Galvanic (or Voltaic) Cells Electrochemistry I.
advertisement

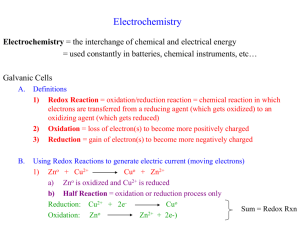
Galvanic (or Voltaic) Cells Electrochemistry = the interchange of chemical and electrical energy = used constantly in batteries, chemical instruments, etc… I. Galvanic Cells A. Definitions 1) Redox Reaction = oxidation/reduction reaction = chemical reaction in which electrons are transferred from a reducing agent (which gets oxidized) to an oxidizing agent (which gets reduced) 2) Oxidation = loss of electron(s) to become more positively charged 3) Reduction = gain of electron(s) to become more negatively charged B. Using Redox Reactions to generate electric current (moving electrons) 1) 16H+(aq) + 2MnO4-(aq) + 5Fe0(aq) 2Mn2+(aq) + 5Fe2+(s) + 8H2O(l) a) Fe2+ is oxidized and MnO4- is reduced b) Half Reaction = oxidation or reduction process only Reduction: 2(8H+ + MnO4- + 5eMn2+ + 4H2O) Sum = Redox Rxn 0 2+ Oxidation: 5(Fe Fe + 2e-) 2) 3) In solution: a) Fe2+ and MnO4- collide and electrons are transferred b) No work can be obtained; only heat is generated In separate compartments, electrons must go through a wire = Galvanic Cell a) Generates a current = moving electrons from Fe2+ side to MnO4- side b) Current can produce work in a motor c) Salt Bridge = allows ion flow without mixing solutions (Jello-like matrix) d) C. Chemical reactions occur at the Electrodes = conducting solid dipped into the solution i) Anode = electrode where oxidation occurs (production of e-) ii) Cathode = electrode where reduction occurs (using up e-) Cell Potential 1) Think of the Galvanic Cell as an oxidizing agent “pulling” electrons off of the reducing agent. The “pull” = Cell Potential a) ecell = Cell Potential = Electromotive Force = emf b) Units for ecell = Volt = V 1 V = 1 Joule/1 Coulomb 2) Voltmeter = instrument drawing current through a known resistance to find V Potentiometer = voltmeter that doesn’t effect V by measuring it II. Standard Reduction Potentials A. Standard Hydrogen Electrode 1) When measuring a value, you must have a standard to compare it to 2) Cathode = Pt electrode in 1 M H+ and 1 atm of H2(g) Half Reaction: 2H+ + 2eH2(g) e1/2 = 0 3) We will use this cathode to find ecell of other Half Reactions 4) Standard Reduction Potentials can be found in your text appendices a) Always given as a reduction process b) All solutes are 1M, gases = 1 atm 5) Combining Half Reactions to find Cell Potentials a) Reverse one of the half reactions to an oxidation; this reverses the sign of e1/2 b) Don’t need to multiply for coefficients = Intensive Property (color, flavor) c) Example: 2Fe3+(aq) + Cuo 2Fe2+(aq) + Cu2+(aq) i. Fe3+ + eFe2+ e1/2 = +0.77 V ii. Cu2+ + 2eCuo e1/2 = +0.34 V iii. Reverse of (ii) added to (i) = -0.34 V + +0.77 V = +0.43 V = ecell B. Direction of electron flow in a cell 1) Cell always runs in a direction to produce a positive ecell 2) Fe2+ + 2eFeo e1/2 = -0.44 V MnO4- + 5e- + 8H+ Mn2+ + 4H2O e1/2 = +1.51 V 3) We put the cell together to get a positive potential a) 5(Feo Fe2+ + 2e-) b) 2(MnO4- + 5e- + 8H+ Mn2+ + 4H2O) 16H+(aq) + 2MnO4-(aq) + 5Feo(s) e1/2 = +0.44 V e1/2 = +1.51 V 2Mn2+(aq) + 5Fe2+(aq) + 8H2O(l) ecell = 1.95V C. The complete description of a Galvanic Cell 1) Items to include in the description a) Cell potential (always +) and the balanced overall reaction b) Direction of electron flow c) Designate the Anode and the Cathode d) Identity of the electrode materials and the ions present with concentration 2) Example: Completely describe the Galvanic Cell based on these reactions Ag+ + eAg eo = +0.80 V Fe3+ + eFe2+ eo = +0.77 V Ag+(aq) + Fe2+(aq) Ago(s) + Fe3+(aq) eocell = +0.03 V Electrochemical Potential, Work, and Energy III. Potential, Work, and Energy A. Units 1) Joule (J) = unit of energy, heat, or work (w) = kg•m2/s2 2) Coulomb (C) = unit of electrical charge (q). 1 e- = 1.6 x 10-19 C 3) Volt (V) Work (J) = electrical potential (e) Charge (C) 4) 1 J of work is produced when 1 C of charge is transferred between two points differing by 1 V of electrical potential 5) 6) Work flowing out of a system (Galvanic Cell) is taken to be negative work Cell Potential is always positive ε 7) -w q or - w qε From last chapter, wmax = DG DG w max - qε max B. Electrochemical Problems 1) When current flows, we always waste some of the energy as heat instead of work w < wmax 2) We can, however, measure emax with a potentiometer, so we can find the hypothetical value of wmax 3) 4) Example: eocell = 2.50 V 1.33 mole e- pass through the wire. eactual = 2.10 V a) 1 Faraday (F) = the charge on 1 mole of electrons = 96,485 C (6.022 x 1023 e-/mol)(1.6 x 10-19 C/e-) = 96,485 C/mol b) c) w = -qe = -(1.33 mol e-)(96,485 C/mole e-)(2.10 J/C) = -2.69 x 105 J wmax = -qemax = -(1.33 mol e-)(96,485 C/mole e-)(2.50 J/C) = -3.21 x 105 J d) Efficiency = w/wmax = -2.69 x105 J/-3.21 x 105 J = 0.838 or 83.8% Free Energy (DG) DG w max - qε max nFε max a) q = nF where n = number of moles, F = 96,485 C/mole b) DG = -nFe (assuming the maximum e) c) Maximum cell potential is directly related to DG between reactants and products in the Galvanic Cell (This lets us directly measure DG) ε - DG Spontaneou s Process 5) Example: Calculate DGo for the reaction Cu2+(aq) + Fe(s) Cu(s) + Fe2+(aq) eo = 0.34 V o E cell = +0.78 V o e = 0.44 V a) Half Reactions: Cu2+ + 2eCuo Feo Fe2+ + 2e- b) DGo = -nFeo = -(2 mol e-)(96,485 C/mol e-)(0.78 J/C) = -1.5 x 105 J Example: Will 1 M HNO3 dissolve metallic gold to make 1 M Au3+? a) Half Reaction: NO3- + 4H+ + 3eNO + 2H2O eo = +0.96 V Auo Au3+ + 3eeo = -1.50 V Au(s) + NO3-(aq) + 4H+(aq) Au3+(aq) + NO(g) + 2H2O(l) eocell = -0.54V b) Since e is negative (DG = +) the reaction will not occur spontaneously 6) C. The Nernst Equation 1) Derivation a) DG = DGo + RTlnQ = -nFe b) DGo = -nFeo c) -nFe = -nFeo + RTlnQ oC, this simplifies to RT εε lnQ nF o 0.0592 εε logQ n o 2) At 25 3) Example: 2Al(s) + 3Mn2+(aq) 2Al3+(aq) + 3Mn(s) eocell = 0.48 V a) Oxidation: 2Al(s) 2Al3+(aq) + 6eb) Reduction: 3Mn2+(aq) + 6e3Mn(s) c) [Mn2+] = 0.5 M, [Al3+] = 1.5 M d) Q = [Al3+]2 / [Mn2+]3 = (1.5)2 / (0.5)3 = 18 0.0592 ε cell 0.48 V log18 0.47 V 6 e) f) As the reaction proceeds, ecell Calculating K: 0 (Q K) = Dead Battery! o 0.0592 nε 0 εo logK logK n 0.0592 IV. Electrolysis = using electric energy to produce chemical change (opposite of cell) A. Example 1) Consider the Cu/Zn Galvanic Cell a) Anode: Zn Zn2+ + 2eb) Cathode: Cu2+ + 2eCu eocell = +1.10 V 2) If we attach a power source of eo > +1.10 V, we can force e- to go the other way a) Anode: Cu Cu2+ + 2eb) Cathode: Zn2+ + 2eZn c) Called an Electrolytic Cell B. Calculations with Electrolytic Cells 1) How much Chemical Change? Is usually the question. 2) Find mass Cuo plated out passing 10 amps (10 C/s) through Cu2+ solution 30 min. a) Cu2+ + 2eCuo(s) b) Steps: current/time, charge (C), moles e-, moles Cu, grams Cu (10 C/s)(30 min)(60 s/min) 18,000 C 1 mol e - (18,000 C) 0.187 mol e 96,485 C 1 mol Cu (0.187 mol e-) 0.0935 mol Cu 2 mol e (0.0935 mol Cu)(63.546 g/mol) 5.94 g Cu Example: How long must a current of 5.00 amps be applied to a Ag+ solution to produce 10.5 g of silver metal? Electrolysis of Water 1) Galvanic: 2H2 + O2 2H2O (Fuel Cell) 2) Electrolytic Cell: a) Anode: 2H2O O2 + 4H+ + 4e-eo = -1.23 V b) Cathode: 4H2O + 4e2H2 + 4OHeo = -0.83 V c) Overall: 6H2O 2H2 + O2 + 4(H+ + OH-) 2H2O 2H2 + O2 eocell = -2.06 V 3) We must add a salt to increase the conductance of pure water [H+] = [OH-] = 10-7 3) C. D. Electrolysis of Mixtures 1) Mixture of Cu2+, Ag+, Zn2+; What is the order of plating out? a) Ag+ + eAg eo1/2 = +0.80 V b) Cu2+ + 2eCu eo1/2 = +0.34 V c) Zn2+ + 2eZn eo1/2 = -0.76 V 2) Reduction of Ag+ is easiest (eo = most positive) followed by Cu, then Zn 3) Example: Ce4+ (eo1/2 = +1.70 V), VO2+ (eo1/2 = +1.00 V), Fe3+ (eo1/2 = +0.77 V)