Engr 270 -- Materials Science and Engineering TEST 4 -
advertisement

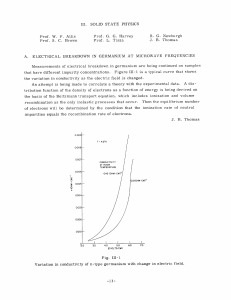
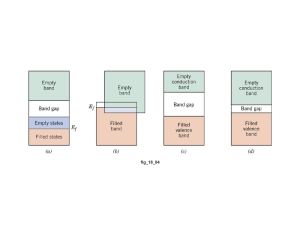
Engr 270 -- Materials Science and Engineering TEST 4 -- Dec. 7, 2006 Name: ________________________ Part 2: Solve each of the following problems completely. 1. A copper wire of commercial purity is needed to conduct 10 A of current with a maximum voltage drop of 0.4 V/m. What must be it minimum diameter? The electrical conductivity of pure copper is 5.85 × 107 (Ωm)-1, compute the maximum diameter of the wire. 2. At room temperature the electrical conductivity and the electron mobility for aluminum are 3.8 × 107 (Ω-m)-1 and 0.0012 m2/V-s, respectively. a) Compute the number of free electrons per cubic meter for aluminum at room temperature. b) What is the number of free electrons per aluminum atom? Assume a density of 2.7 g/cm3. 3. The energy gap of Germanium is 0.66 eV. The electron mobility if 0.364 m2/V-s. The hole mobility is 0.190 m2/V-s. The intrinsic hole concentration at room temperature (300 K) is 2.3 × 1019 holes/m3. a) Calculate the electrical conductivity of germanium (intrinsic) at room temperature. b) Calculate the electrical conductivity of germanium (intrinsic) at 100°C. (|e| = 1.6 × 10-19 C, and the k = 8.62 × 10-5 eV/K) c) Germanium is to be doped with Antimony to produce an extrinsic n-type semiconductor. What atomic density (in atoms/m3) is required to yield a conductivity of 104 (Ωm)-1. (Neglect the conductivity of intrinsic Germanium.) Solution: a) σ = n e (μ + μ ) b) ⎛ Eg σ2 = exp⎜⎜ − σ1 ⎝ 2k e h ⎛ 1 1 ⎞⎞ ⎜⎜ − ⎟⎟ ⎟ ⎟ ⎝ T2 T1 ⎠ ⎠ σ = (2.3 × 1019 )(1.6 × 10 −19 )(0.364 + 0.190) = 2.04 (Ωm ) −1 ⎛σ ⎞ 0.66 1 ⎞ ⎛ 1 − ln⎜⎜ 2 ⎟⎟ = − ⎟ −5 ⎜ 2 8.62 × 10 ⎝ 373 300 ⎠ ⎝ σ1 ⎠ ( ) σ2=24.9 (Ωm)-1 c) 104 = n(1.6 × 10−19 )0.364 n = 1.72 × 1023 electrons / m3 4. Iron oxide (FeO) has the rock salt crystal structure. It has a density of 5.70 g/cm3, and the atomic weights are 55.85 g/mol for Iron, and 16.00 g/mol for Oxygen. a. Calculate the unit cell edge length. b. How does the result in part (a) compare with the edge length as determined from the ionic radii of 0.077 nm for Iron, and 0.140 nm for Oxygen? 5. Show that the minimum cation-to-anion radius ratio for the coordination number 8 is 0.732. the unit cell edge length is 2rA, and from the base of unit cell x 2 ( ) = 2 rA Or x = 2rA 2 2 ( ) + 2rA 2 2 = 8rA Now from the triangle that involves x, y, and the unit cell edge ( ) x 2 + 2 rA (2 rA 2) 2 2 ( = y 2 = 2 rA + 2rC ( + 4rA2 = 2rA + 2 rC ( ) 2 ) 2 ) 2 rA 3 − 1 = 2rC Which reduces to rC = 3 − 1 = 0.732 rA 6. Calculate the atomic packing factor for CsCl. Ionic radii are 0.170 nm for Cs+ and 0.181 nm for Cl-. 7. The repeating mer unit in the polyvinyl chloride is shown. A certain polyvinyl chloride specimen contains equal numbers of macromolecules with 1000 mers, 2000 mers, 3000 mers, and 5000 mers. The atomic weights of C, H, and Cl are , respectively, 12.01, 1.01, and 35.45 g/mol. a. Compute the mer molecular weight. b. Calculate is the number-average molecular weight. c. Calculate the weight-average molecular weight.