 1a while (e) corresponds to the chiton of Fig. 1b; (a) dorsal view displaying position of cross sections L1 and T1; (b) ventral view; (c) side/ventral view; (d) side view; (e) side view displaying position of cross section T1; (a, b, d and e) include complimentary images created using a transparency effect; (f) projected area in the ‘‘y’’–‘‘z’’ plane (open circle and square symbols) and total overlap percentage (filled circle and square symbols) vs. plate number calculated from the plates and plate-to-plate overlapping regions of the transparent images of (d) and (e). Aerial overlap contributions from all adjacent plates were considered in calculations of total overlap percentage. Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx solution at room temperature. The procedure was monitored using stereo, light and fluorescence microscopy (BZ-8000, Keyonce, Japan). Immersion of the isolated organic matrix, inter-plate material, and girdle in 2.5 M NaOH at 37 °C led to an immediate loss of brownish pigment as well as of proteins from these specimens. The fibrous colorless materials were then washed with distilled water five times, dialyzed against deionized water on Roth (Germany) membranes with a MWCO of 14 kDa for 48 h at 4 °C, and finally dried at room temperature. Calcofluor White (Fluorescent Brightener M2R, Sigma) was used to confirm the presence of chitin. Samples were placed in 0.1 M Tris–HCl at pH 8.5 for 30 min, stained using 0.1% Calcofluor White solution for 30 min in darkness, rinsed five times with deionized water, dried at room temperature, and finally observed using fluorescence microscopy (Ehrlich et al., 2007). 2.10. Fourier-transform infrared spectroscopy (FTIR) Infrared spectra were recorded on a Nicolet 210 FT-IR Spectrometer. Two hundred and fifty scans were recorded at a spectral resolution of 2 cm 1. All spectra were baseline corrected with a two-point linear baseline at 845 and 1890 cm 1. An a-chitin standard from the demosponge Ianthella basta (Brunner et al., 2009) was used as a control. 2.11. Amino acid analysis (AAA) Dry, cleaned plates were ground with a pestle and mortar and weighed. The fine powder was suspended in deionized water. In a demineralization step, stoichiometric amounts of 1 M HCl were added dropwise to the solution over the course of 2 days (Treves et al., 2003). After complete demineralization, the solution was centrifuged (3000 rpm, 10 min, Eppendorf Centrifuge 5804 R) to separate soluble material from the insoluble, precipitated material. The soluble material was dialyzed exhaustively (Spectra Pro 7 dialysis membranes, 3500 MWCO, part # 132112) to remove salts and small molecules. The insoluble material was lyophilized and dried overnight in a vacuum oven at 50 °C. Samples were hydrolyzed for AAA, which was performed using a Waters system with Breeze software by Bio-Synthesis, Inc. (Lewisville, TX). 3. Results 3.1. Three-dimensional structure and geometry of the plate assembly of T. marmorea The three-dimensional structure of the plate assemblies of two representative T. marmorea chitons (Fig. 1a and b) were derived from renderings of microCT data. In their curved states, the plate assemblies of the chitons seen in Fig. 1a and b have dimensions 1.7 0.95 0.45 cm and 1.66 1.4 1.38 cm (length (‘‘z’’direction) width (‘‘x’’-direction) height (‘‘y’’-direction)), respectively. The girdle, as well as the inter-plate material (transverse muscle and cuticular layer), were very slightly or non-mineralized and consequently did not absorb X-rays well enough to be clearly visible in the microCT scans. Fig. 2a and b display dorsal and ventral views, respectively, of the plate assembly of the smaller chiton of Fig. 1a along with complimentary transparent images highlighting plate-to-plate overlap (darkened regions). The broad ‘‘u’’ shape of the overlapping regions results from each plate’s (except the first plate) two anterior projections, the apophyses, which imbricate with adjacent anterior plates and are separated by a sinus. Side views of the plate assemblies are shown in Fig. 2d and e, and are accompanied by transparent images. As illustrated by the 5 transparent image of Fig. 2d, in the smaller plate assembly with lower longitudinal curvature (about the ‘‘x’’-axis), plate-to-plate overlap is not limited solely to the apophyses; a portion of each plate’s jugal area (between the sinus, see Supplementary material 1a) is also involved in overlap. In contrast, overlap in the jugal areas of each plate is absent or greatly reduced in the transparent side profile of the larger, defensively postured plate assembly with the greater longitudinal curvature (Fig. 2e). In the plate assembly with the lower longitudinal curvature (Fig. 2d), the total plate overlap percentage of the intermediate plates increases from 52% in plate 2 to 65% in plate 7 (Fig. 2f). In contrast, in the plate assembly with the greater longitudinal curvature (Fig. 2e) the total overlap percentage of the intermediate plates increases from 44% in plate 2 to 58% in plate 7. The average total plate overlap percentages of the two plate assemblies presented in Fig. 2d and e are 62.27 ± 11.45 and 48.00 ± 9.07, respectively. The thickness distribution of the plate assembly of the smaller chiton presented in Fig. 2d (Fig. 3a) shows that the intermediate plates have a similar spatial distribution of thickness, in which the central non-overlapping regions are thickened (0.85 mm) relative to the anterior and posterior overlapping plate edges (0.4 mm) and apophyses (0.2 mm). The thin (green) and thick (red) regions of the plate assembly in Fig. 3b mirror the overlapping and non-overlapping regions, respectively, of the transparent image of the plate assembly in Fig. 2a. The eight plates of the assembly also have both similar average thicknesses (411 ± 31) and average standard deviation of thickness (222 ± 23) (Fig. 3c). Although thickness is relatively constant across the assembly, there is an asymmetric trend in plate volume (Fig. 3c); the head plate is 40% larger than the tail plate by volume. Additional structural data is available in Supplementary material 1, which also labels the anatomy of individual plates and defines structural parameters. Visualization of plate morphology and imbrication is facilitated using a macroscopic prototype of the plate assembly (Supplementary material 2). Fig. 4a and b show the L1 cross sections of the plate assemblies of the chitons presented in Fig. 1a and b, respectively. L1 bisects the jugal sinus of each plate 2–8, so the apophyses are absent. In the plate assembly with lower longitudinal curvature (Fig. 4a), the heterogeneous cross-sectional plate geometry and plate-toplate overlap result in a relatively constant thickness and approximately continuous convex curvature of the plate assembly as a whole. To approximate their longitudinal (about the ‘‘x’’-axis) radii of curvature, 16 landmark points were selected for each assembly from the two end points of each of the eight plates along their longitudinal axes. As illustrated in Fig. 4c and d, circles were fit to these points using a direct least squares fitting algorithm (Pratt, 1987). The longitudinal radii of curvature of the L1 cross sections of Fig. 4a and b are 9.15 and 7.39, respectively. The transverse (about the ‘‘z’’-axis) radii of curvature of the fourth plates of each assembly were approximated using the same method. Eleven landmark points from cross section T1 were used in the calculation for each plate: the midpoint of cross section L1, the midpoints of cross sections L2–L5 (left side) and their analogous midpoints on the right side, and left and right base points of the insertion plates (Fig. 5a and b). The transverse radii of curvature of the T1 cross sections of the fourth plates seen in Fig. 5a and b are 4.60 and 6.71, respectively. To compare the curvature of the two plate assemblies and account for the size disparity, two curvature indices were defined (Fig. 4f). The first index, the longitudinal curvature index (L.C.I), is defined as the length of plate 4 divided by the longitudinal radius of curvature of the plate assembly in cross section L1. This yields L.C.I. values of 0.43 and 0.70 for the plate assemblies of Fig. 4a and b, respectively. The second index, the transverse curvature index (T.C.I), is defined as the width of plate 4 divided by its Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 6 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx (a) Posterior 8 (b) 7 4 6 3 y • 5 x Anterior z 3 mm AP JS AP 0 0.94 mm 4 Total Volume (c) Average Thickness 25 1 20 0.8 15 0.6 10 0.4 5 0.2 2 0 1 Thickness (mm) Volume (mm3) 3 0 1 2 3 4 5 6 7 8 Plate Number Fig.3. Thickness distribution of the armor plate assembly of Tonicella marmorea presented in Fig. 1a; (a) dorsal view of the spatial distribution of thickness for each plate 1–8; (b) dorsal view of the spatial distribution of thickness of the entire plate assembly; (c) total volume (triangle symbols) and average thickness (circle symbols) as a function of plate number. Error bars in (c) refer to standard deviations of the dataset. Terminology: AP, apophyses; JS, jugal sinus. transverse radius of curvature in cross section T1. This yields T.C.I values of 0.48 and 0.50 for the fourth plates of Fig. 5a and b, respectively. Fig. 4e shows how the inter-plate separation distance changes as a function of gap number for the two plate assemblies seen in Fig. 4a and b. Gap number ‘‘n’’ corresponds to the gap between plate number ‘‘n’’ and ‘‘n + 1’’. The inter-plate separation distance of gap ‘‘n’’ was measured along the long axis of plate number ‘‘n + 1’’ in cross section L1. For example, the separation distance between plates 4 and 5 (Gap 4) was measured along the long axis (‘‘l’’) of plate 5 (Fig. 4b). To account for the size disparity between the two assemblies, the separation distances were normalized by dividing each one on by the length of the fourth plate of the respective assembly. As seen in Fig. 4e, the normalized separation Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 7 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx P4 (a) (b) L1 L1 P5 P3 2 mm ℓ x • z Dorsal P2 P6 y Ventral P7 P1 Posterior P8 Anterior (c) (d) L. Radius = 7.39 mm L. Radius = 9.15 mm Normalized Inter-Plate Separation (e) 0.30 0.24 (f) Fig. 6a Fig. 6b L.C.I 0.43 0.70 T.C.I 0.48 0.50 R/t (longitudinal) 10.73 6.44 R/t (transverse) 5.39 5.84 0.18 0.12 (Fig. 6a) 0.06 (Fig. 6b) 0.00 1 2 3 4 5 Gap Number 6 7 Fig.4. L1 (longitudinal bisector) cross-sectional structure of the plate assembly of T. marmorea; (a) cross section L1 of plate assembly of the chiton presented Fig. 1a; (b) cross section L1 of plate assembly the chiton presented in Fig. 1b; (c and d) circles fit to 16 landmark points (the two end points of each of the eight plates along their longitudinal axes) from (a) and (b) using a direct least squares fitting algorithm (Pratt, 1987); (e) normalized inter-plate separation distance vs. gap number. Gap number ‘‘n’’ corresponds to the gap between plate number ‘‘n’’ and ‘‘n + 1’’. The inter-plate separation distance of gap ‘‘n’’ was measured along the long axis of plate number ‘‘n + 1’’ in cross section L1 and normalized by dividing by the length of the fourth plate of the respective assembly; (f) curvature indices and shell thickness ratios. The longitudinal curvature index (L.C.I) is calculated by dividing the length of plate 4 by the longitudinal radius of curvature of the plate assembly in cross section L1. The transverse curvature index (T.C.I) is calculated by dividing the width of plate 4 by its transverse radius of curvature in cross section T1. Shell thickness ratios were calculated by dividing the appropriate radius of curvature by the average of the maximum thicknesses of the fourth plates in cross sections of L1–L5 (Fig. 5a and b). distances of gaps 1 and 2 are similar in both plate assemblies. However, the normalized separation distances of gaps 3, 4, and 7 are 75%, 100%, and 200% greater, respectively, in the plate assembly with a L.C.I. of 0.70 (Fig. 4b) relative to the plate assembly with a L.C.I of 0.43 (Fig. 4a). The normalized separation distance of gaps 5 and 6 are also larger in the plate assembly with the larger L.C.I., although the increase is not as pronounced. In both plate assemblies, the trend of normalized separation distance vs. gap number is asymmetric; the separation distance of gap 2 is 160% larger than that of gap 6. Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 8 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx L1 L2 L1 (a) L2 • L3 L4 • L5 • T1 • • • (b) x • L5 P4 • • • k • • T. Radius = 4.6 mm • • • P5 T1 • y • • • • L4 • k z • L3 T. Radius = 6.71 mm P4 P5 • Dorsal P3 P4 P4 L1 L1 2 mm P5 VTC L2 P3 Ventral P5 ℓ L2 L3 L4 L3 L5 % Total Overlap (Fig. 7a) % Total Overlap (Fig. 7b) Max. Thickness (Fig. 7a) Max. Thickness (Fig. 7b) (c) 1.50 80 1.25 60 1.00 40 0.75 20 L4 Max. Thickness (mm) 100 % Total Overlap AP L5 Posterior 0 Anterior 0.50 1 2 3 4 5 Cross Section (L) Fig.5. Cross-sectional structure of the plate assembly of T. marmorea; (a and b top) T1 cross sections of the fourth plate 4 of the plate assemblies of the chitons presented in Fig. 1a and b, respectively. Black circles indicate the eleven points used to calculate the transverse radius of curvature of the fourth plate of each plate assembly using a direct least squares fitting algorithm (Pratt, 1987); (a and b bottom) Morphological changes of longitudinal cross sections (L1–L5) of the fourth plate of each plate assembly. Cross sections L1–L5 were taken at 20% increments along line segment ‘‘k’’; (c) total overlap percentage and maximum thickness of plate 4 vs. longitudinal cross section number. Total overlap percentage was calculated by projecting the overlapping regions of plates 3 and 5 onto the long axis (‘‘l’’) of plate 4, summing the projected lengths, and expressing the sum as a percentage of the cross-sectional length of plate 4. Maximum cross-sectional thickness was measured perpendicular to the long axis (‘‘l’’) of plate 4. Terminology: AP, apophyses; VTC, ventral tegmental callus. Fig. 5a and b (top) show the dimensions and geometry of transverse cross section T1 of the two plate assemblies. T1 was taken in the region in which plates four and five overlap, and thus includes both plates. Longitudinal cross sections L2–L5 were taken at 20% increments along the line segment which runs diagonally from the midpoint of cross section L1 to the eave of the tegmentum below. A similar line segment was named ‘‘k’’ by Bergenhayn (1930 Fig. 1, page 14), and we adopt this notation (Fig. 5a). Fig. 5c displays how the total overlap percentage of a representative intermediate plate (number four) evolves laterally from L1 to L5. In Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx the both plate assemblies, overlap is heterogeneous in nature, but the heterogeneity is more pronounced in the plate assembly with the higher L.C.I. The total overlap percentage of plate four of the plate assembly with the lower L.C.I. is 60% in cross sections L1 and L2, and 82.5% in cross sections L3–L5. In contrast, the total overlap percentage of plate four of the plate assembly with the higher L.C.I. is 0% in cross sections L1 and L2, and increases approximately linearly from 50% to 90% across cross sections L3–L5. The average total overlap percentages of the fourth plates of the plate assemblies with L.C.I. of 0.43 and 0.70 are 73.9% and 44.6%, respectively. 3.2. Multilayered microstructure of the intermediate plates of T. marmorea The intermediate plates of T. marmorea are composed of six aragonite-based layers (Fig. 6). The dorsal mesostracm was not observed in any plates used in this study, and so we refer to the ventral mesostracum simply as the mesostracum. Fig. 6a and b contain representative longitudinal (L4) and transverse (T1) cross-sections, which are each accompanied by a schematic diagram that illustrates the spatial distribution of the six layers. The key to the schematics (Fig. 6d) relates textures and colors to microstructures and layers. The outermost layer, the tegmentum, has a homogeneous thickness of 155 lm and is infiltrated with the aesthete canal system (Fig. 6a), which constitutes 7.5% of each plate by volume as measured by mercury porosimetry (Supplementary material 1i). Main aesthete channels in the tegmentum run roughly longitudinally, are separated by 20 lm, and have a diameter of 40 lm, which lies in the lower end of the range (40–75 lm) found across genus Tonicella (Vendrasco et al., 2008). Scanning electron microscopy revealed that the tegmentum has a fine-grained homogeneous microstructure (Fig. 7b and g). XRD of the dorsal shell surface found no preferred grain orientations (Fig. 8a). Grains are approximately 1 lm in diameter, but are slightly elongated along one axis ventrally, below the main aesthete channels. Posteriorly, the tegmentum folds underneath the plate and becomes the posterior myostracum (layer 5), which lies in the impression of the transverse muscle (Fig. 6a). The transverse muscle, which runs longitudinally from the eave of the tegmentum to the tip of the apophyses, fills the gap between overlapping plates (Fig. 6c). The thickness of the transverse muscle ranges from 100 to 250 lm. Slit ray and jugal aesthete channels that begin in the tegmentum, pass though the lower layers, and exit on the ventral shell surface are insulated by a fine-grained homogeneous layer that is a couple of microns thick. The second layer, the articulamentum, has a composite prismatic microstructure (Fig. 7c and h). Bundles of prisms fan out dorsally and ventrally from the center of the layer. The length of the prism bundles ranges from approximately 3–10 lm. Individual prisms are rectilinear in shape and have dimensions 0.3 0.3 lm (width height). The thickness of the articulamentum ranges from 0 mm in the jugal area to approximately 0.9 mm laterally, at the start of the insertion plates (Fig. 6b). The articulamentum is followed by the mesostracum, which has a simple crossed-lamellar microstructure (layer 3) (Fig. 7d and i). It branches off from the hypostracum near the plates’ body diagonal, along which the slit ray aesthete channels run (Fig. 6a). The long axes of first order lamellae are oriented at a 45° angle relative to the ventral plate surface and have variable width between 1 and 2 lm. The layer lacks well-defined second order lamellae. Third order lamellae are rectilinear in shape and have dimensions 15 0.3 0.3 lm (length width height); each first order column is 3–6 third order lamellae wide. The angle between bundles of third order lamellae in adjacent first order columns is 40°. Third order lamellae on the interface between adjacent first order 9 columns possess a ‘‘wavy’’ surface topography, which has a wavelength approximately equal to their width (0.3 lm) (Fig. 7i). The periodic surface elevations on one side of the interface align with surface depressions on the opposite side. Layers 4 and 5, the anterior and posterior myostracum, respectively, both have a finegrained homogeneous microstructure with grains approximately 1 lm in diameter (Fig. 7e and j). The anterior myostracum stems from the impressions of the oblique muscles while the posterior myostracum lies in the impression of the transverse muscle. Together, the articulamentum, mesostracum, and anterior myostracum form the apophyses (Fig. 6a). The bottom layer, the hypostracum, has a simple crossed-lamellar microstructure identical to that of mesostracum, except that the long axes of the first order lamellae are oriented perpendicular to the ventral plate surface (Fig. 7f and k). This was supported by XRD of the ventral shell surface, which found two preferred grain orientations corresponding to the two lamellar dip directions (Fig. 8a, top dotted line). First order columns often slightly deviate from the perpendicular orientation, become thinner and disappear, or become thicker and branch. The hypostracums’s transverse distribution of thickness is inverse to that of the articulamentum; it is approximately 0.9 mm thick in the jugal area and absent laterally, at the start of the insertion plates (Fig. 6b). Longitudinally, the hypostracum composes the bulk of the central callus and is absent from the apophyses and insertion plates. 3.3. Chemical composition of the intra-plate organic matrix, girdle, and inter-plate material of T. marmorea The intermediate plates of T. marmorea have an organic matrix content of 2.6 wt.% (aragonite content of 97.4 wt.%), as measured by thermogravimetric analysis (Supplementary material 1i). The organic matrix, which was obtained after a 24 h gentle demineralization using Osteosoft™ solution at room temperature, showed a strong resistance to alkali treatment (Supplementary material 3a). It remained stable after submersion in 2.5 M NaOH at 37 °C for 30 days. The girdle and inter-plate material, which is composed of the transverse muscle and cuticular layer, displayed similar properties. After alkali dissolution of their proteinaceous components, Calcofluor White staining (Supplementary material 3b) and FT-IR spectroscopy indicated the presence of a-chitin within the shell plate organic matrix, girdle, and inter-plate material (Fig. 8b). Amino acid analysis of the intra-plate organic matrix revealed that the total amount of protein in the shell plates is 0.17 wt.% (Supplementary material 4b). 4. Discussion In segmented natural exoskeleton systems, there exists an interplay between the ‘‘inherent materiality’’ of the individual armor sub-units (i.e. microstructure, multilayering, anisotropy, grading, etc.) and the larger length scale morphometric or geometric design to simultaneously achieve the necessary protection and biomechanical flexibility/mobility. This interplay can vary dramatically among different systems. Here, we explore this topic in a model system of the chiton T. marmorea by using a suite of experimental techniques to quantify critical design aspects such as (from smallest to largest length scale): (1) the microstructure, multilayering and material properties of the individual armor sub-units, (2) the geometry of the individual armor sub-units, (3) the interconnections between armor sub-units, (4) the degree and type of armor unit overlap, and the interaction and correlation between these design aspects. We will discuss each of these aspects following. Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 10 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx (a) Dorsal L4 1 1 SR 2 2 4 3 Anterior 6 Ventral 0.5 mm Posterior 5 Transverse Muscle Oblique Muscle 1 (b) T1 6 z x SL x y 3 4 (c) Transverse Muscle 2 AP 0.5 mm 2 mm IP IP (d) Layer 1 2 Name Microstructure Tegmentum Granular Symbol(s) Articulamentum Composite Prismatic 3 Mesostracum Crossed-Lamellar 4 Ant. Myostracum Granular 5 Post. Myostracum Granular 6 Hypostracum Crossed-Lamellar - Muscle Tissue - a) b) a) b) Fig.6. Cross-sectional structure of intermediate plates of Tonicella marmorea by dark-field optical microscopy; (a) composite image of polished longitudinal cross-section (L4) and complimentary schematic; (b) composite image of polished transverse cross-section (T1) and complimentary schematic; (c) longitudinal cross-section displaying transverse muscle between two adjacent intermediate plates; (d) table relating textures in schematics to layers and microstructures. Terminology: AP, apophyses; IP, insertion plate; SR, slit ray. In order to understand functional design, it is important to consider the environment and types of predatory attacks against for which specific natural armor systems are designed. Langer (1978) observed that T. marmorea from the northwestern Atlantic is found most abundantly on subtidal calcareous algae covered rock, and displays cryptic coloration. Field observations led Langer (1978) to suspect that the primary predators of T. marmorea were the sea star Leptasterias littoralis in shallow water (less than 6 m) and the wrasse Tautogolabrus adspersus and winter flounder Pseudopleuronectes americanus at greater depths; secondary predators include the crabs Cancer borealis and Cancer irrorarus and the lobster Homarus americanus. Possible predatory attacks include biting (fish), pinching with claws (lobsters and crabs), drilling with radulas (sea snails), inserting the cardiac stomach underneath or Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 11 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx (a) Dorsal 1- Tegmentum 2- Articulamentum b c (b) (g) 1 µm 1 µm (c) (h) 1 µm 1.5 µm (d) (i) 1 µm 1.5 µm (e) (j) 1 µm 0.5 µm (f) (k) 3 d Cryofractured Samples Polished & Etched Sample L4 0.3 µm Muscle f 6 - Hypostracum 4 e 50 µm Ventral 1 µm 3 µm Fig.7. Spatial distribution of microstructures of intermediate plates of Tonicella marmorea by SEM; (a) composite image of the boxed (white dashes) region of longitudinal cross section L4 of Fig. 6a, which was polished and etched with 0.5 M EDTA for 5 min to increase image contrast. Images (b–f) correspond to magnified regions of (a) and preserve microstructure orientation with respect to cross section L4. Images (g–k) were obtained from cryofractured samples and display true microstructure dimensions and geometry; (b and g) layer 1, tegmentum, fine-grained homogenous; (c and h) layer 2, articulamentum, composite prismatic; (d and i) layer 3, mesostracum, simple crossed lamellar; (e and j) layer 4, anterior myostracum, fine-grained homogenous; (f and k) layer 6, hypostracum, simple crossed lamellar. between the shell plates (sea stars), and smashing against rocks (birds). 4.1. Comparison of the shell plate microstructures of T. marmorea with other Polyplacophora and Mollusca The mesostracum was originally named by Bergenhayn (1930) for its position between the tegmentum and articulamentum in Acanthopleura spinigera. He observed that it was similar in structure to the hypostracum, which is now recognized as having a simple crossed-lamellar microstructure. The mesostracum has been observed lining both the dorsal and ventral sides of the articulamentum in Chiton olivaceous (Kreusch, 1981;Laghi and Russo, 1978; Poulicek and Kreusch, 1983), Leopidopleurus cajentanus (Laghi and Russo, 1980), Liolophura gainardi (Kreusch, 1981; Poulicek and Kreusch, 1983), and Liolophura japonica (Chen, 2010). However, we only observed it lining the articulamentum ventrally in T. marmorea, and Baxter and Jones (1981) did not observe it at all in Lepidochitona cinereus. In addition, Kreusch (1981) observed the mesostracum within the articulamentum instead of lining it dorsoventrally in Cryptochiton stelleri. Laghi and Russo (1978, 1980) and Kreusch (1981) note that in species which Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 12 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx Ventral Surface Dorsal Surface (111) Intensity (102) (112) (a) 25 Aragonite 30 35 40 45 50 55 Two-Theta (deg.) (b) α-Chitin standard from I. basta Organic Matrix after NaOH Treatment Native Inter-Plate Material Inter-Plate Material after NaOH Treatment Native Girdle 947 890 1151 1107 1374 1543 Intensity 1628 1061 1008 Girdle after NaOH Treatment species that contain the dorsal mesostracum may be regarded as having seven, or possibly eight. Laghi and Russo (1978, 1980), Kreusch (1981), and Poulicek and Kreusch (1983) refer to the dorsal and ventral mesostracum layers collectively as the pallial myostracum. With the sole exception of the dorsal mesostracum, the spatial distribution of microstructures in T. marmorea, C. olivaceous (see longitudinal and transverse cross-sectional schematics in Figs. 2 and 4, Laghi and Russo, 1978), and L. gainardi (see longitudinal schematic in Fig. 12, Kreusch, 1981) are very similar. Poulicek and Kreusch (1983) note that in the evolutionary series of chitons, the tegmentum becomes thinner (disappearing almost completely in the more evolved Cryptochiton), the articulamentum becomes thicker, and the hypostracum, mesostracum, and myostracal layers show little variation in proportions. Similar orientation, thickness, and branching irregularities of the first order lamellae of the crossed-lamellar layers of the shell plates of T. marmorea have also been reported in the shells of other mollusks (Currey and Kohn, 1976; Taylor and Reid, 1990; Wilmot et al., 1992). The width of first order lamellae in the crossed-lamellar layers of the shell plates of T. marmorea (1–2 lm), Acanthopleura brevispinosa (1.7–5 lm) (Wilmot et al., 1992), Chiton olivaceus (2–3 lm) (Laghi and Russo, 1978), Lepidopleurus cajetanus (3 lm) (Laghi and Russo, 1980), Liolophura japonica (3 lm) (Chen, 2010), as well as the range found in 36 chiton species representing 19 genera (3–5 lm) (Haas, 1972) lies in the extreme lower end of the range (1–40 lm) found in crossed-lamellar microstructures across Mollusca (Carter and Clark, 1985; Wilmot et al., 1992). As previously observed by Haas (1976, 1981), Poulicek and Kreusch (1983), and Carter and Hall (1990), the simple crossed-lamellar structures present in chiton shell plates are devoid of well-defined second order lamellae. The width of third order lamellae found in the crossed-lamellar layers of T. marmorea, Chiton olivaceus (Laghi and Russo, 1978), and Lepidopleurus cajetanus (Laghi and Russo, 1980) are identical (300 nm), and similar to the range (200–300 nm) observed by Haas (1972). The angle between the two dip directions of third order lamellae in adjacent columns of first order lamellae in T. marmorea is approximately 40°, which is similar to the dip angles previously observed in Chiton olivaceus (50°) (Laghi and Russo, 1978), Lepidopleurus cajetanus (45°) (Laghi and Russo, 1980), and Acanthopleura brevispinosa (32–45°) (Wilmot et al., 1992), and much less than the range (60–85°) observed by Haas (1972). 4.2. Chemical composition of the shell plate organic matrix of T. marmorea 1650 1450 1250 Wavenumber 1050 850 650 (cm-1) Fig.8. Chemical composition of intermediate plates of Tonicella marmorea; (a) X-ray diffraction pattern of the dorsal and ventral surfaces of an intermediate plate; (b) infrared spectra of the intra-plate organic matrix, girdle, and inter-plate material before and after NaOH treatment to remove proteins. contain the dorsal mesostracum, it is split dorsoventrally into two sub-layers by a row of aesthete channels. Thus, while the intermediate plates of T. marmorea have six layers, those of other chiton The intermediate plates of T. marmorea were determined to have an organic matrix content 2.6 wt.% by TGA. Poulicek and Kreusch (1983) found that the shell plate organic matrix content ranged from 0.48 to 1.55 wt.% (greatest in Tonicella lineata) in 11 species; however, they only considered the insoluble portion of the matrix in their measurements. In contrast to the proteinaceous composition of organic matrices of other mollusk shells, those of chiton shell plates possess a very large amount of the aminopolysaccharide chitin (Furuhashi et al., 2009). An earlier study found the range of the weight percent of chitin in the insoluble portion of the shell plate organic matrix, girdle, and organic matrix of the mineralized girdle spicules to be 16–41, 1.6–16, and 0.2–15, respectively (Poulicek and Kreusch, 1983). In addition, the shell plate organic matrix of Acanthopleura villantii was found to contain a chitin/protein ratio of 6.9, which is over a hundred times larger than that found in shells of other mollusks, specifically bivalves (Furuhashi et al., 2009). Our results are consistent with these studies. Because of its resistance to alkali treatment, it is very simple to isolate chitinous material from most composite biomaterials, including cases where chitin is bound to proteins or pigments Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx (Ehrlich, 2010a; Ehrlich et al., 2010). After alkali treatment, the shell plate organic matrix, girdle, and inter-plate material of T. marmorea displayed FTIR spectra very similar to an a-chitin standard from the demosponge I. basta (Fig. 8b). The protein content (0.17 wt.%) of the shell plates of T. marmorea is similar to the range found in the composite prismatic spines and scales (0.07– 0.23 wt.%) of Acanthopleura vaillantii, Acanthopleura spinigera, Nuttalina fluxa, and Ischnochitonina sp. (Treves et al., 2003). This is consistent with results of Taylor and Layman (1972), who found that non-nacreous microstructures including crossed-lamellar, composite prismatic, and homogeneous have a protein content less than 0.4 wt.%. The amino acid profile of the shell plate organic matrix of T. marmorea (Supplementary material 4) is consistent with those of Chiton marmoratus and Acanthopleura granulata (Haas, 1972), with the sole exception of glycine content, which is about 8 mol% greater in C. marmoratus and A. granulata. Calcification of the shell plates is likely controlled by the templating activity of acidic proteins (Ehrlich, 2010b), which may be attached to the chitinous network of the organic matrix (Supplementary material 3c). 4.3. The biomechanical role of the inherent materiality of the individual armor sub-units The multilayered structure of the individual armor sub-units plays a significant role in direct penetration resistance in complex multiaxial loading configurations by predators (Bruet et al., 2008; Wang et al., 2009). The sequence and spatial heterogeneity of the layers of the intermediate plates of T. marmorea suggests a structural response to different loading conditions experienced by different regions of the plates. The transverse distribution of crossed-lamellar hypostracum (900 lm thick in the jugal area and decreasing to zero laterally, Fig. 8b) and orientation of first order lamellae (long axes perpendicular to the ventral plate surface) may function to resist transverse bending (about the ‘‘z’’-axis). Three-point bending tests by Currey and Kohn (1976) on samples of Conus spp. showed that the flexural strength of the crossedlamellar microstructure is highly anisotropic; they determined the flexural strength to be 70 MPa in the axial direction (perpendicular to long axes of first order lamellae) and 200 MPa in the transverse direction (parallel to long axes of first order lamellae). Bending in the axial direction can break the layer by simply pulling the first order lamellae apart from each other, while bending in the transverse direction cannot break the layer without breaking each first order lamellae across its long axis (Currey and Kohn, 1976). The bending tendency of the plates will depend on the boundary conditions at the ambitus (Telford, 1985). Possible ‘‘abutment’’ effects of the girdle and concave substratum, and ‘‘tie rod’’ effects of the muscular system will both increase the bending tendency. Multiple ‘‘channel’’ cracking between first order lamellae is known to be important to overall damage tolerance of shells with inner crossed-lamellar layers (Kuhn-Spearing et al., 1996; Kamat et al., 2000), and is likely an important energy dissipation mechanism in the shell plates of T. marmorea during longitudinal bending deformation. The structure of the first order lamellae found in the two crossed-lamellar layers, the inner hypostracum and middle mesostracum, of the shell plates of T. marmorea permits two toughening mechanisms that may generate high channel crack densities during fracture: crack bridging by irregular first order lamellae (Kuhn-Spearing et al., 1996), and crack bridging by the roughness (‘‘waviness’’) of the third order lamellae on first order interfaces (Kamat et al., 2004). The lateral area of the plates, which likely experiences the largest longitudinal bending deformations, contain two middle layers (the articulamentum and mesostracum) whose first order interfaces differ in orientation from those of the hypostracum. This arrangement permits crack deflection and bridging in the middle layers – a mechanism which is responsible 13 for a large portion of the energy dissipated during fracture of the shell of Strombus gigas (Kamat et al., 2000; Kamat et al., 2004). The aforementioned surface waviness of the third order lamellae may generate a strain hardening mechanism similar to that found in nacre, in which the ‘‘dovetails’’ of individual nacre tablets produce a progressive tablet locking in tension that is responsible for a strain at failure (1%) that is an order of magnitude greater than that of non-biogenic aragonite (Barthelat et al., 2007). The transverse thickness distribution (900 lm thick laterally and decreasing to zero centrally towards the jugal area, Fig. 8b) of the composite prismatic articulamentum, suggests that it functions to resist circumferential (roughly longitudinal, parallel to transverse ‘‘z’’-axis in Fig. 8b) tensile stress and to provide a stiff, hard layer to resist side penetration and pinching. While the prism bundles in the articulamentum are aligned parallel to interfaces of adjacent layers (the tegmentum dorsally and mesostracum ventrally) in the middle of the articulamentum, they are aligned approximately perpendicular to theses interfaces at the layer junctions. This likely serves to provide discrete inter-prism structural pathways for cracks to propagate ventrally for easy arrest by the crossed-lamellar layers (Wang et al., 2009). The inner articulamentum and hypostracum layers may also function to resist drilling attacks. Chiton tuberculatus has been observed alive with drill holes that bore through the tegementum, but left the inner layers intact (Arey and Crozier, 1919). In contrast to the inner shell plate layers, the outer tegmentum’s primary role is likely sensory rather than mechanical in nature; the aesthetes, a complex sensory network of tissue-filled channels, are embedded in the tegmentum. In a secondary mechanical role, the tegmentum may operate as a brittle outer shield, in which the main aesthete channels act as sacrificial stress concentrators that distributes a concentrated load (e.g. biting attack) over a large area of the plate. The homogeneous microstructure of the tegmentum implies that it may also function to resist dissolution. Walter and Morse (1984) showed that finely crystalline microstructures have higher dissolution rates than coarser ones, which lead Taylor and Reid (1990) to suspect that coarse granular or irregular prismatic microstructures are more dissolution resistant than the finely crystalline crossed-lamellar microstructure. As previously proposed by Haas (1981), the homogeneous myostracal layers’ location in the impressions of the latero-pedal, oblique, and transverse muscles suggests that the homogeneous microstructure may be best suited for muscle attachment. 4.4. Geometric design aspects of the armor sub-units of T. marmorea The transverse curvature (curvature of the chiton plates as observed in the ‘‘x’’–‘‘y’’ plane sections) provide an arch-enhanced stiffness to resist bending from both top and lateral loading conditions; this geometry enhancement to mechanical robustness is consistent with the thickness distribution of the articulamentum and hypostracum layers. In less curved conformations (e.g. when attached to a flat substrate) the longitudinal cross-sectional shape of each plate provides a curved outer surface that achieves a nearly continuous outer surface of the plate-assembly. The cross-sectional shape (centrally thickened) and thickness distribution of each unit imparts an overall spatially uniform thickness distribution in the plate-assembly. This effect has also been observed in other segmented natural armor systems including the plate assembly of the marine three spine stickleback Gasterosteus aculeatus (Song et al., 2010) and is likely a universal design principal. Together these features provide the geometric enhancement and coverage for protection from penetration, bending, and pinching attacks. Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 14 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx 4.5. The interconnections between armor units In contrast to physically interconnected joints observed in some other natural exoskeletons (e.g. the peg-and-socket joint of the scales of Polypterus senegalus (Pearson, 1981), the sliding hinge/ ellipsoidal joint of the lateral plates of Gasterosteus aculeatus (Song et al., 2010), the ball-and-socket joint of sea urchin spines (Takahashi, 1967)), T. marmorea utilizes an actuating sandwich structure consisting of two rigid armor plates separated by a more compliant and actuating transverse muscle to achieve biomechanical motion. The transverse muscles are protected by the ventral tegmental callus, which probably functions to reduce the dorsal separation distance between intermediate plates. The contact of the apophyses of the intermediate plates with ventral surface of their anterior neighbors migrates laterally from the L3 cross section to the L5 section as the L.C.I. of the plate assembly increases. The close proximity of the overlapping regions of adjacent plates appears to strongly limit lateral translation (along the ‘‘x’’-axis) and rotation about the ‘‘y’’ axis by the intermediate plates. Longitudinal translation (‘‘z’’ direction) of the plates is likely constrained by the compliance of the underlying muscular system and surrounding girdle. Although the defensive conformation (Fig. 1b) protects the underlying soft parts of T. mamorea from potential predators, it creates vulnerability by dramatically increasing the separation distance of gaps 3 and 4 of the plate assembly by 75% and 100%, respectively, relative to the passive, slightly curved conformation (Fig. 1a). Sea stars have been observed inserting their cardiac stomachs into the gaps between the plates of chitons that rolled into a ball after being captured (Town, 1979). 4.6. The degree and type of armor unit overlap As the L.C.I. of the plate assembly increases, plate-to-plate overlap decreases and becomes more heterogeneous. In the two plate assemblies with a L.C.I. of 0.43 and 0.70, the total overlap percentage of the fourth plate (relative to its long axis ‘‘l’’) ranges from approximately 60–82.5% (average = 73.9%) and 0–90% (average = 44.6%), respectively, between cross sections L1 and L5. In addition, the average plate-to-overlap percentages (calculated from projections of the plates and overlapping regions onto the ‘‘y’’–‘‘z’’ plane) of the two plate assemblies with L.C.I. of 0.43 and 0.70 are 62.27 ± 11.45 and 48.00 ± 9.07, respectively. The reduction of plate-to-plate overlap, along with the aforementioned increase in plate-to-plate separation distance, reduces the protection of plate assembly in the defensive conformation relative to less curved states. Species Cryptochiton stelleri and Chiton virgulatus have occasionally been observed exhibiting a righting behavior in which the plate assemblies undergo a concave curvature and slight twisting motion when the chitons were dislodged and turned upside down by hand, or an aquarium water jet. However, Cryptochiton stelleri always rolled into ball when dislodged and vigorously stimulated by touch. Perhaps in some species the defensive conformation serves as a last resort in the presence of imminent threats when the chiton does not have enough time and/or security to right itself when dislodged. 5. Conclusions In summary, this study investigates the three-dimensional structure and conformational transition of the eight plate shell assembly of the chiton T. marmorea, and considers its biomechanical consequences, as follows. One contribution of this work is the elucidation of the detailed mechanism of conformational change from a passive (flattened or slightly curved, attached to surface) to a defensive (rolled, detached from surface) state of the shell plate assembly. The passive and defensive conformations exhibited differences in longitudinal curvature index (0.43 vs. 0.70), average plate-to-plate overlap (62% vs. 48%), cross-sectional overlap heterogeneity (60–82.5% vs. 0–90%, fourth plate), and plate-toplate separation distance (100% increase in normalized separation distance between plates 4 and 5), respectively. In contrast to physically interconnected joints observed in some other natural exoskeletons (e.g. peg-and-socket, ball-and-socket, etc.), T. marmorea utilizes an actuating sandwich structure consisting of two rigid armor plates separated by a more compliant and actuating transverse muscle to achieve biomechanical motion, analogous to a ‘‘shear lap joint,’’ albeit one that is geometrically structured. This work also provides an understanding of how T. marmorea achieves the required balance between biomechanical mobility and protection in the passive and defensive states. In the passive state, the morphometry of the individual shell plates and plate-to-plate interconnections results in an approximately continuous curvature and constant armor thickness and, hence, spatially homogeneous protection; mobility is limited but armor coverage and protection is maximized. When the animal is detached from a surface in the defensive state, the underlying soft tissues of the foot gain greater coverage and protection by the shell plates and the animal gains mobility through tidal flow, but regions of vulnerability are opened dorsally, due to the increase in plate-to-plate separation distance and decrease in plate-to-plate overlap. Lastly, experiments using optical microscopy, scanning electron microscopy, X-ray diffraction, mercury porosimetry, fourier-transform infrared spectroscopy, and amino acid analysis explore the microstructure and spatial distribution of the six layers within intermediate shell plates, the role of multilayering in resisting predatory attacks, and the detection of chitin as a major component of the intra-plate organic matrix and girdle. Acknowledgments We gratefully acknowledge support of the National Science Foundation MIT Center for Materials Science and Engineering (DMR-0819762), the US Army Research Office through the MIT Institute for Soldier Nanotechnologies (Contract W911NF-07-D-0004), the National Security Science and Engineering Faculty Fellowship Program (N00244-09-1-0064), and the MIT International Science and Technology Initiatives (MISTI-Israel). This work was also partially supported by the DFG (Grant No. EH 394/1-1). The authors would like to thank MIT graduate students Samuel Crawford, Chris Ng, and Simon Choong for their assistance with photography, circle fitting algorithms, and mercury porosimetry, respectively. We also thank E. Rosseeva for great technical assistance. Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jsb.2011.12.019. References Arciszewski, T., Cornell, J., 2006. Bio-inspiration: learning creative design principia. Lect. Notes Artif. Int. 4200, 32–53. Arey, L.B., Crozier, W.J., 1919. The sensory responses of chiton. J. Exp. Zool. 29, 157– 260. Barthelat, F., Tang, H., Zavattieri, P.D., Li, C.-M., Espinosa, H.D., 2007. On the mechanics of Mother-of-Pearl: a key feature in the material hierarchical structure. J. Mech. Phys. Solids 55, 306–337. Baxter, J.M., Jones, A.M., 1981. Valve structure and growth in the Chiton Lepidochitona cinereus (Polyplacophora: Ischnochitonidae). J. Mar. Biol. Ass. UK 61, 65–78. Beedham, G.E., Trueman, E.R., 1967. The relationship of the mantle and shell of the polyplacophora in comparison with that of other mollusca. J. Zool., Lond. 151, 215–231. Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019 M.J. Connors et al. / Journal of Structural Biology xxx (2012) xxx–xxx Bergenhayn, J.R.M., 1930. Die Loricaten von Dr. Sixten Bocks Pazifik-Expedition 1917–1918, mit spezieller Berücksichtigung der Perinotumbildungen und der Schalenstruktur. Göteborgs Kungliga Vetenskaps-och Vitterhets-Samhälles Handlingar Ser. B. 1, 1–52, pls. 1–3. Bøggild, O.B., 1930. The Shell Structure of Mollusks. Det Kongelige Danske Videnskabernes Selskabs Skrifter (Naturvidenskabelig og Mathematisk Afdeling) 9, 235–326. Bouxsein, M.L., Boyd, S.K., Christiansen, B.A., Guldberg, R.E., Jepsen, K.J., et al., 2010. Guidelines for assessment of bone microstructure in rodents using microcomputed tomography. J. Bone Miner. Res. 25, 1468–1486. Bruet, B.J.F., Song, J., Boyce, M.C., Ortiz, C., 2008. Materials design principles of ancient fish armour. Nature Mater. 7, 748–756. Brunner, E., Ehrlich, H., Schupp, P., Hedrich, R., Hunold, S., et al., 2009. Chitin-based scaffolds are an integral part of the skeleton of the marine Demosponge Ianthella basta. J. Struct. Biol. 168, 539–547. Carter, J.G., Clark II, G.R., 1985. Classification and phylogenetic significance of molluscan shell microstructure. In: Bottjer, D.J., Hickman, C.S., Ward, P.D., Broadhead, T.W. (Eds.), Mollusks: Notes for a Short Course. University of Tennessee Department of Geological Sciences Studies in Geology, Knoxville, TN, USA, pp. 50–71. Carter, J.G., Hall, R.M., 1990. Polyplacophora, Scaphopoda, Archaeogastropoda and Paragastropoda (Mollusca). In: Carter, J.G. (Ed.), Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends, vol. 2, Atlas and Index, Van Nostrand Reinhold, New York, pp. 29–31, 49, pls. 122–125 Chen, D., 2010. Microstructure and element composition of the shell plates of Liolophura japonica Wuhan Univ. J. Nat. Sci. 15, 176–184. Currey, J.D., Kohn, A.J., 1976. Fracture in the crossed-lamellar structure of Conus shells. J. Mater. Sci. 11, 1615–1623. Currie, D.R., 1992. Aesthete channel morphology in three species of Australian chitons (Mollusca: Polyplacophora). J. Malac. Soc. Aust. 13, 3–14. Eernisse, D.J., Reynolds, P.D., 1994. Polyplacophora. In: Harrison, F.W., Kohn, A.J. (Eds.), Microscopic Anatomy of Invertebrates, Volume 5. Wiley-Liss, New York, pp. 56–110. Eernisse, D.J., 2007. Chitons. In: Denny, M.W., Gaines, S.D. (Eds.), Encyclopedia of Tidepools and Rocky Shores. University of California Press, Berkeley, California, pp. 127–133. Ehrlich, H., Maldonado, M., Spindler, K.-D., Eckert, C., Hanke, T., et al., 2007. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera). J. Exp. Zool. (Mol. Dev. Evol.) 308, 347– 356. Ehrlich, H., 2010a. Biological Materials of Marine Origin. Invertebrates. SpringerVerlag. Ehrlich, H., 2010b. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 52, 661–699. Ehrlich, H., Ilan, M., Maldonado, M., Muricy, G., Bavestrello, G., et al., 2010. Three dimensional chitin-based scaffolds from verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 47, 132–140. Furuhashi, T., Beran, A., Blazso, M., Czegeny, Z., Schwarzinger, C., et al., 2009. Pyrolysis GC/MS and IR spectroscopy in chitin analysis of molluscan shells. Biosci. Biotechnol. Biochem. 73, 93–103. Gane, P.A.C., Ridgway, C.J., Lehtinen, E., Valiullin, R., Furo9 , I., Schoelkopf, J., Paulapuro, H., Daicic, J., 2004. Comparison of NMR cryoporometry, mercury intrusion porosimetry, and DSC thermoporosimetry in characterizing pore size distributions of compressed finely ground calcium carbonate structures. Ind. Eng. Chem. Res. 43, 7920–7927. Haas, W., 1972. Untersuchungenüber die Mikro- und Ultrastruktur der Polyplacophorenschale. Biomineralisation 5, 3–52. Haas, W., 1976. Observations on the shell and mantle of the placophora. In: Watabe, N., Wilbur, K.M. (Eds.), The Mechanisms of Mineralization in the Invertebrates and Plants. University of South Carolina Press, Columbia, pp. 389–402. Haas, W., 1981. Evolution of Calcareous Hardparts in Primitive Molluscs. Malacologia 21, 403–418. Haszprunar, G., Wanninger, A., 2000. Molluscan muscle systems in development and evolution. J. Zool. Syst. Evol. Res. 38, 157–163. Kaas, P., Van Belle, R.A., 1985. Monograph of Living Chitons (Mollusca: Polyplacophora), vol. 2, Suborder Ischnochitonina, Ischnochitonidae: Schizoplacinae, Callochitoninae and Lepidochitoninae. E.J. Brill, Leiden. Kamat, S., Su, X., Ballarini, R., Heuer, A.H., 2000. Structural basis for the fracture toughness of the shell of the conch Strombus gigas. Nature 405, 1036–1040. Kamat, S., Kessler, H., Ballarini, R., Nassirou, M., Heuer, A.H., 2004. Fracture mechanisms of the Strombus gigas conch shell: II-micromechanics analyses of multiple cracking and large-scale crack bridging. Acta Mater. 52, 2395–2406. Kreusch, B., 1981. Etude Comparée de la Structure et de la Composition Chimique des Formations Squelettiques chez les Mollusques Polyplacophores. Mémoire de Licence en Sciences Zoologiques, Faculté des Sciences, University de Liége, p. 50. 15 Kuhn-Spearing, L.T., Kessler, H., Chateau, E., Ballarini, R., Heur, A.H., et al., 1996. Fracture mechanisms of the Strombus gigas conch shell: implications for the design of brittle laminates. J. Mater. Sci. 31, 6583–6594. Laghi, G.F., Russo, F., 1978. Structtura ed architettura delle piastre di Chiton olivaceus Spengler (Polyplacophora, Mollusca). Bollettinodella Società Paleontologica Italiana 17, 272–291. Laghi, G.F., Russo, F., 1980. Struttura ed architettura delle piastre di Lepidopleurus cajetanus (Poli) (Mollusca: Polyplacophora). Ann. Univ. Ferrara Sezione IX Sci. Geol. Paleontol. 6, 321–338. Langer, P.D., 1978. Some aspects of the biology of three Northwestern Atlantic Chitons: Tonicella rubra, Tonicella marmorea, and Ischnochiton albus (Mollusca: Polyplacophora). Ph.D. Thesis, Department of Zoology, University of New Hampshire, p. 172. Neves, N.M., Mano, J.F., 2005. Structure/mechanical behavior relationships in crossed-lamellar sea shells. Mater. Sci. Eng. C. 25, 113–118. Ortiz, C., Boyce, M.C., 2008. Materials science – Bioinspired structural materials. Science 319, 1053–1054. Pearson, D.M., 1981. Functional aspects of the integument in polypterid fishes. Zoo. J. Linn. Soc. 72, 93–106. Poulicek, E., Kreusch, B., 1983. Evolutionary trends in the skeletal structures of the Polyplacophora. Proceedings of the Eighth International Malacological Congress (Budapest, Hungary), 207–212. Pratt, V., 1987. Direct least-squares fitting of algebraic surfaces. Computer Graphics 21, 145–152. Puchalski, S., Eernisse, D.J., Johnson, C.C., 2008. The effect of sampling bias on the fossil record of chitons (Mollusca, Polyplacophora). Am. Malac. Bull. 25, 87–95. Rasband, W.S., 1997–2011. ImageJ, US National Institutes of Health, Bethesda, Maryland, USA. http://www.imagej.nih.gov/ij/. Runnegar, B., Pojeta, J., Taylor, M.E., Collins, D., 1979. New species of the Cambrian and Ordovician chitons Matthevia and Chelodes from Wisconsin and Queensland: evidence for the early history of polyplacophoran mollusks. J. Paleont. 53, 1374–1394. Schwabe, E., 2005. A catalogue of recent and fossil chitons (Mollusca: Polyplacophora) addenda. Novapex 6, 89–105. Schwabe, E., 2010. Illustrated summary of chiton terminology. Spixiana 33, 171– 194. Sigwart, J.D., 2009. Morphological cladistic analysis as a model for character evaluation in primitive living chitons (Polyplacophora, Lepidopleurina). Am. Malac. Bull. 27, 95–104. Song, J., Reichert, S., Kallai, I., Gazit, D., Wund, M., et al., 2010. Quantitative microstructural studies of the armor of the marine threespine stickleback (Gasterosteus aculeatus). J. Struct. Biol. 171, 318–331. Stebbins, T.D., Eernisse, D.J., 2009. Chitons (Mollusca: Polyplacophora) known from benthic monitoring programs in the Southern California Bight. The Festivus 41, 53–100. Takahashi, K., 1967. The ball-and-socket joint of the sea-urchin spine: geometry and its functional implications. J. Fac. Sci. Univ. Tokyo. Sec. IV 11, 131–135. Taylor, J.D., Layman, M., 1972. The mechanical properties of bivalve (Mollusca) shell structures. J. Paleontol. 15, 73–87. Taylor, J.D., Reid, D.G., 1990. Shell microstructure and mineralogy of the Littorinidae: ecological and evolutionary significance. Hydrobiologia 193, 199–215. Telford, M., 1985. Domes, arches, and urchins: the skeletal architecture of echnoids (Echinodermata). Zoomorphology 105, 114–124. Town, J.C., 1979. Aspects of the biology of Astrostole scabra (Hutton, 1872). Ph.D. Thesis, Department of Zoology, University of Canterbury, p. 200. Treves, K., Traub, W., Weiner, S., Addadi, L., 2003. Aragonite formation in the chiton (Mollusca) girdle. Helvetica Chimica Acta 86, 1101–1112. Vendrasco, M.J., Fernandez, C.Z., Eernisse, D.J., Runnegar, B., 2008. Aesthete canal morphology in the Mopaliidae (Polyplacophora). Am. Malac. Bull 25, 51–69. Vermeij, G.J., 1993. A Natural History of Shells. Princeton University Press, Princteon, NJ. Walter, L.M., Morse, J.W., 1984. Reactive surface area of skeletal carbonates during dissolution: effect of grain size. J. Sediment. Petrol. 54, 1081–1090. Wang, L., Song, J., Ortiz, C., Boyce, M.C., 2009. Anisotropic design of a multilayered biological exoskeleton. J. Mater. Res. 24, 3477–3494. Wilmot, N.V., Barber, D.J., Taylor, J.D., Graham, A.L., 1992. Electron microscopy of molluscan crossed-lamellar microstructure. Phil. Trans. R. Soc. Lond. Biol. 337, 21–35. Wingstrand, K.G., 1985. On the anatomy and relationships of recent Monoplacophora. Galathea Rep. 16, 7–94. Yao, H., Dao, M., Imholt, T., Huang, J.M., Wheeler, K., et al., 2010. Protection mechanisms of the iron-plated armor of a deep-sea hydrothermal vent Gastropod. Proc. Natl. Acad. Sci. 107, 987–992. Zaremba, C.M., Morse, D.E., Mann, S., Hansma, P.K., Stucky, G.D., 1998. Aragonitehydroxyapatite conversion in Gastropod (Abalone) Nacre. Chem. Mater. 10, 3813–3824. Please cite this article in press as: Connors, M.J., et al. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. (2012), doi:10.1016/j.jsb.2011.12.019