Ch. 19-20 Lect. 1 Carboxylic Acids and Derivatives I. Naming Carboxylic Acids
advertisement
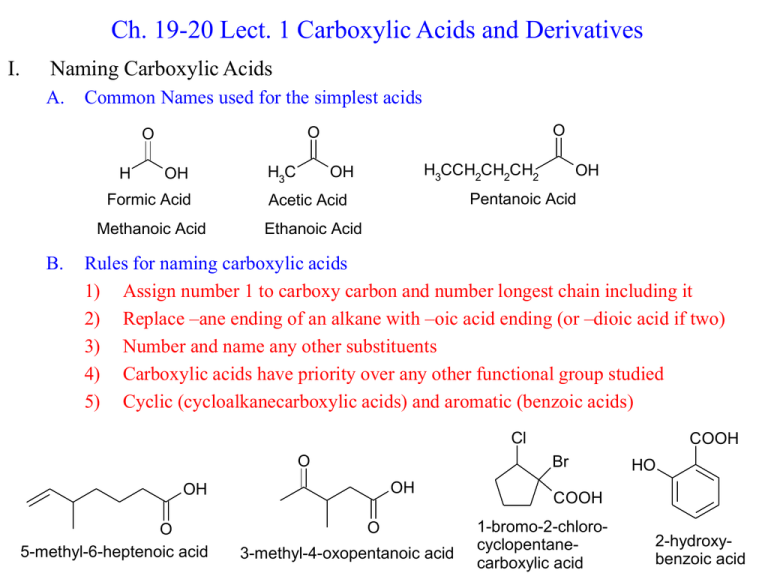
Ch. 19-20 Lect. 1 Carboxylic Acids and Derivatives I. Naming Carboxylic Acids A. Common Names used for the simplest acids H OH Formic Acid Methanoic Acid B. O O O H3C H3CCH2CH2CH2 OH OH Pentanoic Acid Acetic Acid Ethanoic Acid Rules for naming carboxylic acids 1) Assign number 1 to carboxy carbon and number longest chain including it 2) Replace –ane ending of an alkane with –oic acid ending (or –dioic acid if two) 3) Number and name any other substituents 4) Carboxylic acids have priority over any other functional group studied 5) Cyclic (cycloalkanecarboxylic acids) and aromatic (benzoic acids) Cl O Br OH OH O 5-methyl-6-heptenoic acid COOH O 3-methyl-4-oxopentanoic acid HO COOH 1-bromo-2-chlorocyclopentanecarboxylic acid 2-hydroxybenzoic acid II. Structure and Properties of Carboxylic Acids A. Planar sp2 hybridized structure 1) Hydrogen bonded dimers O H O R R O H 2) B. O High melting and boiling points because of hydrogen bonding (Table 19-2) NMR of Carboxylic Acids 1) Proton NMR a) Hydroxy proton strongly deshielded and variable 10-13 ppm b) CH2 next to carbonyl is deshielded due to electronegativity: 2-3 ppm 2) Carbon NMR a) Carbonyl carbon similar to aldehydes and ketones, but more shielded 180ppm O O R OH R + OH O R _ OH + C. IR of Carboxylic Acids 1. Carbonyl stretch at 1700 cm-1 2. O—H stretch at 2500—3300 is broad due to hydrogen bonding D. Acid-Base Characteristics of Carboxylic Acids 1. Fairly strong acids a. Electropositive carbonyl carbon pulls electrons away from O—H bond b. Resulting carboxylate anion is stabilized by resonance c. pKa’s usually between 4 and 5 O R d. e. 2. Base O_ R OH O O R _ O Electron withdrawing substituents increase the acidity: F3CCOOH pKa = 0.23 Carboxylate anion named as an alkanoate: formate, acetate, pentanoate Can be bases if protonated by other strong acids a. Carbonyl oxygen is the most basic (first one protonated) b. Resulting cation is stabilized by resonance (not so if hydroxy is protontated) + R H H O O OH R + OH III. Synthesis of Carboxylic Acids A. Oxidation of primary alcohols and aldehydes by Cr(VI) reagents RCH2 OH 1. Na2Cr2O7 H2SO4, H2O R C OH KMnO4 and HNO3 can also oxidize alcohols and adehydes to carboxylic acids 2 HNO3 + ClCH2CH2CHO B. O ClCH2CH2COOH + 2 NO2 + H2O Organometallic reagents attack carbon dioxide to give carboxylic acids CH3CH2Br + Mg CH3CH2MgBr CH3CH2MgBr + O=C=O CH3CH2COOCH3CH2COO- + H+/H2O CH3CH2COOH C. Hydrolysis of a Nitrile is preferred if other functional groups react with Grignard CH3CH2Br + -C≡N CH3CH2C≡N CH3CH2C≡N 1. OH- 2. H+/H2O CH3CH2COOH Mechanism Later! IV. The Addition-Elimination Mechanism A. The carboxy carbon is attacked by nucleophiles (like other carbonyls) 1) In aldehydes and ketones, addition is followed by aqueous workup to give alcohol 2) In Carboxylic Acids and derivatives, there is a potential leaving group: elimination O R O L R Carboxylic Acid Nu B. + H O H L C. X R Alkanoyl Halide OCOR R Anhydride R OR Ester NR2 Amide Acid Catalyzed Addition-Elimination Mechanism + O R R OH O O O O R O Nu H H R L Nu + H L O R H L H Nu + Base Catalyzed Addition-Elimination Mechanism _ O O O -Nu R L R Nu L R Nu H O+ R Nu O B R Nu D. Notes on Addition-Elimination Reactions 1. Hydroxide (and other derivitives) are generally poor leaving groups 2. Acidic Carboxylic Acid will protonate most basic nucleophiles 3. Formation of Carboxylate anion with strongly basic nucleophilies is irreversible O R 4. E. Base OH O O R O_ _ R O Weak bases (alcohols, amines, other neutral nucleophiles) can proceed in substitution without deprotonating the acid Relative Reactivities 1. Alkanoyl halides > Anhydrides > Esters > Acids > Amides 2. The potential leaving group decides the reactivity a. Electronegative leaving group activates the carbonyl carbon b. Resonance stabilizes the carbonyl group O R + X Reactive: Alkanoyl halide O R O .. NR2 R Unreactive: Amide _ NR2 +